Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Surgical Strategy for Resecting Hepatocellular Carcinoma in the Caudate Lobe: Isolated or Combined Lobectomy? A Single-Center Study and Meta-Analysis
Authors Xu ZG , Zhang FH, Sun DW, Zheng QT, Ji GW, Wang K
Received 19 November 2021
Accepted for publication 13 January 2022
Published 26 January 2022 Volume 2022:9 Pages 13—25
DOI https://doi.org/10.2147/JHC.S349335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Zheng-Gang Xu,1– 3,* Fei-Hong Zhang,1– 3,* Dong-Wei Sun,1– 3,* Qi-Tong Zheng,1– 3 Gu-Wei Ji,1– 3 Ke Wang1– 3
1Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 2Key Laboratory of Liver Transplantation, Chinese Academy of Medical Sciences, Nanjing, People’s Republic of China; 3NHC Key Laboratory of Living Donor Liver Transplantation (Nanjing Medical University), Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ke Wang; Gu-Wei Ji
Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, People’s Republic of China, Tel +86 18061675088; +86 15951758275, Fax +86 68136450, Email [email protected]; [email protected]
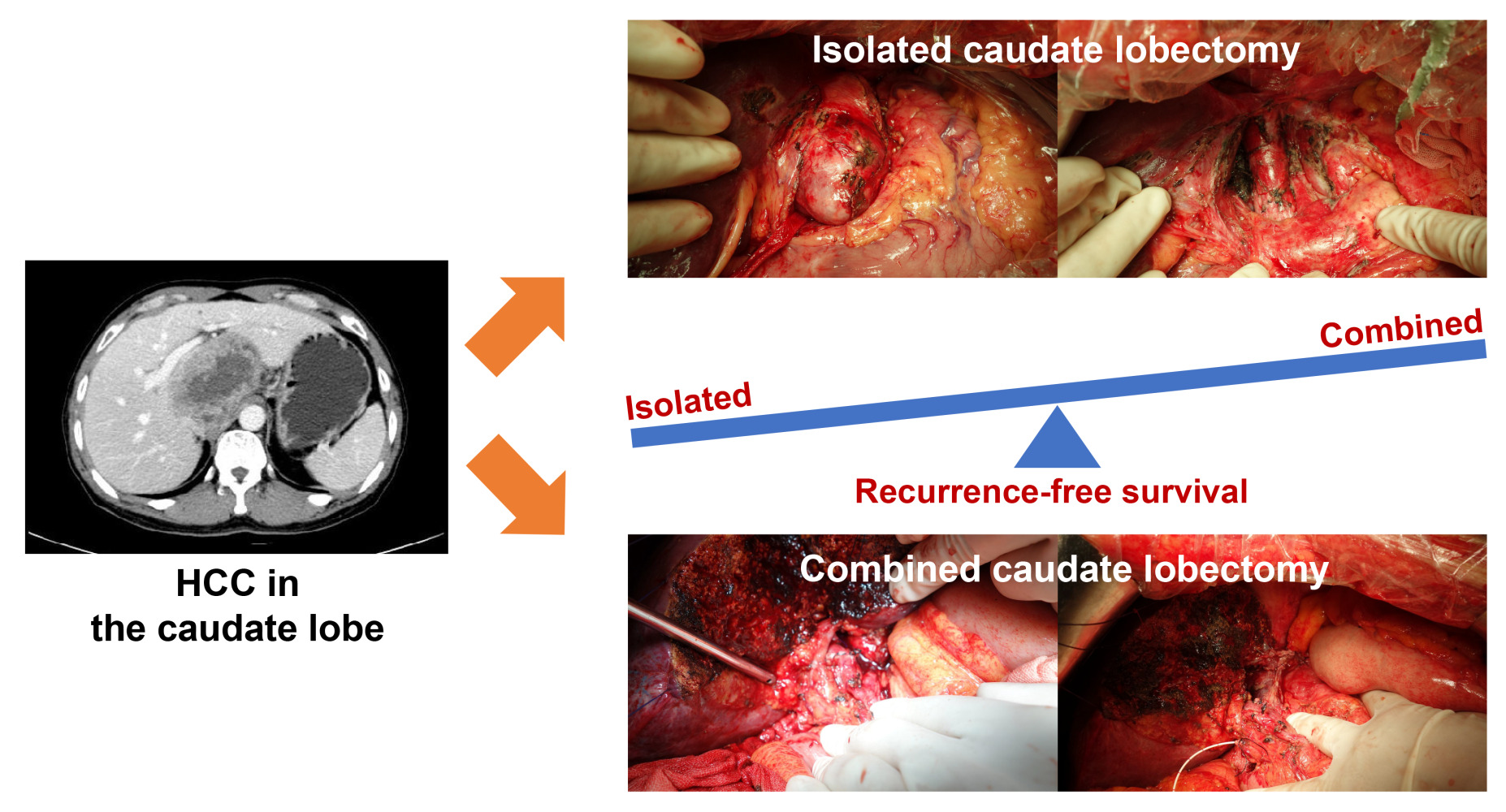
Background: Resection of hepatocellular carcinoma (HCC) originating in the caudate lobe remains challenging, while the optimal extent of resection is debated. We aimed to evaluate the relative benefits of combined caudate lobectomy (CCL) versus isolated caudate lobectomy (ICL) for caudate HCC.
Methods: Patients who underwent curative-intent resection for caudate HCC between January 2010 and December 2018 were identified from a single-center database. Surgical outcomes of the two strategy groups were analyzed before and after propensity score matching. A systematic review with meta-analysis was also performed to compare outcomes of CCL versus ICL for caudate HCC.
Results: A total of 28 patients were included: 11 in the CCL and 17 in the ICL group. Compared with ICL, the CCL group contained patients with larger tumors and a higher incidence of vascular invasion. After propensity score matching, 6 pairs of patients were selected. In the well-matched cohort, CCL demonstrated significantly improved recurrence-free survival (RFS) (P = 0.047) compared with ICL; no significant differences were noted for overall survival (OS), operation time, blood loss and morbidity rate. A total of 227 patients from nine eligible studies and ours were involved in the systematic review. Meta-analysis revealed that CCL provided better RFS (hazard ratio 0.54, 95% confidence interval 0.31– 0.92) than ICL; no significant differences were observed in OS, operation time, blood loss and morbidity rate.
Conclusion: CCL confers superior RFS over ICL without compromise of perioperative outcomes and should be prioritized for patients with caudate HCC when feasible, especially for those with large-sized tumors.
Keywords: hepatocellular carcinoma, caudate lobe, surgery, outcomes, meta-analysis
Graphical Abstract:
Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary liver malignancy and ranks sixth most common malignancy, with an increasing incidence worldwide.1,2 Surgical resection is considered to be the mainstay of potentially curative therapy for patients with localized HCC and preserved liver function.1–3 Over the past decade, advances in surgical techniques, patient selection, and perioperative management have significantly reduced surgical mortality to below 3% at specialized hepatobiliary centers.4 Despite the progress in liver surgery, resection of HCC originating in the caudate lobe of the liver remains technically challenging due to its deep location, tight confines, and close association with major vessels.5,6
After Couinaud’s nomenclature, the caudate lobe is now well defined anatomically and subdivided into three portions according to Kumon’s definition: (1) Spiegel lobe: situated on the left side of the inferior vena cava (IVC), dorsal to the Arantius’ ligament and the lesser omentum; (2) paracaval portion: situated on the ventral side of the IVC, covered by the right and middle hepatic vein cranially; (3) caudate process: situated between the right side of the IVC and the left side of the right posterior section, dorsal to the right portal pedicle.5 Different procedures to isolated caudate lobectomy (ICL), such as high dorsal resection, transhepatic anterior resection, and ventral approach, have been reported to improve the curability of caudate lobe tumors.6–8 Although such operations are relatively safe and potentially curative, it seems tough to achieve sufficient surgical margins via ICL, and combined caudate lobectomy (CCL) with adjacent hemi-liver or other segment is preferred in patients with acceptable liver function.6
Several studies have compared CCL with ICL in terms of postoperative outcomes, recurrence and survival; however, the reported data are rather contradictory leaving uncertainty and boosting long-lasting debates.9,10 Therefore, the aim of this study was to evaluate and validate the potential benefits of CCL compared with ICL for caudate HCC based on a single-center retrospective analysis as well as a systematic review with meta-analysis.
Materials and Methods
Patients
Patients who underwent curative-intent resection for caudate HCC between January 2010 and December 2018 at First Affiliated Hospital of Nanjing Medical University (Nanjing, China) were identified. Patients with incomplete clinicopathological information or missing follow-up data were excluded, as were patients who had any treatment (i.e., previous liver resection, local ablation, transarterial chemoembolization) before surgery and resection for ruptured tumor. The study was approved by the Institutional Review Board and in accordance with the Declaration of Helsinki. Patient consent was waived as retrospective anonymous data were analyzed.
Demographics, clinicopathological characteristics, intra-and post-operative parameters were retrospectively reviewed, including age, sex, etiology, cirrhosis, liver function indexes, alpha-fetoprotein (AFP) level, tumor size, tumor number, vascular invasion, tumor grade, intraoperative blood loss, blood transfusion, operative time, surgical margin status, postoperative complications and hospital stays. Macrovascular invasion was defined as the invasion of the vessel that was visible on gross examination, whereas microvascular invasion was defined as the invasion that was identifiable only on microscopy.11 All patients underwent surveillance with AFP, liver function, chest and abdominal imaging every 3 months for the first 2 years and every 6 months thereafter in accordance with the clinical practice guidelines of the European Society for Medical Oncology.12 Overall survival (OS) and recurrence-free survival (RFS) were defined as the time from the date of surgery to the date of death and the date of first tumor recurrence/metastasis, respectively. Survival data were censored on December 15, 2020.
Operative Technique
The resection approaches (left-side, right-side, bilateral or anterior) and resection extents (CCL or ICL) were predominantly determined by tumor size, location, liver function and, most importantly, presence of macroscopic vascular invasion. Specifically, left-side or right-side approach was respectively indicated for Spiegel or process HCC while bilateral approach or anterior approach was indicated for paracaval HCC or complete resection of the caudate lobe. ICL was mainly indicated for HCC with marginal localization while CCL was mainly indicated for HCC with center localization or suspicion of vascular invasion as well as acceptable liver function. Nevertheless, the surgical procedure may be altered after dissecting to guarantee safety and curability. Hepatic parenchymal transection was performed using clamp-crushing method with Pringle maneuver under ultrasonographic guidance.
Literature Search and Selection Criteria
We performed a comprehensive literature search in four databases (PubMed, the Cochrane Library, the Web of Science and Embase) through May 2021 by using the following algorithm:
(‘carcinoma, hepatocellular’[MeSH]) AND (caudate) AND (hepatectomy OR hemi-hepatectomy OR lobectomy OR segmentectomy OR excision OR resection OR surgical OR surgery)
Studies included in this meta-analysis met the following inclusion criteria: (1) full-text publications written in English; (2) pathologically confirmed HCC in the caudate lobe; (3) surgical strategy (CCL or ICL) specified in the publication. The exclusion criteria were as follows: (1) reported in abstract form only; (2) studies not comparing CCL versus ICL. Figure 1 shows the schematic diagram of study selection.
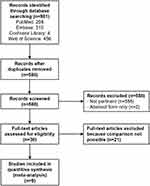 |
Figure 1 Flow diagram of systematic review. |
Data Extraction, Quality Assessment, and Outcomes
Data for study-, patient-, and surgery-related characteristics were independently extracted by two investigators (Z.G.X and F.H.Z) using a pre-designed template. The GetData Graph Digitizer software (version 2.26; http://getdata-graph-digitizer.com) was employed to digitize and extract data from the published Kaplan-Meier curves where necessary. We estimated the sample mean and standard deviation from the reported skewed data using the Box-Cox and Quantile Estimation methods described by McGrath et al.13 Discrepancies between two investigators were resolved by referencing to the original article, with any further disagreement arbitrated by senior investigator (G.W.J). Publication bias was evaluated statistically using Egger’s regression test, and graphically using Funnel plot. The trim-and-fill method was fitted for adjustment when we observed a statistically significant bias. The Newcastle-Ottawa scale (NOS) was applied to assess study quality and the score ≥ 6 was defined as high quality. We performed additional analysis after excluding low-quality studies to verify the stability of study results. The primary outcome of interest was long-term survival, including OS and RFS. Intraoperative parameters (operation time and blood loss) and postoperative complications (morbidity and mortality) were secondary outcomes.
Statistical Analysis
Categorical variables were presented as number (percentage) unless otherwise indicated and compared using χ2 or Fisher’s exact test when appropriate. Continuous variables were reported as median (range) and compared using Student’s t-test or Mann-Whitney U-test when appropriate. Survival curves were calculated using Kaplan–Meier method and compared using Log rank test. The associations between variables and survival were analyzed by using Cox regression analysis; variables that achieved statistical significance in univariate analysis were included in multivariate analysis to identify the most reliable prognostic factor. We note that patient selection bias and confounding variables between CCL and ICL could impact on the comparison of outcomes; therefore, propensity score matching (PSM) was performed with one-to-one nearest neighbor matching and fixed caliper width of 0.2 to identify matched sets using variables found to be significantly different between two strategies. A two-sided P<0.05 was considered statistically significant.
In terms of the meta-analysis, survival endpoints (OS and RFS) were assessed using the hazard ratio (HR) with corresponding 95% confidence interval (CI) and continuous endpoints (operation time and blood loss) were assessed using the weighted mean difference (WMD) with 95% CI. Dichotomous endpoints (morbidity and mortality) were assessed using the risk difference (RD) with 95% CI because risk ratios were not available in several studies. Statistical heterogeneity was explored by using the I2 statistic and the Q test. A random-effect model was adopted when P < 0.1 and I2 > 50%, suggesting substantial heterogeneity; otherwise, a fixed-effect model was applied. All analyses were conducted using SPSS (version 26.0; SPSS, Inc. Chicago, IL) and Stata MP (version 14.0; Stata Corporation, Parallel Edition).
Results
Patient Characteristics and Surgical Procedures
A total of 28 patients who underwent curative-intent resection of caudate HCC were recruited into this study after the exclusion criteria were applied. The median patient age was 58 years (range, 41–78 years) and 24 patients (85.7%) were men. All patients were of Child-Pugh grade A and ICG-R15<10%. We performed a complete resection of the caudate lobe in 8 patients (28.6%) and partial resection in 20 patients (71.4%).
There were 11 patients (39.3%) in the CCL group and 17 patients (60.7%) in the ICL group. Table S1 summarizes the surgical procedures in each treatment group. Baseline characteristics of patients in the original (unmatched) cohort are shown in Table 1. Compared with patients treated with ICL, those who received CCL had larger tumors (P=0.048) and a higher incidence of vascular invasion (P=0.009), especially macrovascular invasion; other characteristics were comparable between groups.
 |
Table 1 Baseline Characteristics |
Surgical Outcomes Before and After PSM
In the unmatched cohort, after a median follow-up of 24.9 months (range, 4.4–83.6 months), the 5-year OS rates were 51.9% (95% CI, 28.7–93.9%) and 58.4% (95% CI, 34.0–100%) in the CCL and ICL groups, respectively; the 5-year RFS rates were 54.5% (95% CI, 31.8–93.6%) and 9.8% (1.7–57.1%) in the CCL and ICL groups, respectively. No significant differences were found in both OS (P=0.245) and RFS (P=0.253) between the two strategies. Kaplan–Meier survival curves stratified by surgical strategy are shown in Figure 2. In an unmatched cohort of patients treated with these strategies, CCL was associated with significantly longer operation time (P<0.001), more blood loss (P=0.005) and increased length of stay (P<0.001) compared with ICL. No significant differences were observed between the groups in blood transfusion rate, morbidity rate and mortality rate.
We noted that patients with larger tumor or vascular invasion were less likely to receive ICL. PSM was then performed to match these patients on tumor size and vascular invasion. A well-matched cohort of 12 patients produced by PSM are given in Table 1. The median follow-up time was 38.9 months (range, 9.6–72.9 months). The OS rates were comparable in the matched CCL and ICL groups (P=0.215); however, CCL was associated with improved RFS compared with ICL (P=0.047). No statistically significant differences were noted for operation time, blood loss, morbidity rate, and mortality rate among patients undergoing CCL versus ICL in the matched cohort.
Prognostic Factors for Caudate HCC
We next sought to explore the independent prognostic factors in the original cohort using Cox regression modeling (Table 2). The univariate analysis indicated that high bilirubin level (HR, 1.089; 95% CI, 1.001–1.185; P=0.046) and vascular invasion (HR, 4.810; 95% CI, 1.218–19.00; P=0.025) were significantly associated with poor OS while vascular invasion (HR, 4.726; 95% CI, 1.795–12.44; P=0.002) and liver cirrhosis (HR, 2.664; 95% CI, 1.041–6.821; P=0.041) were significantly associated with early recurrence. The multivariate analysis demonstrated that vascular invasion was the only independent prognostic factor for OS (HR, 4.788; 95% CI, 1.176–19.50; P=0.029) and RFS (HR, 4.039; 95% CI, 1.293–12.61; P=0.016).
 |
Table 2 Prognostic Variables |
Meta-Analysis of Main Outcome Measures
A total of nine studies, among the many identified by initial search, were included, involving 199 patients.9,10,14–20 All these studies were retrospective, with median NOS score of 7 (range, 5–8) (Table S2). We combined the extracted data with our own data for the full meta-analysis of main outcomes (Tables 3 and S3).
 |
Table 3 Summary of Reports on Oncological Outcomes of Resected Caudate HCC |
Of the nine selected studies, six reported OS as an outcome,9,10,14–16,19 while five reported RFS as an outcome.9,10,14–16 Regarding OS, a total of 137 patients were analyzed and the pooled HR was 0.87 (95% CI, 0.46–1.62) with low statistical heterogeneity (I2=10.0%; P=0.353), suggesting no benefit of CCL over ICL (Figure 3A). We did not find evidence of publication bias based on Egger’s test (t=0.37; P=0.723) (Figure 3B). Similar results were obtained after excluding two studies of low quality (Figure 3C). Regarding RFS, a total of 114 patients were analyzed and the pooled HR was 0.54 (95% CI, 0.31–0.92) with low statistical heterogeneity (I2=0.0%; P=0.881), demonstrating a benefit of CCL over ICL (Figure 4A). No significant publication bias was identified via Egger’s test (t=0.66; P=0.543) (Figure 4B). The RFS benefit of CCL over ICL was further confirmed after excluding two low-quality studies (Figure 4C).
Among the nine selected studies, seven provided information on intraoperative parameters (operation time and blood loss),10,15–20 while six reported postoperative complications (morbidity and mortality).10,15,16,18–20 Regarding operation time, a total of 192 patients were analyzed and the pooled WMD calculated by means of a random effects model (P<0.001 for the test of heterogeneity; I2=89.9%) was 24.91 (95% CI, −41.81–91.63), indicating that CCL operations were not significantly longer in duration than ICL operations (Figure S1A). Significant publication bias was evidenced by Egger’s test (t=2.51; P=0.046) (Figure S1B). We therefore used the trim-and-fill method to recalculate the pooled WMD (−23.87; 95% CI, −84.96–37.22), with three missing studies imputed to produce a symmetrical Funnel plot (Figure S1C). Similar results were obtained after excluding two low-quality studies (Figure S1D). Regarding blood loss, a total of 192 patients were analyzed and the pooled WMD fitted by a random effects model (P<0.002 for the test of heterogeneity; I2=68.6%) was 75.15 (95% CI, −284.06–434.35), indicating that CCL operations were not significantly higher in blood loss than ICL operations (Figure S2A). Significant publication bias was evidenced by Egger’s test (t=2.99; P=0.024) (Figure S2B). The trim-and-fill method was adopted to recalculate the pooled WMD (−270.65; 95% CI, −657.58–116.29), with four missing studies imputed to produce a symmetrical Funnel plot (Figure S2C). Similarly, blood loss did not differ significantly between strategies after excluding two low-quality studies (Figure S2D).
On the other hand, a total of 153 patients were enrolled in the meta-analysis of the association between surgical strategy and postoperative complication. When postoperative morbidity was examined, no significant difference was found between strategies, with the pooled RD of −0.08 (95% CI, −0.23–0.06) and low statistical heterogeneity (I2=15.3%; P=0.313) (Figure S3A). No significant publication bias was observed via Egger’s test (t=1.33; P=0.240) (Figure S3B). A similar trend was observed after excluding two low-quality studies (Figure S3C). When postoperative mortality was examined, no significant difference was noted between strategies, with the pooled RD of 0.01 (95% CI, −0.07–0.09). No significant between-study heterogeneity (I2=0.0%; P=0.979) as well as publication bias (Egger’s test: t=0.26; P=0.807) were observed (Figure S4A and B). Similar results were obtained after excluding two studies of low quality (Figure S4C).
Discussion
Complete resection represents the potentially curative treatment for caudate HCC. Among various types of hepatectomy, caudate lobectomy represents the highly advanced level. Different surgical approaches have been described to guarantee surgical safety and oncological curability of caudate HCC.6–8 The ideal one should optimize locoregional control with sufficient surgical margins to reduce recurrence and improve survival, yet preserve as much remnant liver parenchyma to minimize postoperative liver dysfunction. Therefore, CCL or ICL represents the core level in a decision-making system for surgical procedure.6 Although previous reports have compared the surgical outcomes of CCL with those of ICL, all published studies are single-institutional with limited sample sizes and no meta-analysis has tried to integrate the results of these studies. Based on the PSM analysis of a single-center HCC database as well as a systematic review with meta-analysis, we showed that CCL was associated with improved RFS compared with ICL; there were no significant differences between strategies in OS, operation time, blood loss, morbidity rate and in-hospital mortality.
From the surgical viewpoint, CCL is mainly indicated for caudate HCC patients with macrovascular invasion and well-preserved liver function; ICL is preferred for small-sized and marginally located tumors. In the original cohort, we observed that CCL did not confer a survival benefit over ICL while multivariate Cox analysis demonstrated that vascular invasion was the only independent determinant of prognosis. However, the CCL group contained more patients with larger tumors and macrovascular invasion, which are well-established prognostic factors and associated with surgical difficulty.1–4 Therefore, the two resection strategies may be compared only by PSM method to correct for potential confounding. So, when we utilized PSM, it resulted in the removal of patients with small-sized tumor (≤3 cm) and macrovascular invasion. In the well-matched cohort, we underlined that CCL reduced the risk of tumor recurrence compared with ICL, which may be attributed to a reduced incidence of tumor exposure during liver parenchymal transection, a wider negative margin at the cut-stump, and the avoidance of repeated squeezes and turning over of the tumor-bearing caudate lobe that may enhance the spread of tumor cells into the circulation in CCL strategy.6 Nevertheless, CCL did not provide a significantly better OS even in the well-matched cohort, which can be explained by the fact that various factors other than tumor burden and liver function are related to survival time, such as performance status, other cause of death, recurrence pattern and treatment.1–4 Compared with ICL, CCL was also not associated with prolonged operation time, increased blood loss, and higher morbidity in the matched cohort. Notably, these findings highlighted by our own data are consistent with the results of our meta-analysis.
Recently, Takayama et al.6 have first established an algorithm that consists of three clinical factors (tumor sublocation, size, and ICG-R15) to provide a recommendation of the resection strategy for caudate HCCs with various background livers. The “Takayama algorithm” prioritizes combined hemi-hepatectomy over isolated resection for patients with caudate HCC≥3cm and ICG-R15<10%. Interestingly, in the well-matched cohort, our results showed the potential oncological superiority of CCL over ICL while only patients with large HCC (>3 cm) and good ICG-R15 (<10%) were included; hence, our results confirmed the validity of the “Takayama algorithm”.
Several limitations of the present study should be acknowledged. First, the sample size of our study cohort is small. Nevertheless, consistent results between our PSM cohort and meta-analysis were obtained. Second, although our meta-analysis included a relatively large number of caudate HCC patients treated with resection, meta‐regression or subgroup analysis was not performed to parse the significant heterogeneity among studies due to insufficient details of the studies involved. Third, all eligible studies were derived from Asian, and most patients had hepatitis B virus-related HCC. Our results require future validation in non-Asian populations for the generalizability. Fourth, our results were composed of expert surgeons’ experiences and may not be applicable for every surgeon because short-term and long-term outcomes of caudate HCCs can be influenced by the surgeon’s skills.
Conclusions
In conclusion, our study demonstrates the oncological superiority of CCL over ICL without compromise of perioperative outcomes based on the PSM analysis of a single-center HCC database and a systematic review with meta-analysis. We recommend the prioritization of CCL over ICL for patients with caudate HCC and acceptable liver function, especially for those with large-sized tumors.
Abbreviations
HCC, hepatocellular carcinoma, IVC, inferior vena cava; ICL, isolated caudate lobectomy; CCL, combined caudate lobectomy; AFP, alpha-fetoprotein; OS, overall survival; RFS, recurrence-free survival; NOS, Newcastle-Ottawa scale; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; WMD, weighted mean difference; RD, risk difference.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, Ke Wang, upon reasonable request.
Ethics Approval and Informed Consent
This study protocol was approved by the Institution Review Board of First Affiliated Hospital of Nanjing Medical University and in accordance with the Declaration of Helsinki. Written informed consent was waived because retrospective anonymous data were analyzed.
Funding
This study was supported by Key Program of the National Natural Science Foundation of China (31930020), National Natural Science Foundation of China (82102150) and Natural Science Foundation of Jiangsu Province (BK20210968).
Disclosure
The authors declare no potential conflicts of interest in this work.
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
3. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
4. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi:10.1053/j.gastro.2018.08.065
5. Kumon M. Anatomical study of the caudate lobe with special reference to portal venous and biliary branches using corrosion liver casts and clinical application. Liver Cancer. 2017;6(2):161–170. doi:10.1159/000454682
6. Takayama T, Midorikawa Y, Higaki T, et al. Algorithm for resecting hepatocellular carcinoma in the caudate lobe. Ann Surg. 2021;273(6):e222–e229. doi:10.1097/SLA.0000000000003384
7. Higaki T, Takayama T, Midorikawa Y. Ventral approach for resecting hepatocellular carcinoma in the caval portion of the caudate lobe. Surgery. 2018;163(6):1245–1249. doi:10.1016/j.surg.2018.01.002
8. Midorikawa Y, Takayama T. Caudate lobectomy (segmentectomy 1) (with video). J Hepatobiliary Pancreat Sci. 2012;19(1):48–53. doi:10.1007/s00534-011-0450-1
9. Tanaka S, Shimada M, Shirabe K, et al. Surgical outcome of patients with hepatocellular carcinoma originating in the caudate lobe. Am J Surg. 2005;190(3):451–455. doi:10.1016/j.amjsurg.2004.12.005
10. Liu P, Qiu BA, Bai G, et al. Choice of approach for hepatectomy for hepatocellular carcinoma located in the caudate lobe: isolated or combined lobectomy? World J Gastroenterol. 2012;18(29):3904–3909. doi:10.3748/wjg.v18.i29.3904
11. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi:10.1053/j.gastro.2009.06.003
12. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871–873. doi:10.1093/annonc/mdy510
13. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A; DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520–2537. doi:10.1177/0962280219889080
14. Yamamoto T, Kubo S, Shuto T, et al. Surgical strategy for hepatocellular carcinoma originating in the caudate lobe. Surgery. 2004;135(6):595–603. doi:10.1016/j.surg.2003.10.015
15. Shimada M, Matsumata T, Maeda T, Yanaga K, Taketomi A, Sugimachi K. Characteristics of hepatocellular carcinoma originating in the caudate lobe. Hepatology. 1994;19(4):911–915. doi:10.1002/hep.1840190417
16. Yang MC, Lee PO, Sheu JC, Lai MY, Hu RH, Wei CK. Surgical treatment of hepatocellular carcinoma originating from the caudate lobe. World J Surg. 1996;20(5):562–565. doi:10.1007/s002689900087
17. Peng SY, Li JT, Liu YB, et al. Surgical treatment of hepatocellular carcinoma originating from caudate lobe–a report of 39 cases. J Gastrointest Surg. 2006;10(3):371–378. doi:10.1016/j.gassur.2005.09.026
18. Sakoda M, Ueno S, Kubo F, et al. Surgery for hepatocellular carcinoma located in the caudate lobe. World J Surg. 2009;33(9):1922–1926. doi:10.1007/s00268-009-0110-7
19. Zhou Y, Zhang X, Wu L, Xu D, Li B. Surgical outcomes of hepatocellular carcinoma originating from caudate lobe. ANZ J Surg. 2013;83(4):275–279. doi:10.1111/j.1445-2197.2012.06232.x
20. Shimada S, Kamiyama T, Yokoo H, et al. Prognoses and clinicopathological characteristics for hepatocellular carcinoma originating from the caudate lobe after surgery. World J Surg. 2019;43(4):1085–1093. doi:10.1007/s00268-018-4869-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



