Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Surgical Strategies Affect the Long-Term Prognosis of Patients with Hepatocellular Carcinoma Adjacent to the Left Branch of the Portal Vein
Authors Bai S, Shen X, Liu J , Lu C, Wang J, Liu L, Wang C, Wang H, Liu K, Sun Y, Xue F
Received 25 October 2023
Accepted for publication 13 December 2023
Published 27 December 2023 Volume 2023:10 Pages 2355—2366
DOI https://doi.org/10.2147/JHC.S443137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Shilei Bai,1,* Xiaojing Shen,2,* Jianwei Liu,1,* Caixia Lu,1 Jie Wang,1 Liu Liu,1 Chunyan Wang,1 Huifeng Wang,3 Kai Liu,4 Yanfu Sun,1 Feng Xue1
1Department of Hepatic Surgery II, the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Naval Medical University), Shanghai, People’s Republic of China; 2Department of Pathology, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China; 3Department of Hepatic Surgery, the Fifth Clinical Medical College of Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 4Department of Biliary Tract Surgery IV, the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Naval Medical University), Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feng Xue; Yanfu Sun, Department of Hepatic Surgery II, the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Naval Medical University), 225 Changhai Road, Shanghai, 200433, People’s Republic of China, Tel +86-21-81875513, Fax +86-21-65562400, Email [email protected]; [email protected]
Purpose: When hepatocellular carcinoma (HCC) is closely associated with the left branch of the portal vein, there is still controversy regarding the surgical approach.
Methods: This study enrolled 330 HCC patients with tumors adjacent to the left branch of the portal vein. Among them, 85 patients underwent left hemihepatectomy (LH), while the remaining 235 underwent liver lobectomy (LL), which included left medial segmentectomy or left lateral sectionectomy. Perioperative complications, time to recurrence and overall survival (OS) were compared using propensity score matching.
Results: LH resulted in a lower 5-year recurrence rate and higher 5-year OS rate than LL (56.5% vs 74.0%, p=0.002; 67.4% vs 53.5%, p=0.029). The LL group showed a higher tendency for early recurrence (ER) and intrahepatic recurrence (IR). The cumulative IR rates at 1- 3-, and 5-years for the LH group and the LL group were 17.0%, 36.7%, 45.1% and 33.8%, 57.1%, 63.7%, respectively, with a p-value of 0.007. There was no statistically significant difference in the cumulative ER rates between the two groups at 1-, 3-, and 5- years. Furthermore, the LH group and the LL group had similar perioperative complications, and no cases of liver failure occurred.
Conclusion: LH, compared to LL, reduced the IR rate and ER rate in HCC patients with tumor adjacent to the left branch of the portal vein. It improved the OS outcome of the patients, and there was no significant difference in perioperative complications between the two groups.
Keywords: hepatocellular carcinoma, left hemihepatectomy, liver lobectomy, propensity score matching, recurrence, overall survival
Introduction
Liver cancer is one of the most common and deadly malignancies, causing over 780,000 deaths worldwide each year.1 Hepatocellular carcinoma (HCC) is the predominant histological type, accounting for approximately 80% of all liver cancers.2,3 For early to intermediate-stage HCC, anatomical liver resection is the preferred treatment for patients with good liver function, as it can effectively reduce intrahepatic metastasis by removing the portal vein system.2–6 However, there are still challenges regarding high postoperative recurrence rates and poor overall survival, especially in patients with perivascular HCC.7,8
When the tumor is located in the left medial lobe or left lateral lobe adjacent to the left branch of the portal vein, there are generally two surgical options: left hemihepatectomy (LH) or liver lobectomy (LL: left medial sectionectomy or left lateral sectionectomy).9,10 Currently, there is still controversy regarding which surgical approach is most suitable for this type of patient. LH can provide better surgical margins, but HCC patients often have underlying liver cirrhosis caused by hepatitis B or C, and excessive liver tissue resection may increase the risk of postoperative complications. On the other hand, LL often have surgical margins close to the tumor, which may increase the risk of recurrence and metastasis, affecting the long-term prognosis of these patients.11,12 Therefore, further evaluation is needed to assess the impact of these two surgical approaches on HCC patients with tumors adjacent to the left branch of the portal vein.
In this study, we retrospectively evaluated a group of HCC patients with tumor adjacent to the left branch of the portal vein who underwent LH or LL. We used propensity score matching (PSM) to match the clinical and pathological characteristics of these cases and compared the short-term and long-term outcomes of the two surgical approaches for HCC patients with involvement of major blood vessels.
Patients and Methods
This study consecutively enrolled a total of 3315 HCC patients who underwent liver resection at the Third Affiliated Hospital of Naval Medical University (Eastern Hepatobiliary Surgery Hospital) from January 2013 to December 2017. The inclusion criteria were as follows: (1) Pathologically confirmed as a solitary HCC; (2) Tumor located only in the left lateral lobe or left medial lobe; (3) The tumor is adjacent to the left branch of the portal vein, with a distance of less than 1cm; (4) Liver function classified as Child-Pugh A or B7; (5) No major vascular invasion or extrahepatic metastasis; (6) Surgical resection classified as R0. Exclusion criteria: (1) Tumor not closely adjacent to the left branch of the portal vein; (2) Tumor involving both liver lobes (left lateral lobe and left medial lobe; left medial lobe and right anterior lobe or caudate lobe); (3) Multiple HCCs; (4) Surgical resection classified as R1 or R2; (5) Presence of portal vein tumor thrombus or hepatic vein tumor thrombus; (6) Incomplete clinical data. In the end, LH was performed on 85 HCC patients, while LL was conducted on 245 HCC patients. This study was approved by the hospital’s ethics committee (EHBHKY2021-K-036), and informed consent was obtained from each patient to use their data for scientific research.
Surgical Procedure
Standard preoperative examinations encompass liver function, renal function, coagulation function, tumor markers (AFP, CEA, CA19-9), HBV and HCV antigen/antibody, HBV-DNA, and a standard electrocardiogram. Patients with concurrent cardiac conditions undergo additional dynamic electrocardiogram and echocardiography. Imaging assessments comprise a chest X-ray or chest computed tomography (CT), abdominal ultrasound, and contrast-enhanced CT or magnetic resonance imaging (MRI) of the upper abdomen. Preoperative diagnosis follows the guidelines set by the American Association for the Study of Liver Diseases (AASLD).13
Liver resection surgeries are performed by our surgical team consisting of experienced surgeons (Y.F.S., W.P.Z., F.S., K.W., J.M.Yet al) with over ten years of expertise in liver operations. Anatomical resection is typically performed in patients with perivascular hepatocellular carcinoma. However, for patients with severe liver cirrhosis or tumors in peripheral locations, non-anatomical resection is performed.
Postoperative Follow-Up
Patients were scheduled for follow-up visits every 2 months during the initial 6-month period, and subsequently every 6 months. Follow-up evaluations included monitoring peripheral blood tumor markers (such as AFP), ultrasound examinations, and contrast-enhanced CT or MRI of the abdomen. Additional contrast-enhanced CT or MRI scans of the abdomen were conducted if tumor recurrence or metastasis was suspected. In cases where typical signs of liver cancer were detected on imaging, it was classified as intrahepatic recurrence or metastasis. PET-CT scans or bone scans were performed if necessary to confirm the diagnosis. The study endpoints were overall survival (OS) and time to recurrence (TTR). OS was calculated from the date of liver resection to the date of death or last follow-up, while TTR was calculated from the date of liver resection to the date of first HCC recurrence or last follow-up. Tumor progression is categorized as either intrahepatic or extrahepatic recurrence. Early recurrence was defined as recurrence within 2 years after surgery. Intrahepatic recurrence (IR) is defined as the appearance of new tumors within the liver, regardless of contact with the resection margin. Extrahepatic recurrence (ER) includes all metastasis lesions diagnosed outside the liver.14
Statistical Analysis
The statistical analysis was performed using R software, version 4.0.0 (http://www.r-project.org). Categorical variables were presented as numbers (n) and percentages (%). For categorical variables, either the Chi-square test or Fisher’s exact test was used for comparisons, depending on the circumstances. Propensity score matching (PSM) using a 1:1 nearest-neighbor matching method was applied to adjust for confounding factors between the two groups based on baseline variables. Overall survival (OS) and time to recurrence (TTR) were calculated using the Kaplan-Meier method with Log rank tests. Univariate and multivariate Cox regression analyses were conducted to identify independent risk factors for OS and TTR. In the multivariate Cox regression model, variables with a significance level of P<0.05 in the univariate analysis were selected using forward stepwise variable selection. A statistical significance level of P<0.05 was considered in all analyses.
Results
Patient Characteristics
Inclusion and exclusion criteria for patients are presented in Figure 1. Among the included 330 patients, there were 85 patients who underwent LH, and 245 patients who underwent LL. The median follow-up time was 58.4 months (range, 6.3–75.6 months) in the LH group and 54.0 months (range, 3.0–73.5 months) in the LL group. Compared to patients who underwent LL, patients who underwent LH had a higher proportion of age <60 years (85.9% vs 72.7%), platelet count >100*10^9/mL (89.4% vs 78.4%), absence of ascites (97.6% vs 89.8%), and HCC located in the left medial section (48.2% vs 33.5%). To eliminate biases caused by baseline differences, we conducted PSM separately for the two groups. PSM was performed using a 1:1 nearest-neighbor matching method, resulting in 85 HCC patients being included in both the LH group and the LL group (Table 1).
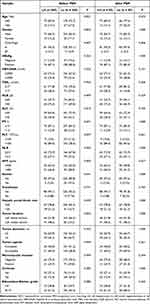 |
Table 1 Basal Clinicopathological Characteristics of HCC Patients Before and After PSM |
 |
Figure 1 Flow chart of patient’s inclusion. |
Perioperative Outcomes
Perioperative outcomes of patients undergoing LH and LL are shown in Table 2. There were no significant differences between the LH group and the LL group in terms of pleural effusion, ascites, postoperative bleeding, surgical site infection, respiratory complications, postoperative liver failure, bile leakage, and postoperative mortality.
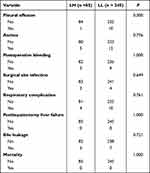 |
Table 2 Perioperative Outcomes of HCC According to the Operative Procedure |
Recurrence and OS Before and After PSM
During the follow-up period, 22 out of 85 patients (22/85) who underwent LH died, while 87 out of 245 patients (87/245) who underwent LL died. The cumulative recurrence rates at 1-, 3-, and 5- years in the two groups were 21.4%, 46.6%, and 56.5% for the LH group, and 37.8%, 62.5%, and 69.7% for the LL group, respectively, with a P-value of 0.006 (Figure 2A). The cumulative OS rates at 1-, 3-, and 5- years in the two groups were 96.4%, 85.8%, and 67.4% for the LH group, and 89.2%, 65.8%, and 54.9% for the LL group, respectively, with a P-value of 0.022 (Figure 2B). After PSM, 31 deaths occurred in the LL group. The cumulative recurrence rates at 1-, 3-, and 5- years for the LH group were 21.4%, 46.6%, and 56.5%, respectively, while for the LL group, they were 43.9%, 67.4%, and 74.0%, respectively, with a P-value of 0.002 (Figure 2C). The cumulative OS rates for the two groups at 1-, 3-, and 5- years were 96.4%, 85.8%, and 67.4% for the LH group, and 91.6%, 64.4%, and 53.5% for the LL group, respectively, with a P-value of 0.029 (Figure 2D).
Patterns of Recurrence After Liver Resection Before and After PSM
The patterns of recurrence in HCC patients are shown in Table 3, during the follow-up period, among the 85 patients in the LH group, 43 patients experienced recurrence, including 31 cases of IR and 12 cases of ER. In the 245 patients in the LL group, 153 patients experienced recurrence, including 113 cases of IR and 40 cases of ER. The cumulative rates of IR at 1-, 3-, and 5- years were 17.0%, 36.7%, and 45.1% in the LH group, and 29.8%, 52.5%, and 57.9% in the LL group, respectively (P=0.015) (Figure 3A). The cumulative rates of ER at 1-, 3-, and 5- years were 5.3%, 16.7%, and 20.7% in the LH group, and 11.3%, 21.0%, and 28.0% in the LL group, respectively (P=0.220) (Figure 3B). In the LH group, there were 29 cases of early recurrence, while the LL group had 121 cases of early recurrence. Compared to the LL group, the LH group achieved a lower rate of early recurrence (34.1% vs 49.3%, p=0.021). (Table 3) After PSM, 85 patients were included in each group. In the LL group, 57 out of 85 patients (57/85) experienced recurrence, including 41 cases of IR and 16 cases of ER (Table 3). The cumulative IR rates at 1-, 3-, and 5- years for the LH group were 17.0%, 36.7%, and 45.1%, respectively, while for the LL group, they were 33.8%, 57.1%, and 63.7%, respectively, with a P-value of 0.007 (Figure 3C). The cumulative ER rates for the two groups were 5.3%, 16.7%, and 20.7% for the LH group, and 15.2%, 24.1%, and 28.3% for the LL group at 1-, 3-, and 5- years, respectively, with a P-value of 0.120 (Figure 3D). In the LH group, there were 29 cases of early recurrence, while the LL group had 45 cases of early recurrence. Compared to the LL group, the LH group achieved a lower rate of early recurrence (34.1% vs 52.9%, p=0.020) (Table 3).
 |
Table 3 Patterns of Recurrence After Different Liver Resection |
Treatment-Related Prognostic Factors
In the multivariate analysis of all patients (n=330), factors associated with recurrence were LL (hazard ratio [HR], 1.74; 95% CI, 1.23–2.46; p=0.002), positive HBsAg (HR, 1.57; 95% CI, 1.05–2.35; p=0.029), ALT ≥ 44 U/L (HR, 1.43; 95% CI, 1.05–1.94; p=0.025), tumor diameter ≥ 5cm (HR, 1.84; 95% CI, 1.37–2.46; p<0.001), incomplete tumor capsule (HR, 1.46; 95% CI, 1.08–1.98; p=0.013), and positive MVI (HR, 1.58; 95% CI, 1.16–2.16; p=0.004) were independent risk factors for recurrence (Table 4).
 |
Table 4 Univariate and Multivariate Cox Regression Analysis of Recurrence in HCC Patients Before PSM |
LL (HR, 1.63; 95% CI, 1.02–2.63; p=0.043), tumor diameter ≥ 5cm (HR, 1.95; 95% CI, 1.33–2.87; p=0.001), incomplete tumor capsule (HR, 1.79; 95% CI, 1.19–2.70; p=0.006), and positive MVI (HR, 1.91; 95% CI, 1.28–2.84; p=0.001) were independent risk factors for OS (Table 5).
 |
Table 5 Univariate and Multivariate Cox Regression Analysis of Overall Survival in HCC Patients Before PSM |
Discussion
This study confirms that for HCC patients with tumors adjacent to the portal vein, LH provides lower recurrence rates and better OS outcomes compared to LL. It reduces intrahepatic recurrence and early recurrence rates without increasing perioperative complications. This is the first article to report on the short-term and long-term effects of different surgical approaches on HCC patients with tumors adjacent to the portal vein.
The surgical treatment of HCC aims to completely remove the tumor to achieve pathological negativity, clear any possible invasion of blood vessels and adjacent organs, and/or prevent potential intrahepatic or extrahepatic metastasis beyond the tumor.15 When patients have good liver function, anatomical liver resection is the preferred surgical approach for early-to-intermediate stage liver cancer, as it effectively reduces intrahepatic recurrence by removing the portal vein system and improves overall survival.15–19 However, the surgical margin is also a key factor affecting long-term prognosis in patients undergoing anatomical liver resection.20,21 Zhang et al study showed that among MVI-positive HCC patients undergoing anatomical liver resection, those with a surgical margin less than 1 cm had poorer 1-, 3-, and 5-year recurrence-free survival rates and overall survival rates compared to patients with a surgical margin greater than 1 cm.20 When tumors are adjacent to major blood vessels, LL often does not provide an ideal surgical margin as important vascular structures need to be preserved, resulting in poorer long-term prognosis for patients. In this study, the 1-, 3-, and 5- years recurrence rates were 21.4%, 46.6%, and 56.5% for the LL group and 43.9%, 67.4%, and 74.0% for the LH group. The 1-, 3-, and 5- years overall survival rates were 96.4%, 85.8%, and 67.4% for the LL group and 91.6%, 64.4%, and 53.5% for the LH group, showing statistically significant differences. Multivariate Cox regression analysis also confirmed that LL was an independent risk factor influencing recurrence and overall survival in HCC patients with tumors adjacent to the left portal vein. These results provide guidance for the selection of surgical approaches in clinical practice. If the tumor is adjacent to the left portal vein and liver function allows, LH is recommended.
The LH group, compared to the LL group, showed a reduction in early recurrence rate. After propensity score matching (PSM), 52.9% of the recurrence cases in the LL group were early recurrences, while only 34.1% of the cases in the LH group were early recurrences. The LH group also demonstrated a lower intrahepatic recurrence rate. After PSM, among 85 patients who underwent LL, 41 experienced intrahepatic recurrence, whereas among 85 patients who underwent LH, only 31 experienced intrahepatic recurrence. Moreover, the LH group had lower 1-, 3-, and 5- years intrahepatic recurrence rates compared to the LL group. These results may be attributed to the reason that some microsatellite nodules that form through the peritumor portal system may not be detected on pretreatment imaging and therefore cannot be effectively removed by lobectomy. This is because only the tumor and some of the surrounding liver tissue are removed, while most of the liver segments carrying the tumor are not removed. LH group removing more liver tissue close to the tumor, thus clearing potential intrahepatic recurrence foci and microvascular invasion.14,22,23
In this study, there was no statistically significant difference in perioperative complications between the two groups of patients. This is because both the LH and LL are well-established surgical procedures. Additionally, the LH involves removing only approximately 30% of the total liver volume, which is relatively smaller compared to the right hepatectomy. Surasak et al analyzed 155 cases of donor livers and found that the average volume ratios of the left lateral lobe, left medial segment, caudate lobe, right anterior segment, and right posterior segment were 17%, 14%, 2%, 37%, and 30%, respectively.24 Therefore, in patients with Child-Pugh A or B7 liver function, the left hepatectomy does not increase the risk of postoperative liver failure.
This study has the following limitations: Firstly, it is a retrospective study, which has inherent limitations. Better designed prospective studies should be conducted to further evaluate the impact of LH and LL on these patients. Secondly, the majority of patients in this study had HCC associated with hepatitis B infection, and it is uncertain whether the results obtained in this study can be extrapolated to HCC related to hepatitis C or alcohol. Lastly, although a single-center study can achieve standardization of surgical approaches, the fact that this study is conducted at a single center is also a limitation as it may limit the generalizability of our findings.
In conclusion, LH compared to LL, reduces the intrahepatic recurrence rate and early recurrence rate in HCC patients with involvement of the left branch of the portal vein. It improves the overall survival outcome of patients, and there is no significant difference in perioperative complications between the two procedures.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
Retrospective review and collection of patient data was approved by The study was approved by the Eastern Hepatobiliary Surgery Hospital ethics committee. No further ethical approval was required for this retrospective study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Supported by the National Natural Science Foundation for Young Scholars (82002459).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Galle PR. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
3. Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surgery Nutrition. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
4. Marubashi S, Gotoh K, Akita H, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015;102(7):776–784. doi:10.1002/bjs.9815
5. Jiao S, Li G, Zhang D, et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surgery. 2020;80:243–255. doi:10.1016/j.ijsu.2020.05.008
6. Jung DH, Hwang S, Lee YJ, et al. Small Hepatocellular Carcinoma With Low Tumor Marker Expression Benefits More From Anatomical Resection Than Tumors With Aggressive Biology. Ann Surg. 2019;269(3):511–519. doi:10.1097/SLA.0000000000002486
7. Wu F, Chen B, Dong D, et al. Phase 2 Evaluation of Neoadjuvant Intensity-Modulated Radiotherapy in Centrally Located Hepatocellular Carcinoma: a Nonrandomized Controlled Trial. JAMA Surgery. 2022;157(12):1089–1096. doi:10.1001/jamasurg.2022.4702
8. Yu W, Wang W, Rong W, et al. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surgeons. 2014;218(3):381–392. doi:10.1016/j.jamcollsurg.2013.11.030
9. Zou J, Li S, Wang Q, et al. Surgical strategies for hepatocellular carcinoma located in the left lateral lobe: a propensity score-matched and prognostic nomogram study. Cancer Med. 2021;10(10):3274–3287. doi:10.1002/cam4.3894
10. Orimo T, Kamiyama T, Kakisaka T, et al. Central Hepatectomy Versus Major Hepatectomy for Centrally Located Hepatocellular Carcinoma: a Propensity Score Matching Study. Ann Surg Oncol. 2021;28(11):6769–6779. doi:10.1245/s10434-021-09751-z
11. Bai S, Yang P, Liu J, et al. Surgical Margin Affects the Long-Term Prognosis of Patients With Hepatocellular Carcinoma Undergoing Radical Hepatectomy Followed by Adjuvant TACE. oncologist. 2023;28(8):e633–e644. doi:10.1093/oncolo/oyad088
12. Aoki T, Kubota K, Hasegawa K, et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg. 2020;107(1):113–120. doi:10.1002/bjs.11329
13. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
14. Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi:10.1097/SLA.0000000000000710
15. Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64(3):594–600. doi:10.1016/j.jhep.2015.10.015
16. Kato Y, Sugioka A, Kojima M, et al. Minimally Invasive Anatomic Liver Resection for Hepatocellular Carcinoma Using the Extrahepatic Glissonian Approach: surgical Techniques and Comparison of Outcomes with the Open Approach and between the Laparoscopic and Robotic Approaches. Cancers. 2023;15(8):2219. doi:10.3390/cancers15082219
17. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. doi:10.1097/01.sla.0000231758.07868.71
18. Zhong XP, Zhang YF, Mei J, et al. Anatomical versus Non-anatomical Resection for Hepatocellular Carcinoma with Microscope Vascular Invasion: a Propensity Score Matching Analysis. J Cancer. 2019;10(17):3950–3957. doi:10.7150/jca.32592
19. Hokuto D, Nomi T, Yasuda S, et al. Does anatomic resection improve the postoperative outcomes of solitary hepatocellular carcinomas located on the liver surface? Surgery. 2018;163(2):285–290. doi:10.1016/j.surg.2017.08.024
20. Zhang XP, Xu S, Lin ZY, et al. Significance of anatomical resection and resection margin status in patients with HBV-related hepatocellular carcinoma and microvascular invasion: a multicenter propensity score-matched study. Int j Surgery. 2023;109(4):679–688. doi:10.1097/JS9.0000000000000204
21. Liu J, Zhuang G, Bai S, et al. The Comparison of Surgical Margins and Type of Hepatic Resection for Hepatocellular Carcinoma With Microvascular Invasion. oncologist. 2023.
22. Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131(3):311–317. doi:10.1067/msy.2002.121892
23. Sasaki A, Kai S, Iwashita Y, et al. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103(2):299–306. doi:10.1002/cncr.20798
24. Leelaudomlipi S, Sugawara Y, Kaneko J, et al. Volumetric analysis of liver segments in 155 living donors. Liver Transplantation. 2002;8(7):612–614. doi:10.1053/jlts.2002.33731
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


