Back to Journals » Infection and Drug Resistance » Volume 16
Surgical Resection to Treat a Japanese Patient with Pulmonary Coccidioidomycosis
Authors Abe T, Yamaguchi F , Sakakura S, Shiratori Y, Mase A, Funaki T, Kamio Y, Suzuki T, Shikama Y , Hoshino Y
Received 16 December 2022
Accepted for publication 27 April 2023
Published 8 May 2023 Volume 2023:16 Pages 2787—2791
DOI https://doi.org/10.2147/IDR.S401752
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Takashi Abe,1,* Fumihiro Yamaguchi,1,* Shunsuke Sakakura,1 Yo Shiratori,1 Ayaka Mase,1 Toshitaka Funaki,1 Yoshito Kamio,2 Takashi Suzuki,2 Yusuke Shikama,1 Yasutaka Hoshino3
1Department of Respiratory Medicine, Showa University Fujigaoka Hospital, Yokohama, Japan; 2Departments of Thoracic Surgery, Showa University Fujigaoka Hospital, Yokohama, Japan; 3Department of Chemotherapy and Mycoses, National Institute of Infectious Diseases, Tokyo, Japan
*These authors contributed equally to this work
Correspondence: Fumihiro Yamaguchi, Department of Respiratory Medicine, Showa University Fujigaoka Hospital, 1-30 Fujigaoka, Aoba-ku, Yokohama, 227-8501, Japan, Tel +81-45-971-1151, Email [email protected]
Abstract: Coccidioidomycosis is an endemic disease that is particularly prevalent in the United States. However, its geographic distribution is becoming widespread. Here, we present a Japanese male who resided in the United States for 1 year, where he was diagnosed with pulmonary coccidioidomycosis that was accompanied by cavity formation. He did not tolerate antifungal therapy and consequently underwent partial resection of the upper lobe of his left lung upon his return to Japan. The patient’s symptoms improved after surgery. The trend toward global networking and logistics means that a diagnosis of coccidioidomycosis should be considered in routine practice in nonendemic areas. Due to the rarity of surgical treatment for this disease, prolonged follow-up is necessary. During the last follow-up, the patient was symptom-free.
Keywords: coccidioidomycosis, Coccidioides immitis, Coccidioides posadasii, endemic disease
Introduction
Coccidioidomycosis is caused by fungi in the genus Coccidioides, which comprises two species: C. immitis and C. posadasii.1 The disease is endemic to the United States, Central America, and South America. In particular, there are areas of high endemicity in the United States, including the San Joaquin Valley in California and the south-central region of Arizona.2,3 Nonetheless, the geographic distribution and the number of new cases reported from East Asia, India, Canada, and Europe have shown a recent increase.4 The majority of coccidioidal infections are acquired through inhalation of airborne arthroconidia. Reportedly, 60% infections are completely asymptomatic.5 Furthermore, the onset of pulmonary coccidioidomycosis typically resolves without any sequelae and with apparent long-lived immunity in spite of no antifungal therapy;6 however, radiographic abnormalities, including pulmonary nodules, are occasionally detected after the acute phase, and they can be difficult to distinguish from malignancies following imaging examination. Persistent or chronic pulmonary coccidioidomycosis is characterized by cavities and accompanied by prolonged symptoms owing to immune-mediated responses, including fatigue, fever, erythema nodosum, and arthralgias, with the latter three known as the triad of desert rheumatism.4 In those cases, some therapeutic intervention including antifungal therapy and surgical resection may be required. Generally, amphotericin B is used for severe or emergency cases and azole antifungals for chronic diseases. Herein, we present a case of surgical resection to treat a Japanese patient with pulmonary coccidioidomycosis involving cavity formation as a result of the rarity of surgical treatment for this disease.
Method
Resection samples were cultured on potato dextrose agar medium at 25°C. DNA was extracted from clinical isolates by hot-water extraction method. Primers for nucleic acid amplification were designed as indicated in Supplemental Figure 1. PCRs were performed to amplify some regions in the internal transcribed spacer (ITS) region and the D1/D2 region of the 28S ribosomal RNA gene. All PCR assays were carried out in 50-µL volumes containing template DNA, 1 unit of DNA polymerase, and 50 µM of each primer. Cycling parameters were 10 sec at 98°C, 15 sec at 55°C, and 30 sec at 68°C for 35 cycles. The amplified products were purified and sequenced. The identified sequences were subjected to homology searches in the GenBank and MycoBank databases (Supplemental Figure 2). Institutional approval was required to publish the case details (approved number 22–224-B).
Case Report
A 58-year-old Japanese male who was an ex-smoker and living in Bakersfield, California, in the United States for 1 year for business, presented with fatigue, myalgia, and arthralgia. He was serologically diagnosed with pulmonary coccidioidomycosis following a positive test for Coccidioides that showed a positive IgM and IgG of anti-Coccidioides antibodies. The coccidioidal complement fixation test showed a titer of 1:2. Sputum cultures were not performed. He was started on oral antifungal medication (fluconazole, 800 mg/day) soon after diagnosis. He returned to Japan 6 months thereafter and immediately visited our hospital and discontinued any antifungal therapy because of the side effect of hair loss. He had no past medical history including a compromised immune system. Serum gamma globulin level was within normal limit and HIV antibody test was negative. No eosinophilia (4% of all leukocytes; 232 /μL), and serum (1–3)-β-D-glucan levels were not elevated (1.5 pg/mL). A computed tomography scan revealed a well-circumscribed nodule, 20 mm in size, in the upper lobe of the left lung (Figure 1A). The nodule had formed a cavity with a maximum diameter of 8 mm during the course of the disease (Figure 1B). Although a bronchoscopic examination was performed, the resultant specimens were culture-negative. The nodule was also suspected of pulmonary malignancy. Ultimately, we partially resected the left upper lobe. The resected samples were sectioned and subsequently stained using hematoxylin and eosin as well as Grocott methenamine silver staining. Histological examination revealed necrosis of lung tissue (Figure 2A), spherical bodies characteristic of Coccidioides (Figure 2B), and some endospores (Figure 2C). The culture derived from the mashed nodule eventually became positive for C. immitis in 11 days (Figure 3), which was confirmed by DNA sequencing of the ITS region and the D1/D2 region of the 28S ribosomal RNA gene. The patient’s symptoms improved after surgery. Follow-up examination was performed every 2 months without antifungal therapy, and the patient has been asymptomatic for 2 years after surgery with no worsening outcomes on imaging examinations (Figure 4).
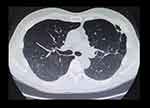 |
Figure 4 Computed tomography scan results of our patient 2 years after resection. No new lesions and no lung deterioration. |
Discussion
We presented the case of a Japanese patient initially diagnosed with pulmonary coccidioidomycosis in the United States by serologic testing. The diagnosis was eventually confirmed upon his return to Japan by fungal culture derived from resected samples following surgery at our hospital. The patient had been living in the city of Bakersfield, which is situated near the San Joaquin Valley, an area that is highly endemic for coccidioidomycosis. Even if infection is established, 60% of patients remain asymptomatic, whereas coccidioidomycosis appears to confer lifelong immunity.4 However, the progression of pulmonary coccidioidomycosis should be constantly monitored in cases of non-indigenous individuals because, unlike the indigenous population, non-indigenous individuals may not harbor any immunity to coccidioidomycosis. Symptomatic coccidioidomycosis can occur even in individuals with normal immunity because type 2 immune responses exacerbate the disease by reducing interferon-γ. Indeed, the efficacy of type 2 immune response blockade in coccidioidomycosis has been reported.7 In addition, elevated eosinophil count is supposed as a predictive feature of coccidioidomycosis.8
Most cases of focal pulmonary coccidioidomycosis do not require treatment, even if cavities form.4 However, our patient had suffered from the disease for over 6 months despite continuous administration of an antifungal agent. Azoles, including fluconazole, are key drugs for coccidioidomycosis treatment in terms of oral and prolonged administration.9,10 However, our patient could not tolerate the side effects of the antifungal therapy. Furthermore, imaging findings did not completely eliminate the possibility of pulmonary malignancy. Additionally, increased age is a supposed risk factor for disease progression,11 and our patient was middle-aged. He underwent a partial lobectomy expecting a complete recovery. According to the clinical practice guidelines on the management of coccidioidomycosis published by the Infectious Diseases Society of America, patients with a healthy immune who have symptomatic chronic pulmonary coccidioidomycosis with cavity lesions should be treated with an oral antifungal agent. However, they also state that surgery should be considered when the cavities are intractable to antifungal treatment.12 Meanwhile, the long-term outcomes after surgery remain poorly defined because such treatment for this condition is rare. A previous postoperative study reported that antifungal therapy was administered to ≥40% of patients.13 Although our patient has not required postoperative therapeutic intervention to date, prolonged follow-up monitoring of radiographic changes and sputum culture results is warranted.
This study had several limitations. First, serological tests were not performed after resection and the transfer of antibodies was unknown. Second, the follow-up period lasted for only 2 years after surgery and further follow-up is needed.
Ethical Approval
Official approval for the study was obtained in advance from the Ethics Committee at Showa University (approved number 22-224-B).
Patient Consent for Publication
The patient provided written informed consent for publication of the case details and any accompanying images.
Acknowledgments
We thank the Department of Chemotherapy and Mycoses, National Institute of Infectious Diseases, Tokyo, Japan, and the Department of Pathology, National Institute of Infectious Diseases, Tokyo, Japan, for performing the fungal culture and DNA sequencing and the pathological diagnosis, respectively.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding sources.
Disclosure
We declare that we have no conflict of interest in connection with this paper, and we received no payment or services from a third party in relation to this study.
References
1. Sharpton TJ, Stajich JE, Rounsley SD, et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19(10):1722–1731. doi:10.1101/gr.087551.108
2. Brown J, Benedict K, Park BJ, Thompson GR. Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013;5:185–197. doi:10.2147/CLEP.S34434
3. Boro R, Iyer PC, Walczak MA. Current Landscape of Coccidioidomycosis. J Fungi. 2022;8(4):413. doi:10.3390/jof8040413
4. Crum NF. Coccidioidomycosis: a Contemporary Review. Infect Dis Ther. 2022;11(2):713–742. doi:10.1007/s40121-022-00606-y
5. Wilson L, Ting J, Lin H, et al. The rise of valley fever: prevalence and cost burden of Coccidioidomycosis infection in California. Int J Environ Res Public Health. 2019;16(7):1113. doi:10.3390/ijerph16071113
6. Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36(12):1394–1402. doi:10.2105/AJPH.36.12.1394
7. Tsai M, Thauland TJ, Huang AY, et al. Disseminated coccidioidomycosis treated with interferon-γ and dupilumab. N Engl J Med. 2020;382(24):2337–2343. doi:10.1056/NEJMoa2000024
8. Ramadan FA, Ellingson KD, Canales RA, Bedrick EJ, Galgiani JN, Donovan FM. Cross-sectional study of clinical predictors of Coccidioidomycosis, Arizona, USA. Emerg Infect Dis. 2022;28(6):1091–1100. doi:10.3201/eid2806.212311
9. Ampel NM. Coccidioidomycosis: changing concepts and knowledge gaps. J Fungi. 2020;6(4):354. doi:10.3390/jof6040354
10. Bajwa AK, Rongkavilit C. Update on Coccidioidomycosis in the United States and beyond. Glob Pediatr Health. 2020;7:2333794X20969282. doi:10.1177/2333794X20969282
11. Diep AL, Hoyer KK. Host response to Coccidioides infection: fungal immunity. Front Cell Infect Microbiol. 2020;10:581101. doi:10.3389/fcimb.2020.581101
12. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of Coccidioidomycosis. Clin Infect Dis. 2016;63(6):e112–e146. doi:10.1093/cid/ciw360
13. Jaroszewski DE, Halabi WJ, Blair JE, et al. Surgery for pulmonary coccidioidomycosis: a 10-year experience. Ann Thorac Surg. 2009;88(6):1765–1772. doi:10.1016/j.athoracsur.2009.07.075
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



