Back to Journals » Drug Design, Development and Therapy » Volume 14
Suppression of Cisplatin-Induced Hepatic Injury in Rats Through Alarmin High-Mobility Group Box-1 Pathway by Ganoderma lucidum: Theoretical and Experimental Study
Authors Hassan HM , Al-Wahaibi LH, Elmorsy MA, Mahran YF
Received 10 February 2020
Accepted for publication 5 May 2020
Published 11 June 2020 Volume 2020:14 Pages 2335—2353
DOI https://doi.org/10.2147/DDDT.S249093
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Hanan M Hassan,1 Lamya H Al-Wahaibi,2 Mohammed A Elmorsy,3 Yasmen F Mahran4,5
1Department of Pharmacology and Biochemistry, Faculty of Pharmacy, Delta University for Science & Technology, Gamasa City, Dakhliya, Egypt; 2Department of Chemistry, College of Sciences, Princess Nourah bint Abdulrahman University, Riyadh, KSA, 11671, Saudi Arabia; 3Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt; 4Department of Pharmacology and Toxicology, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt; 5Department of Pharmaceutical Sciences, Faculty of Pharmacy, Princess Nourah bint Abdulrahman University, Riyadh, KSA, Saudi Arabia
Correspondence: Hanan M Hassan Tel +2 050 2770140
Fax +20502770145
Email [email protected]
Purpose: Drug-induced liver injury (DILI) is the most common cause of acute liver failure. The aim of this study was to investigate the molecular mechanisms by which Ganoderma lucidum mushroom (GLM) may ameliorate cisplatin (CP)-induced hepatotoxicity theoretically and experimentally.
Materials and Methods: Thirty-six male Sprague-Dawley (SD) rats were divided into six groups, two of them are normal and Ganoderma lucidum control groups. Liver injury was induced by a single dose of CP (12 mg/kg i.p) in four groups, one of them is CP control group. Besides cisplatin injection in day 1, rats in groups (4– 6) were subjected to GLM (500 mg/kg/day) either every other day or daily oral dose or via i.p injection for 10 consecutive days.
Results: In this study, GLM supplementation caused significant reduction of elevated high-mobility group box-1 (HMGB-1) with a concurrent decline in TNF-α and upregulation of IL-10 compared to the CP group (P< 0.05). The histopathological and fibrosis evaluation significantly confirmed the improvement upon simultaneous treatment with GLM. Moreover, immunohistochemical examination also confirmed the recovery following GLM treatment indicated by downregulation of NF-κB, p53 and caspase-3 along with upsurge of B-cell lymphoma 2 (Bcl-2) expression (P< 0.05). GLM treatment significantly decreased serum levels of hepatic injury markers; ALT, AST, T. bilirubin as well as oxidative stress markers; MDA and H2O2 with a concomitant increase in hepatic GSH and SOD. Also, the performed docking simulation of ganoderic acid exhibited good fitting and binding with HMGB-1 through hydrogen bond formation with conservative amino acids which gives a strong evidence for its hepatoprotective effect and may interpret the effect of Ganoderma lucidum.
Conclusion: GLM attenuated hepatic injury through downregulation of HMGB-1/NF-kB and caspase-3 resulted in modulation of the induced oxidative stress and the subsequent cross-talk between the inflammatory and apoptotic cascade indicating its promising role in DILI.
Keywords: drug-induced liver injury, Ganoderma lucidum mushroom, ganoderic acid, cisplatin-induced hepatotoxicity, inflammatory cytokines, high-mobility group box-1
Introduction
Drug-induced liver injury (DILI) is the most common cause of acute liver failure worldwide.1,2 Cisplatin (CP) is one of the most common and potent anti-neoplastic agents used against testicular, ovarian, cervical, as well as bladder malignancies.3–6 Despite its effectiveness, CP use is limited due to its serious toxic side effects such as nephrotoxicity,7 ototoxicity and neurotoxicity.8 Some studies had ascertained that hepatotoxicity is another important dose-limiting side effect of CP-based chemotherapy.9,10 During the aggressive treatment protocols, higher doses of CP that may be required for effective tumor suppression which could lead to hepatotoxicity that is also encountered during low-dose repeated CP therapy.11 In addition, CP induces direct breaks in DNA strands of the basal epithelium, causing release of reactive oxygen species (ROS) and direct damage to the cells.12 Indeed, oxidative stress plays an important role in this toxicity, and it is a sign of mitochondrial damage.13 Furthermore, inflammation, an oxidative stress complication, is characterized by excessive production of cytokines, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6).11,14 Accordingly, stress can simultaneously provoke both adaptive and apoptotic responses; the integration of these programmed survival and death signals determines the fate of the cell. Bcl-2 and p53 are key proteins in the regulation of apoptosis following stress.15,16 The immanent common finding of the hepatotoxicity among cancer patients who are treated with CP boosts the urgent need of an ideal hepatoprotective natural product that improves clinical outcome among those cancer patients. Therefore, development of an effective intervention is seen as a high priority in oncological supportive care.
High-mobility group box-1 (HMGB-1) is a ubiquitous protein, identified as an alarmin molecule, participating in the pathogenesis of acute as well as chronic liver injuries.1,17 HMGB-1 protein contains two similar DNA-binding domains A-box and B-box, each with three α helices that fold into an L- or V-shaped structure containing a negatively charged acidic tail that interacts with specific residues within and between the HMG boxes and thus regulates the 3D structure and DNA binding of the protein.18 Hepatocytes actively secrete HMGB-1 in response to oxidative stress induced by pro-oxidants, and appear to be the major cellular source of HMGB-1 in the damaged liver.19 Alarmins, upon binding their respective receptors, mobilize and activate immune cells, which will further take part in host defense and tissue repair,20 a property that explains the immunomodulatory activities of HMGB-1 upon which it is classified as an “endokine.”21 Furthermore, HMGB-1 itself, or in conjunction with other pro-inflammatory cytokines as interleukin1-beta (IL1-β), interferon gamma (IFNγ) and TNF-α amplifies the inflammatory cascade by stimulating the production of certain cytokines.22,23
Ganoderma lucidum mushroom (GLM) is a valuable source of biologically active substances.24 It has a hepatoprotective action against alcohol, cadmium and tert-butyl hydroperoxide (t-BHP)-induced liver injury through anti-oxidative and anti-inflammatory mechanisms.25–27 Chemically, GLM comprises terpenes, proteins, polysaccharides, amino acids, flavonoids, alkaloids, steroids, mannitol, and other minor compounds.28 Ganoderic acid (GA) is one of the most explored terpenes of the GLM in which GA-A and GA-H effectively suppress the processes of cell proliferation, metastasis, and adhesion by modulating the expression of nuclear transcription factors as activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κB).29 Although the beneficial properties of GLM had been well defined, the potential mechanisms by which it may protect the liver against the cytotoxic effects have not been fully exploited yet. Thus, this study is designed to investigate the molecular mechanisms by which GLM may attenuate hepatotoxicity experimentally and theoretically.
Materials and Methods
Drugs and Chemicals
Cisplatin was obtained from (Sigma Chemical Co., St. Louis, MO, USA). Ganoderma lucidum was obtained from (DXN Pharmaceutical SDN, BHD Malaysia) as a powder form.
Animals
Male Sprague-Dawley rats (120–140 g) were obtained from Experimental Animal Center, Mansoura University, and were housed in certified animal cages, under controlled environmental conditions, at room temperature 22 ±2◦C and a 12-hour light-dark cycle, food and water were provided ad libitum. The rats were allowed to acclimatize to the laboratory environment for 7 days before the start of the experiment. The Ethical Committee of the Faculty of Pharmacy, University of Delta for Sciences and Technology approved the experimental protocols (FPDU1/2019), in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
Experimental Design
A total of thirty six healthy adult male Sprague-Dawley (SD) rats (120–240 g) body weight obtained from experimental animal center, Mansoura University were used for the experiments. The rats were housed under standard light conditions (12 h light/12 h dark) with food and water available ad libitum and were divided into six groups: (n = 6)/each.
Control Group (Cont)
Rats were received distilled water (1000 mg/dl) daily for 10 consecutive days using oral gavage.
Ganoderma lucidum Group (GL)
Rats were received GLM (500 mg/kg/day) in distilled water (1000 mg/dl) daily for 10 consecutive days using oral gavage and served as GL control group.
Cisplatin Group (CP)
Rats were injected with a single dose of Cisplatin (12 mg/kg i.p) on day 1 only and served as cisplatin group.
The following groups were injected with a single dose of Cisplatin (12 mg/kg i.p) only on day 1, and were co-treated with GLM by different regimens for 10 consecutive days as follows:
Every Other Day Group (EOD)
Besides cisplatin injection on day 1, rats were subjected to GLM (500 mg/kg/day) in distilled water on days 1, 3, 5, 7 and 9 using oral gavage and served as cisplatin and oral GLM every other day treated group.
Everyday Group (Daily)
Besides cisplatin injection on day 1, rats were subjected to GLM (500 mg/kg/day) in distilled water daily using oral gavage, starting from day one for 10 consecutive days and served as cisplatin and oral daily GLM treated group.
Intraperitoneal Group (i.p)
Besides cisplatin injection on day 1, rats were co-treated with GLM (500 mg/kg/day i.p) in distilled water just twice throughout the whole study, at days 2 and 6 and served as cisplatin and i.p GLM (i.p).
The doses and time course of experiments used for cisplatin and Ganoderma lucidum were in the range of those used in other studies with a modification of increasing the study period for 10 days.30–32 Furthermore, doses were determined after preliminary experiments.
The different regimen choice was based on the concern of toxicity in case of high dose and which was in accordance with others33 who suggested that in such a case, an every other day dose is recommended until the successful dose of treatment is recognized.
Animal Sacrifice and Sample Collection
On day 10 of the experiment, blood samples of overnight-fasted rats were collected via puncture of the retro-orbital venous plexus using capillary hematocrit tubes, allowed to stand, centrifuged at 3000 rpm for 5 mins, serum samples were separated and stored at −80°C until they were analyzed. Then, the animals were sacrificed by cervical dislocation; rat livers were removed, cleaned, weighed, and chilled on crushed ice. Liver tissues were isolated, rinsed in ice-cold saline and homogenized (10% w/v) in ice-cold, sodium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl. The homogenates were centrifuged at 3000 rpm for 20 min at 4◦C; the supernatant was collected and stored at −80◦C until used for biochemical testing.
Assessment of Liver Function
The biochemical markers of liver injury, including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (T. bilirubin) levels were assessed using commercial colorimetric assay kits purchased from (Creative BioMart, NY, USA) according to the manufacturer’s instructions.
Assessment of Oxidative Stress Markers
Malondialdehyde (MDA) concentration in liver homogenate was measured as previously described by our group, it is based on the reaction of thiobarbituric acid with MDA to form thiobarbituric acid reactive species (TBARs), measured colorimetrically at 534 nm and relative to MDA content.34,35 Also, hydrogen peroxide concentration was estimated based on the reaction of H2O2 with 3,5-dichloro-2-hydroxybenzensulfonic (DHBS) acid and 4-aminophenazone in the presence of peroxidase enzyme to form a chromophore which is measured at 510 nm colorimetrically.36
Assessment of Anti-Oxidant Activity
Hepatic superoxide dismutase (SOD) activity was determined depending on the ability of the enzyme to inhibit phenazine methosulfate-mediated reaction of nitroblue tetrazolium, which was measured colorimetrically at 560 nm.37,38 Moreover, the concentration of hepatic glutathione (GSH) in the liver homogenate was measured depending on the reaction of 5,5ʹ dithiobis 2-nitrobenzoic acid (DTNB) with glutathione and development of a relatively stable yellow color measured at 412 nm spectrophotometrically.39,40
Enzyme-Linked Immunosorbent Assay
The hepatic levels of HMGB-1 and TNF-α were assessed using (Bioassay Technology Laboratory, Shanghai, China) and interleukin-10 (IL-10) (eBioscience Inc., San Diego, CA, USA) ELISA kits according to the manufacturers’ instructions.
Histopathological, Immunohistochemical Examination
Hepatic tissue samples were placed in 4% paraformaldehyde for 72 hrs, embedded in paraffin wax, cut into 3 µm sections, and stained with hematoxylin-eosin (H, E). In addition, the liver sections embedded in paraffin were cut, stained with Masson’s trichrome stain for evaluation of the collagen deposition around central veins and in portal areas. Staining intensity was assessed using the scores from (0 to 3) as follows: (0), negative; (1), weak; (2), moderate and (3), strong staining.41
Immunohistochemical (IHC) evaluation was conducted, as other set of paraffin-embedded tissues were cut into 4 µm thick sections, deparaffinized in xylene and rehydrated with aqueous alcohol solutions.42 For antigen retrieval of NF-kB,43 P53,44 Bcl-2,45 and caspase-3,46 tissue sections were placed in glass jars containing 0.01 M sodium citrate buffer (pH 6.0) and boiled for 5 mins twice to enhance immunoreactivity. After cooling, the slides were rinsed with phosphate buffer saline (pH7.2) the samples were then incubated overnight at 4°C against the different primary antibodies for; NF-kB (1:300),43 P53 (1:300),44 Bcl-2 (1:300),45 and caspase-3 (1:400),46 (Santa Cruz Biotechnology Inc, CA, USA). Slides were washed and incubated with relative secondary antibodies, finally, the sections were washed with tris buffered saline and the immunoreaction was visualized using 3,3ʹ-diaminobenzidine tetrahydrochloride (Genemed Power-Stain, 1.0 Poly HRP DAB kit, Sakura Finetek Inc USA). Sections were then washed under running tap water for 10 mins, counterstained with Mayer’s haematoxylin and mounted for assessment. The levels of IHC staining intensity after adding NF-kB, P53, Bcl-2 antibodies were assessed using the scores from (0 to 3) as follows: (0), negative; (1), weak; (2), moderate and (3), strong staining.47
In addition, the percentages of IHC staining intensity of the bright brown cytoplasmic caspase-3 were considered mild when positive staining is in < 25–50% of examined fields, moderate when positive staining is in 25–50% of examined fields and strong when positive staining is in 50–75% of examined fields and very strong when positive staining is in >75% of examined fields. A pathologist blinded to the different animal groups using a light microscope assessed grading of necroinflammation, quantification of fibrosis and IHC estimation.
Qualitative Phytochemical Analysis of GLM
A total phytochemical screening was performed to reveal the bioactive constituents of GLM. Methanol was used as a solvent for preparing the extract from the mushroom powder. 20 g of GLM powder was dissolved in100 mL of methanol, after the completion of extraction, the supernatant was filtered through Whatman #1 filter paper. The filtrate was evaporated to dryness to obtain a residue, which was stored at 4°C in airtight containers for further investigations. The residue was reconstituted in methanol and subjected to qualitative chemical tests for assessing the following various phytoconstituents; Triterpenes and/or Steroids, cardiac glycosides, flavonoids, saponins, alkaloids and/or nitrogenous bases, tannins, and carbohydrates.48 Free and combined anthraquinones were also estimated.49
Assessment of Total Polyphenols, Flavonoids Content of GLM and Docking Study
Total Polyphenols Content
The total phenolic content of GLM powder was determined using Folin–Ciocalteu reagent method.50 Aliquots of 0.1 mL of the GLM solution in distilled water (0.1g/mL) were mixed with 2.8 mL of distilled water, 2 mL of 2% (w/v) sodium carbonate and finally 0.1 mL of 50% (v/v) of Folin–Ciocalteu reagent was added. This mixture was incubated for 30 mins at room temperature and the absorbance of the resulting color was measured at 750 nm against distilled water as blank, using Spekol 11 (Carl Zeiss-Jena) spectrophotometer. For quantitative determination, a standard curve of Gallic acid (0–200mg/l) was prepared by the same method.
Total Flavonoids Content
The total flavonoids content of GLM powder was determined colorimetrically using aluminum chloride.51 A 0.5 mL of the GLM solution (0.1 g/mL distilled water) was mixed with 1.5 mL of 95% ethyl alcohol, 0.1 mL of 10% aluminum chloride (AlCl3), 0.1mL of 1M potassium acetate (CH3COOK) and 2.8 mL of distilled water. After incubation for 40 min at room temperature, the absorbance of the reaction mixture was measured at 415 nm against distilled water as blank, using Spekol 11 (Carl Zeiss-Jena) spectrophotometer. Quercetin was chosen as a standard of flavonoids for making the standard curve (0–50mg/l).
Docking Study
The various Ganoderma lucidum constituents were built in 2D using ChemBioOffice (CBO) suite and geometrically optimized,52 then subjected to docking simulation using the Molecular Operating Environment (MOE) software,53 where the protein molecule was kept rigid but with flexible binding site and each compound had to go through 50 runs of docking process. Finally studying the docking results for each compound separately to determine the best fitting compounds to the provided protein molecule, our dock workflow went through the following stages:
Conformational Analysis: to generate conformations from a single 3D conformer.
Placement: 50 poses were generated for each compound using “Triangle Matcher” technique.
Rescoring: scoring functions emphasize favourable hydrophobic, ionic and hydrogen bond contacts. We followed “London dG” method from MOE package.
Refinement: Poses can be refined using force field method.
The protein file used for our docking study was chosen from the protein data bank (pdb code: 2YRQ) representing human HMGB-1.54
Statistical Analysis
Data were statistically analyzed using Prism software (Graph Pad 8, San Diego, CA, USA), differences between groups were tested for significance using one-way analysis of variance (ANOVA), followed by Tukey-Kramer multiple comparison test, once the differences exist among the means. Results were presented as means ± standard error mean (SEM), values p<0.05 were regarded as statistically significant.
Histopathological scores of collagen deposition in the examined sections describing nonparametric variables were analyzed by Kruskal–Wallis test followed by Dunn’s tests for comparison six groups. P value < 0.05 was considered statistically significant.
Statistical analyses of IHC staining intensity scores were also performed using prism software. Because the variables did not fit the normal distribution, non-parametric comparisons were performed by Kruskal–Wallis test followed by Dunn’s method to compare all means. The significance level was set when p < 0.05.
Percentages of IHC staining intensity of caspase-3 were analyzed using one-way ANOVA followed by Tukey’s test to compare all means.
Results
Hepatoprotective Effect of GLM on Liver Function Biomarkers
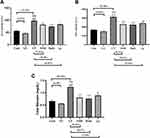
Figure 1A–C shows that CP caused a significant elevation in serum levels of liver enzymes ALT, AST and T. bilirubin as compared to the control groups (cont and GL) p<0.001. On the other hand, supplementation with GLM together with CP significantly decreases liver enzymes as compared to the CP group, and these effects where highly reversed in daily group p<0.001 as compared to the CP group.
Hepatoprotective Effect of GLM on Oxidative Stress Markers
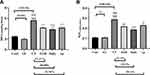
Figure 2A,B reveals that CP administration, dramatically increased hepatic MDA and H2O2 contents, respectively, as compared to the cont and GL groups (p<0.001), suggesting remarkable oxidative stress and lipid peroxidation. Meanwhile, GLM treatment in animals co-treated with CP significantly attenuated the hepatic oxidative stress and lipid peroxidation when compared with the CP group (p<0.05), considering the fact that daily treatment gave the best results.
Remarkable Anti-Oxidant Activity of GLM
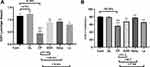
Figure 3 confirms the antioxidant defense of GLM in hepatic tissue; (a) shows a dramatic depletion of hepatic reduced GSH after CP administration. (b) Displays SOD % inhibition as compared to the Cont group. On the other hand, both hepatic GSH and SOD activity were significantly restored due to supplementation with (500 mg/kg/day) GLM together with CP either in EOD at p<0.01, daily at p<0.001 or i.p at p<0.05 as compared to CP only treated group.
Effect of GLM on Hepatic Inflammatory Response
In Figure 4A, B a prominent significant increase in hepatic HMGB-1 and TNF-α levels were noticed in the group treated with CP as compared to that of Cont and GL groups p<0.001. However, a significant downregulation of hepatic HMGB-1 and TNF-α concentrations were observed in all groups treated with CP and co-treated with (500 mg/kg/day) GLM either EOD at p<0.01, daily at p<0.001 or i.p at p<0.05 as compared with CP-treated group. Correspondingly, the anti-inflammatory and immunosuppressive cytokine Il-10 expression in Figure 4C was dramatically diminished in CP group as compared to the cont and GL groups p<0.001, contrarily hepatic Il-10 levels were extremely significant in EOD at p<0.01, daily at p<0.001 and i.p p<0.05groups in comparison to CP-treated group.
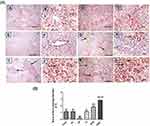
Histopathological and Immunohistochemical Assessment
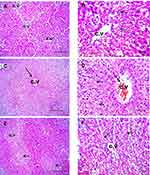
Histopathological Examination
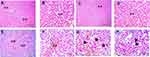
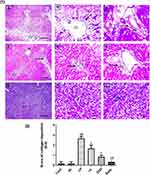
Liver sections stained with hematoxylin-eosin (H,E) and Masson’s trichrome revealed a normal architecture of liver tissues in Cont and GL rats (Figures 5A–D and 7A–D). Conversely, CP treatment induced significant liver injury characterized by necroinflammation and collagen deposition (Figures 5E–H and 7E–H). In contrast, treatment with GLM together with CP significantly attenuated hepatic injury as indicated by a significant reduction in the inflammation score (p<0.01) compared with the CP-only-treated group (Figure 6), showing the best result in the daily group. In addition, GLM treatment markedly reduced the fibrosis score (p<0.01), compared with rats treated with CP group, with the fact that daily group showed the best results as compared to EOD and i.p groups Figure 8(1,2).
Immunohistochemical Expression of NF-kB, P53, Bcl-2 and Caspase-3
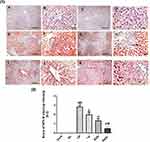
In Figures 9(1, 2) and 10(1,2), immunohistochemical detection of liver sections stained using Mayer’s hematoxylin for expression of NF-kB and P53 revealed a negative nuclear staining in control groups 9 (A, B), 10 (a, b) and GL groups 9 (C, D), 10 (c, d). However, strong positive nuclear staining in CP groups 9 (E, F) and 10 (E, F) were also detected in NF-kB and P53 expression, respectively. Remarkably, treatment with GLM together with CP triggered a significant downregulation of NF-kB and P53 expression P< 0.05 indicated by; moderate positive nuclear staining in i.p treated group Figures 9G, H and 10G, H, mild positive nuclear staining in EOD groups 9 (I, J), 10 (I, J) and very mild positive nuclear staining in daily groups 9 (K, L),10 (K, L).
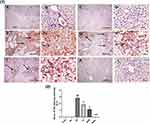
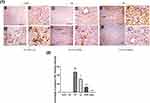
In addition, cisplatin treatment revealed a significant downregulation of Bcl-2 expression in liver sections stained using Bcl-2 antibody Figure 11E, F as compared to control group (A, B) and GL group (C, D) P< 0.05. Alternatively, treatment with GLM ameliorates Bcl-2 expression ranging from mild positive staining score (1) in i.p group (G, H), moderate positive staining score (2) in EOD group (i, j), and strong positive staining score (3) in daily-treated group (K, L) Figure 11(1,2).
Immunohistochemical analysis of caspase-3 Figure 12(1) confirmed the ability of GLM to attenuate CP-induced apoptosis in hepatic tissues, which were markedly mitigated in the daily group as compared to CP-treated group P< 0.05, expressed as a percentage of staining intensity Figure 12(2).
Results of Qualitative Phytochemical Analysis of GLM
The phytochemical screening of the methanolic extract of GLM revealed that it contains; triterpenoids, glycosides, flavonoids, alkaloids and/or nitrogenous bases, tannins, and carbohydrates as presented in Table 1.
 |
Table 1 Qualitative Phytochemical Analysis of GLM Methanolic Extract |
The Total Polyphenol, and Flavonoid Content of GLM
Table 2 shows the total polyphenol content of GLM expressed as milligram gallic acid equivalent (GAE)/g based on dry weight. In addition, the total flavonoid content was expressed as milligram quercetin equivalent (QE)/g based on dry weight. These results together may confirm that GLM is a source of natural antioxidant compounds and can be used as excellent food supplement.
 |
Table 2 Total Polyphenol, Flavonoid Content of GLM |
Molecular Modeling Results
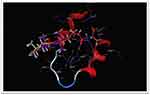
Table 3 presents the binding scores of the different Ganoderic acid constituents with HMGB-1. Also, Figure 13A-F presents the binding between the constituents (ganoderic acid) with the highest scores with HMGB-1. Likewise, Figure 14 shows the overlay of compounds 2, 5 and 9 in the binding site.
 |
Table 3 The Binding Scores of the Different Ganoderic Acid Constituents with HMGB-1 (pdb Code: 2YRQ) |
 |
Figure 13 (A–F) The binding between ganoderic acid with the highest scores with HMGB-1. |
Discussion
The present study investigated the possible protective effects of GLM against CP-induced hepatotoxicity through anti-oxidative and anti-inflammatory effect. To induce acute hepatotoxicity, a single dose of CP (12 mg/kg) was injected i.p, and it produced serious array of events of liver injury where significant increase in serum ALT (+73.75%), AST (+87.08%) and T. bilirubin (+86.93%) was recognized as compared to the control group. In addition, significant increase in hepatic oxidative stress and inflammatory markers was found. These events were consistent with other studies who confirmed the changes related to the CP-induced hepatotoxicity.9,55 Our results showed that supplementation with GLM causes a significant decrease in serum levels of liver injury markers; ALT, AST and T. bilirubin as compared to the CP group, which indicate a promising role of GLM in CP-induced acute liver injury.
Besides, CP-induced liver injury was also confirmed by (+121.4%) increment in MDA and (+146.22%) in H2O2 levels with a concomitant decline in hepatic antioxidants such as reduced GSH (−61.58%) and SOD activity (−60%). These results were consistent with previous studies, which affirmed the induction of potential oxidative stress because of CP injection, leading to cell death in liver tissue.56–58 Accordingly, severe hepatocellular injury such as mitochondria which releases excess amount of ROS when the cytochrome P450 catalytic cycle is interrupted. Interestingly, administration of GLM caused reversal of alterations in GSH as well as SOD levels. We suggested that the antioxidant activity of GLM may be attributed to their content of total polyphenol (132.74 mg GAE/g) and total flavonoids (23.18 mg QE/g) as were found in our study. Our results were in line with other studies reported that the total phenolic content of edible mushrooms had shown to be intimately related to their antioxidant capability to scavenge free radicals.59–61 Indeed, this study revealed that the daily administration of GLM had a superior protective effect when compared to EOD or i.p administration that may be due to less fluctuation of GLM concentration in plasma.
In addition to the biochemical investigation, the histopathological examinations confirmed that devastating events of hepatocytes, which was induced by the injection of CP and characterized by centrilobular areas of necrosis with vacuolar, and hydropic degeneration in hepatocytes, showed a significant improvement upon simultaneous treatment with GLM, which give a strong evidence for its defensive effect. Furthermore, this study confirmed a remarkable attenuating effect of GLM in CP-induced hepatic fibrosis, as indicated by fibrosis scoring. Noteworthy, our results verified previous reports of anti-fibrotic effect of GLM in models of liver fibrosis.56,62
In our study, CP-induced upregulation of the upstream as well as the downstream inflammatory signaling, which is confirmed by a significant increment of NF-κB, TNF-α and reduction in IL-10. These results may be due to that TNF-α intensifies the hepatic tissue inflammation via chemotaxis of immune cells and activation of other cytokines.63 Also, the decline in IL-10 exacerbates hepatic lesion that downregulate pro-inflammatory cytokine release.64 These findings were in accordance with a previous study about tangeretins’ protective effect against cisplatin-induced acute hepatic injury in rats.65 Our results also showed that GLM afforded a significant protection against CP-induced inflammation mainly through the reduction of the proinflammatory NF-κB and TNF-α and upregulation of anti-inflammatory IL-10. In this context, daily GLM administration had shown to have a significant higher anti-inflammatory effect than the EOD group or i.p injection when compared to the CP group. Notably, an important previous finding confirmed that sTNF-α induces resistance to cisplatin chemotherapy in malignant pleural mesothelioma, thus inhibition of TNF-α activity reduces the chemo-resistance to cisplatin.66 Accordingly, Ganoderma lucidum might provide a sustained chemotherapeutic response if it is added to cisplatin-based therapy.
It is well known that HMGB-1 is a redox-sensitive protein considered as one of the several cellular molecules involved in the crosstalk between apoptosis and autophagy.67 Our study highlighted that the intense upregulation of HMGB-1 was associated with TNF-α activation and a sharp decline in IL-10. Besides, it plays an important role in signaling pathways leading to tissue injury in the CP group. This could be attributed to the extracellular HMGB-1 which might act as both; a chemoattractant for leukocytes, and a pro-inflammatory mediator leading to the release of TNF-α, IL-1 and IL-6.68–70 Furthermore, the performed docking simulation of 15 ganoderic acids in Ganoderma lucidum in our study exhibited good fitting and binding within the protein under investigation through hydrogen bond formation with conservative amino acids on the A box; mainly cysteine 30 (Cys30) and Histidine 34 (His34) that may interpret the effect of Ganoderma lucidum on HMGB-1.
The current study also revealed that CP-induced apoptotic cell death in hepatic tissue detected by upregulation of p53 along with the reduction of Bcl-2 and were confirmed by assessing the main apoptotic caspases; caspase-3 expression in rat hepatic tissues. Our findings were in accordance with a study suggested that the decreased Bcl-2 immunoexpression in CP-treated rat hepatic tissues was accompanied by caspase 3/7 upregulation.65 Moreover, apoptotic cells, like necrotic cells could stimulate inflammation via the HMGB-1 release. It works through Toll-like receptor 4 (TLR4) activating c-Jun N-terminal kinase (p38/pJNK) and NF-κB, producing the pro-inflammatory cytokine TNF-α.71 Our data would suggest that HMGB-1 protein has a crucial role in triggering inflammatory responses implicated in CP-induced acute hepatic injury through p53-mediated mechanism and propose a relationship between HMGB-1 release and the incidence of apoptosis. This suggestion was aligned with those stated that the cell mediates the activation of either survival or pro-apoptotic pathways, including NF-kB, the tumor suppressor p53 and JNK in response to CP treatment.72 Furthermore, our results showed that GLM treatment elicited modulation of inflammatory cytokines and suppression of apoptosis in CP-induced hepatotoxicity, which could be due to decreased oxidative stress-mediated apoptosis. This cross-talk between the inflammatory and apoptotic signaling is normalized by administration of GLM in daily-, EOD- and the i.p-treated groups. This study sheds lights for the first time on the incorporation of HMGB-1/pJNK/NF-κB signaling pathway in the protective mechanisms offered by GLM against Cp-induced hepatic injury. Noteworthy, a recent study demonstrated that GLM induced oxidative DNA damage selectively in colorectal cancer cell lines, thus it may reduce the effective curative dose of anticancer drugs. However, it protected non-malignant cells from the accumulation of reactive oxygen species.73 This probably sounds promising as GLM does not oppose the anticancer effect of the chemotherapy but it may sensitize cancer cells instead.
Conclusion
The present study succeeded in providing structural and functional evidences for the suppressing effect of GLM on HMGB-1 using molecular modeling technique and linking these docking results to the experimental findings. Our study is the first to provide a suggestion that suppression of HMGB-1/pJNK/NF-κB signaling pathway by GLM has a key role against CP-induced hepatotoxicity in vivo. Our theoretical and experimental results may ascertain that GLM might modulate hepatic injury through attenuation of oxidative stress and the subsequent cross-talk between the inflammatory and oxidative stress-mediated apoptosis cascades (Figure 15). Therefore, the present study may open a new scenario for the clinical usefulness of adding GLM to chemotherapy-based treatment of cancer patients, to enhance treatment efficacy and delaying the adverse effects induced treatment discontinuation.
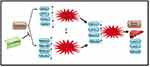 |
Figure 15 Summary of effects of (500 mg/kg/day) GLM in cisplatin-induced hepatotoxicity in male Sprague-Dawley rats. GLM: Ganoderma lucidum mushroom |
Acknowledgments
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program. The authors would like to thank Dr. Walaa Awadin, Ass. Prof. of Pathology, Faculty of Veterinary Medicine, Mansoura University, Egypt, for performing the histopathological and immunohistochemical examination. In addition, we remain grateful to Dr. Amany M. Marzouk Prof. Of Pharmacognosy, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa City, Egypt for her help.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gaskell H, Ge X, Nieto N. High-mobility group box-1 and liver disease. Hepatol Commun. 2018;2(9):1005–1020. doi:10.1002/hep4.1223
2. Lee WM. Acute liver failure. S.E.Min. Respir. Crit. Care Med. 2012;33(01):36–45. doi:10.1055/s-0032-1301733
3. Nitzsche B, Gloesenkamp C, Schrader M, et al. Anti-tumour activity of two novel compounds in cisplatin-resistant testicular germ cell cancer. Br J Cancer. 2012;107:1853–1863. doi:10.1038/bjc.2012.481
4. Meng F, Sun G, Zhong M, et al. Anticancer efficacy of cisplatin and trichostatin A or 5-aza-20 -deoxycytidine on ovarian cancer. Br. J. Cancer. 2013;08(3):579–586. doi:10.1038/bjc.10
5. Gonza´lez-Sa´nchez I, Lira-Rocha A, Navarrete A, et al. Synergistic anticancer activity of Thiazolo[5,4-b]quinoline derivative D3CLP in combination with cisplatin in human cervical cancer cells. Anticancer Res. 2012;32:5159–5165.
6. Pinto-Leite R, Arantes-Rodrigues R, Ferreira R, et al. Temsirolimus improves cytotoxic efficacy of cisplatin and gemcitabine against urinary bladder cancer cell lines. Urol Oncol. 2014;32:
7. Parlakpinar H, Sahna E, Ozer MK, et al. Physiological and pharmacological concentrations of melatonin protect against cisplatin-induced acute renal injury. J Pineal Res. 2002;33:161–166.
8. Gulec M, Oral E, Dursun OB, et al. Mirtazapine protects against cisplatin-induced oxidative stress and DNA damage in the rat brain. Psychiatry Clin. Neurosci. 2013;67(1):50–58. doi:10.1111/j.1440-1819.2012.02395.x
9. Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol. Sci. 2006;89(2):515–523. doi:10.1093/toxsci/kfj031
10. Naqshbandi A, Khan W, Rizwan S, Khan F. Studies on the protective effect of flaxseed oil on cisplatin-induced hepatotoxicity. Human Exp Toxicol. 2012;31(4):364–375. doi:10.1177/0960327111432502
11. Dkhil MA, Al-Quraishy S, Aref AM, et al. The potential role of Azadirachta indica treatment on cisplatin-induced hepatotoxicity and oxidative stress in female rats. Oxid Med Cell Longev. 2013:741817. doi:10.1155/2013/741817.
12. Zsengelle´r ZK, Ellezian L, Brown D, et al. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem. 2012;60(7):521–529. doi:10.1369/0022155412446227
13. Halliwell B. Free radicals and antioxidants: updating a personal. view. 2012;70:257–265. doi:10.1111/j.1753-4887.2012.00476.x
14. Ingawale DK, Mandlik SK, Naik SR. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): a critical discussion. Environ Toxicol Pharmacol. 2014;37,118–133. doi:10.1155/2013/741817.
15. Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290(5493):989–992. doi:10.1126/science.290.5493.989
16. Warren C, Wong-Brown M, Bowden N. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019. doi:10.17710.1038/s41419-019-1407-6
17. Inkaya AC, Demir NA, Kolgelier S, et al. Is serum high-mobility group box 1 (HMGB-1) level correlated with liver fibrosis in chronic hepatitis B? Medicine (Baltimore). 2017;96(36):e7547. doi:10.1097/MD.0000000000007547
18. VanPatten S, Al-Abed Y. High Mobility Group Box-1 (HMGb1): current wisdom and advancement as a potential drug target. J. Med. Chem. 2018;61(12):5093–5107. doi:10.1021/acs.jmedchem.7b01136
19. Ge X, Antoine DJ, Lu Y, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J. Biol. Chem. 2014;289(33):22672–22691. doi:10.1074/jbc.M114.552141
20. Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–365. doi:10.1016/j.coi.2005.06.002
21. Arshad MI, Piquet-Pellorce C. Samson M IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 2012;32(8):1200–1210. doi:10.1111/j.1478-3231.2012.02802.x
22. Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000;192(4):565–570. doi:10.1084/jem.192.4.565
23. Sha Y, Zmijewski J, Xu Z, et al. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi:10.4049/jimmunol.180.4.2531
24. Siwulski M, Sobieralski K, Golak-Siwulska I, Sokół S, Sękara A. Ganoderma lucidum (Curt.: fr.) Karst. – health-promoting properties. A review. Bot Med Res. 2015;61:2015–2026. doi:10.1515/hepo-2015-0026
25. Zhao C, Fan J, Liu Y, et al. Hepatoprotective activity of Ganoderma lucidum triterpenoids in alcohol-induced liver injury in mice, an iTRAQ-based proteomic analysis. Food Chem. 2019;271:148–156. doi:10.1016/j.foodchem.2018.07.115
26. Jin H, Jin F, Jin JX, et al. Protective effects of Ganoderma lucidum spore on cadmium hepatotoxicity in mice. Food Chem Toxicol. 2012;52:171–175. doi:10.1016/j.fct.2012.05.040
27. Ha Do T, Oh J, Khoi NM, et al. In vitro and in vivo hepatoprotective effect of ganodermanontriolagainst t-BHP-induced oxidative stress. J. Ethnopharmacol. 2013;150(3):875–885. doi:10.1016/j.jep.2013.09.039
28. Gill BS, Navgeet Kumar S. Ganoderma lucidum targeting lung cancer signaling: A review. Tumor Biol. 2017;39(6):1010428317707437. doi:10.1177/1010428317707437
29. Jiang J, Grieb B, Thyagarajan A, et al. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-κB signaling. Int. J. Mol. Med. 2008;21(5):577–584.
30. Al-Malki AL, Sayed BM. Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. Compl Alter Med. 2014;14(1):282. doi:10.1186/1472-6882-14-282
31. Sikandar KS, Rehman UK, Muhammad IB, Touqeer AR, Tasveer ZB, Mohammad S and Shahana UK. Diuretic Activity and cytotoxic study of various extracts of Ganoderma lucidum. World Appl Sci J. 2013;26(7):964–967.
32. Lee EB, Cheon SA, Kim SM, et al. General pharmacology of G009, a polysaccharide isolated from Ganoderma lucidum IY 009. J Appl Pharmacol (Korean). 1994;2:369–375.
33. Helton-Rhodes K. Feline immunomodulators. In: Bonagura JD, editor. Current Veterinary Therapy XII. 1995:581–588.
34. Hassan HM, Al-Gayyar MM, El-Gayar AM, Ibrahim TM. Effect of simvastatin on inflammatory cytokines balance in air pouch granuloma model, inflammation. Allergy Drug Targets. 2014;13:74–79.
35. Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90(1):37–43. doi:10.1016/0009-8981(78)90081-5
36. Aebi H. Methods Enzymol. 1984;105:121–126.
37. Baehner RL, Boxer LA, Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976;48:309–313. doi:10.1182/blood.V48.2.309.309
38. DeChatelet LR, McCall CE, McPhail LC, Johnston JR. Superoxide dismutase activity in leukocytes. J Clin Invest. 1974;53(4):1197–1201. doi:10.1172/JCI107659
39. Alsheblak MM, Elsherbiny NM, El-Karef A, El-Shishtawy MM. Protective effects of L-carnosine on CCl4 –induced hepatic injury in rats. Eur Cytokine Netw. 2016;27(1):6–15. doi:10.1684/ecn.2016.0372
40. Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi:10.1016/0003-9861(59)90090-6
41. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi:10.1016/0168-8278(95)80226-6
42. Potočnjak I, Broznić D, Kindl M, Kropek M, Vladimir-Knežević S, Domitrović R. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of ERK1/2, STAT3, and NF-κB activation. Food Chem Toxicol. 2017;107(Pt A):215–225. doi:10.1016/j.fct.2017.06.043.
43. Meyer R, et al. Cloning of the DNA-binding subunit of human nuclear factor κB: the level of its mRNA is strongly regulated by phorbol ester or tumor necrosis factor a. Proc Natl Acad Sci USA. 1991;88:966–970. doi:10.1073/pnas.88.3.966
44. Banks L, Hatada EN, Hohmann HP, et al. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986;159(3):529–534. doi:10.1111/j.1432-1033.1986.tb09919.x
45. Royuela M, De Miguel MP, Bethencourt FR, et al. IL-2, its receptors, and Bcl-2 and Bax genes in normal, hyperplastic and carcinomatous human prostate: immunohistochemical comparative analysis. Growth Factors. 2000;18:135–146. doi:10.3109/08977190009003239
46. Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(1):1–16. doi:10.1042/bj3260001
47. Arab HH, Salama SA, Omar HA, Arafael SA, Maghrabi IA. Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS One. 2015;10(3):e0122417. doi:10.1371/journal.pone.0122417.
48. Naveen KC, Srikumar R, Swathi S, Chidambaram R, Muthukrishnan G, Prabhakar R. Phytochemical analysis and antifungal activity of Ganoderma lucidum. Ind J Public Health Res Devel. 2018;9:12.
49. Evans W.Pharmacognosy.
50. Lin J, Tang C-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–147. doi:10.1016/j.foodchem.2006.01.014
51. Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182.
52. ChemBio Office version 13. Perkin Elmer. Cambridge, MA.
53. Molecular Operating Environment (MOE). 2013.08, Chemical Computing Group ULC. Montreal, QC, Canada; 2018.
54. Tomizawa T, Koshiba S, Watanabe S, Harada T, Kigawa T, Yokoyama S. Solution structure of the alpha adaptinC2 domain from human Adapter-related protein complex 1 gamma 2 subunit. RIKEN Struct Genomics Proteomics Init (RSGI). 2007. doi:10.2210/pdb2E9G/pdb
55. Waseem M, Bhardwaj M, Tabassum H, Raisuddin S, Parvez S. Cisplatin hepatotoxicity mediated by mitochondrial stress. Drug Chem Toxicol. 2015;38:452–459. doi:10.3109/01480545.2014.992437
56. Soares AA, de Sá-nakanishi AB, Bracht A, et al. Hepatoprotective effects of mushrooms. Molecules. 2013;18:7609–7630. doi:10.3390/molecules18077609
57. Zeng P, Zhihua G, Zeng X, et al. J Cell Mol Med. 2018;22(7).
58. Choi MJ, Kang H, Lee YY, et al. Cisplatin-induced ototoxicity in rats is driven by RIP3-dependent necroptosis. Cells. 2019;2;8(5). doi:10.3390/cells8050409
59. Liang Z, Yuan Z, Guo J, et al. Ganoderma lucidum polysaccharides prevent palmitic acid-evoked apoptosis and autophagy in intestinal porcine epithelial cell line via restoration of mitochondrial function and regulation of MAPK and AMPK/Akt/mTOR signaling pathway. Int J Mol Sci. 2019;20(3):E478. doi:10.3390/ijms20030478
60. Lutz M, Fuentes E. Roles of phenolic compounds in the reduction of risk factors of cardiovascular. Dis Mol. 2019;24.
61. Guo YJ, Deng GF, Xu XR, et al. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012;3:1195–1205.
62. Wu YW, Fang HL, Lin WC. Post-treatment of Ganoderma lucidum reduced liver fibrosis induced by thioacetamide in mice. Phytother Res. 2010;24(4):494–499. doi:10.1002/ptr.2949
63. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi:10.1101/cshperspect.a001651
64. Koppelman B, Neefjes JJ, de Vries JE, de Waal MR. Interleukin-10 down-regulates MHC class II alpha beta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7(6):861–871. (). doi:10.1016/S1074-7613(00)80404-5
65. Omar HA, Mohamed WR, Arab HH, Arafa EA. Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: targeting MAPKs and apoptosis. PLoS One. 2016;11:e0151649. doi:10.1371/journal.pone.0151649
66. Gordon GJ, Mani M, Mukhopadhyay L, et al. Inhibitor of apoptosis proteins are regulated by tumour necrosis factor‐α in malignant pleural mesothelioma. J Pathol. 2007;211:4. doi:10.1002/path.2120
67. Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi:10.1016/j.bbamcr.2013.06.001
68. Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–5560. doi:10.1093/emboj/cdg516
69. Yang Q, Wang J, Li J, et al. High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cerebral Blood Flow Metab. 2010;30:243–254. doi:10.1038/jcbfm.2009.202
70. Jiang W, Bell C, Pisetsky D. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J. Immunol. 2007;178(10):6495–6503. doi:10.4049/jimmunol.178.10.6495
71. Chen XL, Sun L, Guo F, et al. High-mobility group box-1 induces proinflammatory cytokines production of Kupffer cells through TLRs dependent signaling pathway after burn injury. PLoS One. 2012;7(11):e50668. doi:10.1371/journal.pone.0050668
72. Fontes A, Alemany-Pagès M, Oliveira PJ, Hans Zischka J, Azul AM. Antioxidant versus pro-apoptotic effects of mushroom-enriched diets on mitochondria in liver disease. Int J Mol Sci. 2019;20(16):3987. doi:10.3390/ijms20163987
73. Opattova A, Horak J, AcVodenkova S, et al. Ganoderma Lucidum induces oxidative DNA damage and enhances the effect of 5-Fluorouracil in colorectal cancer in Vitro and in vivo. Mutation Res Genet Toxicol Environ Mutagenesis. 2019;845:403065. doi:10.1016/j.mrgentox.2019.06.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.