Back to Journals » OncoTargets and Therapy » Volume 15
Superselective Prostate Artery Embolization for Treatment of Severe Haematuria Secondary to Rapid Progression of Treatment-Induced Neuroendocrine Prostate Cancer: A Case Report
Authors Deng L, Li C, He Q, Huang C, Chen Q, Zhang S, Wang L, Gan Y, Long Z
Received 29 October 2021
Accepted for publication 11 January 2022
Published 20 January 2022 Volume 2022:15 Pages 67—75
DOI https://doi.org/10.2147/OTT.S345193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Liang Deng,1 Chao Li,1 Qiangrong He,1 Chenghui Huang,2 Qian Chen,3 Shengwang Zhang,4 Long Wang,1 Yu Gan,5 Zhi Long1
1Andrology Center, Department of Urology, The Third Xiangya Hospital of Central South University, Changsha, 410013, People’s Republic of China; 2Department of Medical Oncology, The Third Xiangya Hospital of Central South University, Changsha, 410013, People’s Republic of China; 3Department of Pathology, The Third Xiangya Hospital of Central South University, Changsha, 410013, People’s Republic of China; 4Department of Radiation Oncology, The Third Xiangya Hospital of Central South University, Changsha, 410013, People’s Republic of China; 5Department of Urology, Xiangya Hospital of Central South University, Changsha, 410008, People’s Republic of China
Correspondence: Yu Gan, Department of Urology, Xiangya Hospital of Central South University, 87 Xiang Road, Changsha, Hunan, People’s Republic of China, Tel +86 15111140206, Fax +86 73184327332, Email [email protected]; Zhi Long, Andrology Center, Department of Urology, The Third Xiangya Hospital of Central South University, 138 Tongzipo Road, Changsha, Hunan, People’s Republic of China, Tel +86 13755076226, Fax +86 73188618028, Email [email protected]
Background: Treatment-induced neuroendocrine prostate cancer (t-NEPC) represents a highly aggressive subtype of castration-resistant prostate cancer that commonly arises from prostate adenocarcinoma (AdPC) after continuous androgen deprivation therapy (ADT). However, current treatments for t-NEPC are limited and far from satisfactory. According to our limited knowledge, report regarding the management of t-NEPC related hemorrhage is rare. Here, we report a case of t-NEPC formation after chronic hormonal therapy accompanying with severe bleeding in primary tumor and share our experiences to deal with the severe hematuria resulting from the progression of t-NEPC tumor.
Case Presentation: An 80-year-old man with a significantly high prostate-specific antigen was diagnosed via pathology as advanced AdPC due to multiple bone metastases. He then received ADT including bicalutamide and goserelin. After 20 months of stable disease, the cancer rapidly progressed and presented with severe gross hematuria caused by bleeding of the primary tumor. The histopathologic analysis of a secondary biopsy of the primary tumor confirmed neuroendocrine prostate cancer, and subsequent genetic testing revealed germ-line mutations in the RB1 and FOXA1. To control the bleeding and relieve symptoms, the patient was treated with superselective prostate artery embolization (PAE). After the left internal pudendal artery and the right prostatic artery were embolized, hematuria was quickly alleviated and disappeared. However, the patient was not a suitable candidate to platinum-based chemotherapy due to weak constitution. Goserelin was continuously applied to maintain castration level of serum testosterone. Meanwhile, palliative radiotherapy to the prostate tumor, high-risk lymph node drainage areas (including iliac and para-aortic lymph nodes, internal iliac lymph nodes, presacral lymph nodes and obturator nerve lymph nodes) and bone metastases (right sacroiliac joint and thoracic vertebra) was performed and relieved the pain. Unfortunately, this patient eventually died of cachexia and multiple organ failure nearly 27 months after initial diagnosis.
Conclusion: To treat severe hematuria caused by progression of t-NEPC, superselective PAE may be a rapid and efficient way to stop bleeding.
Keywords: prostate cancer, androgen deprivation therapy, treatment-induced neuroendocrine prostate cancer, superselective prostate artery embolization, radiotherapy
Background
Treatment-induced neuroendocrine prostate cancer (t-NEPC) represents a highly aggressive subtype of castration-resistant prostate cancer (CRPC) that commonly arises from prostate adenocarcinoma (AdPC) after continuous androgen deprivation therapy (ADT).1,2 It is generally characterized by the following clinical features, including unresponsiveness to ADT, disproportionately low PSA level and expression of one or more neuroendocrine markers, such as chromogranin A, synaptophysin and neuron-specific enolase (NSE), which usually occurs in the late-stage of prostate cancer and inevitably indicates a poor prognosis.3 Moreover, neovascularization is another distinctive characteristic of t-NEPC.4 The rapid progression of a tumor is probably accompanied by an imbalance between a fast-growing demand of blood supply and the formation of new blood vessels, which may result in tumor necrosis and hemorrhage and has become a challenging clinical problem.5 However, current treatments for t-NEPC are limited and far from satisfactory.6 According to our limited knowledge, report regarding the management of t-NEPC related hemorrhage is rare. Here, we report a case of t-NEPC formation after chronic hormonal therapy accompanying with severe bleeding in primary tumor. The genetic testing revealed that mutations of RB1 and FOXA1 may provide a genetic predisposition for the transdifferentiation from AdPC to t-NEPC in this case.
Case Presentation
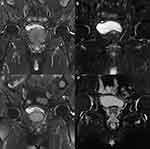
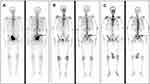
An 80-year-old man was admitted to our institute for lower urinary tract symptoms (LUTS) in April 2018. International Prostate Symptom Score (IPSS) of the patient was evaluated as 30, while Quality of life (QoL) was scored as 5 points and Eastern Cooperative Oncology Group (ECOG) performance status was 0. Digital rectal examination revealed a grade III enlarged prostate, with palpable hard nodules on the surface obviously and the central sulcus disappeared. The value of serum prostate- specific antigen (PSA) was 145.6 ng/mL (normal value: 0–4 ng/mL), testosterone (T) was 354.5 ng/dl (normal value: 193–740 ng/dl), hemoglobin (Hb) was 119 g/l (normal value: 130–175 g/l), serum creatinine (SCr) was 67 μmol/l (normal range: 57–111 μmol/l). The pelvic enhanced magnetic resonance imaging (MRI) showed that a high possibility of prostate cancer, and the seminal vesicles and pelvic bones were invaded (Figure 1A). Single-photon emission computed tomography (SPECT) indicated tumor had metastasized to multiple bones including the right ilium and the right sacroiliac joint (Figure 2A). Transrectal ultrasound-guided biopsy revealed poorly differentiated AdPC after pathological examination, with a Gleason score of 9 (4 + 5), PSA (+), synaptophysin (-), chromogranin A (-) and Ki67 (15%) (Figure 3A–C). The clinical stage was determined as stage IV (T3b N0 M1b) and this patient agreed to accept the ADT comprising bicalutamide (50 mg, once a day) combined with goserelin (10.8 mg, once every three months) since April 2018. The patient had a good response to the aforementioned therapy with the LUTS improved (IPSS 15, QoL 3) and serum PSA level decreased to 0.077 ng/mL (normal value: 0–4 ng/mL). Meanwhile, subsequent pelvic enhanced MRI (Figure 1B) and SPECT (Figure 2B) suggested the volume of the primary tumor and metastases was significantly reduced at the follow-up in June 2019.
However, 6 months later, the patient gradually presented with gross hematuria, urination pain, anemia, tachycardia and pale lips, and thus was admitted to our institute again in December 2019. Hb decreased continuously from 115 g/l to 79 g/l in four days after admission, and showed no obvious improvement even after blood transfusions. At that point, serum PSA was 0.416 ng/mL (normal value: 0–4 ng/mL), T was 4.33 ng/dl (normal value: 193–740 ng/dl), SCr was 86 μmol/l. Although SPECT indicated no significant change in bone metastases (Figure 2C), the pelvic enhanced MRI suggested that the primary prostate cancer tumor was enlarged with bladder invasion and the parailiac lymph nodes were involved (Figure 1C). An emergency contrast-enhanced computed tomography (CT) of the chest and abdomen further suggested that the tumor had metastasized to thoracic vertebra without urinary obstruction and ureterohydronephrosis. To sum up, the disease has progressed to the more aggressive CRPC stage.
In consideration of the relative stable PSA level and the rapid progression of the disease, we tested the serum NSE with a value of 170.8 ng/mL (normal value: 0–17 ng/mL). All these results indicated a possible formation of t-NEPC. Therefore, a secondary biopsy of the enlarged primary tumor was performed. The results of pathology showed small-cell neuroendocrine carcinoma (Figure 3D). The immunohistochemical staining contained features of t-NEPC, which had an intensive Ki67 expression (70%), was negative for PSA staining and positive for synaptophysin and CD56 (Figure 3E and F and Supplementary Figure). Furthermore, a genetic testing of the blood sample showed germ-line mutations of RB1 and FOXA1 (Table 1), both of which are tightly associated with the formation of t-NEPC.7–9
 |
Table 1 Gene Mutation Sites and Significance |
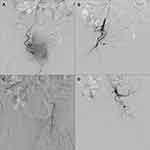
The effect of conservative treatments (eg, continuous bladder irrigation, fluid infusion and blood transfusion) for hematuria was limited. To control the bleeding of the primary tumor and relieve symptoms, the patient was treated with superselective prostate artery embolization (PAE) after multi-disciplinary consultations in January 2020. During the surgery, digital subtraction angiography (DSA) revealed extravasation of contrast medium from branches of right prostatic artery (Figure 4A). Then, superselective PAE was performed to terminate the bleeding. Polyvinyl alcohol particles (PVA) were successfully injected into the right prostatic artery (Figure 4B). We also found that a large amount of contrast medium overflowed at the terminal of the left prostatic artery (Figure 4C). However, the left internal pudendal artery was selected to be embolized with infusion of PVA, gelatin sponge and spring coil due to the malformation of the left prostatic artery (Figure 4D). Finally, the hematuria was controlled immediately with no obvious discomfort after operation.
After analyzing all test results and conducting a comprehensive evaluation, we have realized that the patient was not suitable for platinum-based chemotherapy due to his weak constitution. For this reason, to further control the disease and relieve pain of the patient, palliative radiotherapy (intensity modulated radiation therapy, IMRT) to the pelvic tumor (60.2 Gy/2.15 Gy/28 fractions), the lymph drainage area (50.4 Gy/1.8Gy/28 fractions) and bone metastases (30Gy/3Gy/10 fractions) was performed in the Department of Tumor Radiotherapy of our institution in February 2020. The LUTS were relieved effectively after the palliative radiotherapy. The tumor biomarkers decreased after the superselective PAE and the radiation therapy (NSE from 170.8 ng/mL to 32 ng/mL; PSA from 0.416 ng/mL to 0.058 ng/mL). Moreover, enhanced MRI in May 2020 (Figure 1D) showed that the primary tumor was smaller than that in December 2019 (Figure 1C). Unfortunately, the patient could not withstand the pain of the disease and then refused the follow-up treatments. He eventually died of cachexia and multiple organ failure at home on July 12, 2020.
Discussion and Conclusions
In our case, the patient was initially diagnosed as AdPC with a Gleason score of 9. After a persistent course of hormonal therapy, the disease progressed into CRPC stage with a relatively stable PSA, an elevated serum NSE, intolerant symptoms (chronic pain and hematuria) and increased tumor burden. The latest Comprehensive Cancer Network (NCCN) guideline for metastatic CRPC has already recommended a biopsy of metastatic sites to re-evaluate the histology of the advanced disease10. In this case, tumor burden in the primary site, but not in metastatic sites, was increased significantly with the disease progression. Therefore, we performed the second biopsy in the prostate to confirm the histology of the CRPC tumor. From the results of cell morphology and the immunostaining of neuroendocrine markers, t-NEPC was finally diagnosed.
Preclinical studies support the idea that AdPC evolves into t-NEPC in the presence of androgen receptor inhibition.11 In this case, the time from initial ADT treatment to the diagnosis of t-NEPC was 20 months, which was in accordance with the report from a previous study.12 The formation of t-NEPC is a consequence of multiple context-dependent mechanisms, including the selective pressure from ADT, the cellular plasticity, epigenetic modulations and gene mutations.13,14 A high Gleason score (>8) of the patient is also a risk factor of t-NEPC formation after ADT.12 Moreover, the genetic testing detected germ-line mutations of RB1 and FOXA1 in this case. RB1 is a classical tumor suppressor gene,15 loss of which is one characteristic of t-NEPC and contributes to elevation of lineage plasticity of tumor cells.7,16 The mutation of the transcriptional factor FOXA1 can block luminal differentiation and activate a mesenchymal and neuroendocrine transcriptional program in prostate epithelial cells.9,17 Unfortunately, we only detected the mutation sites rather than whole exon sequencing in consideration of the economic conditions of the patient, the latter could have provided much more useful information.
t-NEPC commonly loses the dependency of AR signaling, establishment of which often indicates an indolent response to ADT.3 Unfortunately, no targeted treatment has been well proved to be effective for t-NEPC. Therefore, t-NEPC unexceptionally progresses rapidly in accompany with the formation of a large number of new blood vessels, which represents a critical phenotype of this aggressive disease.5 Once tumor grows without sufficient nutrition and blood supply, tumor tissue may be necrotic, which may cause hematuria.18 Chronic hematuria induces repeated pain in the lower abdomen and impairs the quality of life, while refractory hematuria can result in severe anemia and becomes a threat to life.19 A similar case was also reported, of which a 70-year-old male was diagnosed with clinical stage T2c AdPC, and received treatments of ADT and radiotherapy. However, after 44 months since radiotherapy, the disease progressed with heavy haematuria which was lasting even after a transurethral surgery. Eventually, the patient deteriorated and died 52 months after radiotherapy.20 Therefore, alleviating the severe bleeding secondary to advanced prostate cancer is a thorny problem. PAE is one of the desirable treatment types of LUTS secondary to benign and/or malignant prostate enlargement and of localized prostate cancer.21 PAE is also commonly used to relieve the refractory hematuria from advanced prostate cancer and other pelvic urological malignancies, which is minimally invasive and has a high success rate of hemostasis and a low incidence of recurrence.18 However, reports introducing the experience of using PAE in treating severe bleeding from t-NEPC tumors are rare. In this case, the disease stepped into the CRPC stage and caused refractory hematuria that failed to response to conservative treatments. Superselective PAE was successfully carried out in the patient with good tolerance and a satisfactory outcome, resulting in the main blood supply of the tumor blocked, bleeding stopped, symptoms well relieved and Hb level stabilized. More importantly, the patient avoided a surgery and got an opportunity for further treatments.
The first-line treatment recommended by NCCN guideline for t-NEPC is chemotherapy based on cisplatin or carboplatin with sufficient supportive care.10 However, the efficacy is limited with an overall survival of 8.9–26.1 months.22 Moreover, the adverse reactions of chemotherapy are inevitable, such as granulocytopenia, severe diarrhea, anemia, and uncontrollable hematuria.23 In consideration of the weak constitution and the hemorrhage tendency of the patient, we recommended the palliative radiotherapy based on a castration level of serum testosterone. Wang HT and colleagues reported that radiotherapy was one of the most common types of treatment after t-NEPC diagnosis in clinical practice (8.6%) and was significantly associated with a longer survival.12 Moreover, the palliative radiotherapy is effective in palliating pelvic symptoms such as hematuria, pain, with acceptable toxicity.24 The adenocarcinoma part of a lump or metastases with mixed histology may have a sensitive response to radiotherapy, which benefits t-NEPC patients. Although the case we reported here eventually died within 7 months after t-NEPC diagnosis, radiotherapy to the pelvic tumor, lymph drainage area and bone metastases was beneficial, even temporarily, for tumor and symptom control.
In this case, high-grade pathology and distinct genetic background of the primary tumor and long-term ADT together contributed to the formation of t-NEPC. To treat the severe hematuria resulting from the progression of t-NEPC tumor, superselective PAE may be a rapid and effective way to stop bleeding and an operation was avoided. In clinical practice, a secondary biopsy for primary tumor or metastases and genetic testing should be taken into consideration when t-NEPC was suspected. Once t-NEPC is diagnosed, an individualized treatment strategy should be carried out to prolong the survival of patients.
Abbreviations
t-NEPC, Treatment-induced neuroendocrine prostate cancer; AdPC, prostate adenocarcinoma; ADT, androgen deprivation therapy; PAE, prostate artery embolization; PSA, prostate-specific antigen; CT, computed tomography; MRI, magnetic resonance imaging; SPECT, Single-photon emission computed tomography.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the third Xiangya hospital of Central South University (Changsha, China, 410013), and written informed consent was acquired from the patient’s relatives.
Consent for Publication
Informed consent was obtained in both written and verbal forms from the patient’s relatives to publish this case report and any accompanying images.
Acknowledgments
We thank Mr. Xuesen Dong and Ms. Ning Xie from the Vancouver Prostate Centre for their theoretical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundations of China (81902606 to Y.G.); the Natural Science Foundations of Hunan province (2020JJ5891 to Y.G.); and the Scientific Research Fund of Hunan Provincial Health and Family Planning Commission (C2016071 to Z.L.).
Disclosure
The authors declare that they have no competing interests.
References
1. Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. 2017;7:9. doi:10.1101/cshperspect.a030452
2. Long Z, Deng L, Li C, et al. Loss of EHF facilitates the development of treatment-induced neuroendocrine prostate cancer. Cell Death Dis. 2021;12(1):46. doi:10.1038/s41419-020-03326-8
3. Beltran H, Tagawa ST, Park K, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol. 2012;30(36):e386–e389. doi:10.1200/JCO.2011.41.5166
4. Zhang Y, Zheng D, Zhou T, et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun. 2018;9(1):4080. doi:10.1038/s41467-018-06177-2
5. Russo G, Mischi M, Scheepens W, De la Rosette JJ, Wijkstra H. Angiogenesis in prostate cancer: onset, progression and imaging. BJU Int. 2012;110(11c):E794–E808. doi:10.1111/j.1464-410X.2012.11444.x
6. Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36(24):2492–2503. doi:10.1200/JCO.2017.77.6880
7. Tan HL, Sood A, Rahimi HA, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. 2014;20(4):890–903. doi:10.1158/1078-0432.CCR-13-1982
8. Lee AR, Gan Y, Tang Y, Dong X. A novel mechanism of SRRM4 in promoting neuroendocrine prostate cancer development via a pluripotency gene network. EBioMedicine. 2018;35:167–177. doi:10.1016/j.ebiom.2018.08.011
9. Adams EJ, Karthaus WR, Hoover E, et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature. 2019;571(7765):408–412. doi:10.1038/s41586-019-1318-9
10. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. doi:10.6004/jnccn.2019.0023
11. Lovnicki J, Gan Y, Feng T, et al. LIN28B promotes the development of neuroendocrine prostate cancer. J Clin Invest. 2020;130(10):5338–5348. doi:10.1172/JCI135373
12. Wang HT, Yao YH, Li BG, et al. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol. 2014;32(30):3383–3390. doi:10.1200/JCO.2013.54.3553
13. Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol. 2018;15(5):271–286. doi:10.1038/nrurol.2018.22
14. Ge R, Wang Z, Montironi R, et al. Epigenetic modulations and lineage plasticity in advanced prostate cancer. Ann Oncol. 2020;31(4):470–479. doi:10.1016/j.annonc.2020.02.002
15. Lee JO, Russo AA, Pavletich NP. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391(6670):859–865. doi:10.1038/36038
16. Ku SY, Rosario S, Wang Y, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355(6320):78–83. doi:10.1126/science.aah4199
17. Kim J, Jin H, Zhao JC, et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene. 2017;36(28):4072–4080. doi:10.1038/onc.2017.50
18. Tapping CR, Crew J, Proteroe A, Boardman P. Prostatic artery embolization (PAE) for prostatic origin bleeding in the context of prostate malignancy. Acta Radiol Open. 2019;8(6):2058460119846061. doi:10.1177/2058460119846061
19. Chen JW, Shin JH, Tsao TF, et al. Prostatic arterial embolization for control of hematuria in patients with advanced prostate cancer. J Vasc Interv Radiol. 2017;28(2):295–301. doi:10.1016/j.jvir.2016.10.010
20. Williams SG, Aw Yeang HX, Mitchell C, et al. Immune molecular profiling of a multiresistant primary prostate cancer with a neuroendocrine-like phenotype: a case report. BMC Urol. 2020;20(1):171. doi:10.1186/s12894-020-00738-8
21. Parikh N, Keshishian E, Manley B, et al. Effectiveness and safety of prostatic artery embolization for the treatment of lower urinary tract symptoms from benign prostatic hyperplasia in men with concurrent localized prostate cancer. J Vasc Interv Radiol. 2021;32(7):1053–1061. doi:10.1016/j.jvir.2021.03.534
22. Conteduca V, Oromendia C, Eng KW, et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. 2019;121:7–18. doi:10.1016/j.ejca.2019.08.011
23. Oudard S, Latorzeff I, Caty A, et al. Effect of adding docetaxel to androgen-deprivation therapy in patients with high-risk prostate cancer with rising prostate-specific antigen levels after primary local therapy: a randomized clinical trial. JAMA Oncol. 2019;5(5):623–632. doi:10.1001/jamaoncol.2018.6607
24. Cameron MG, Kersten C, Vistad I, et al. Palliative pelvic radiotherapy for symptomatic incurable prostate cancer - a prospective multicenter study. Radiother Oncol. 2015;115(3):314–320. doi:10.1016/j.radonc.2015.05.021
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.