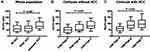Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Sulfatase 2 Along with Syndecan 1 and Glypican 3 Serum Levels are Associated with a Prognostic Value in Patients with Alcoholic Cirrhosis-Related Advanced Hepatocellular Carcinoma
Authors Mouhoubi N, Bamba-Funck J, Sutton A, Blaise L, Seror O, Ganne-Carrié N, Ziol M, N'Kontchou G, Charnaux N, Nahon P, Nault JC, Guyot E
Received 21 July 2022
Accepted for publication 7 October 2022
Published 28 December 2022 Volume 2022:9 Pages 1369—1383
DOI https://doi.org/10.2147/JHC.S382226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Nesrine Mouhoubi,1 Jessica Bamba-Funck,1,2 Angela Sutton,1,2 Lorraine Blaise,3 Olivier Seror,4 Nathalie Ganne-Carrié,3,5 Marianne Ziol,6,7 Gisèle N’Kontchou,3 Nathalie Charnaux,1,2 Pierre Nahon,3,5 Jean-Charles Nault,3,5,* Erwan Guyot1,2,*
1Université Sorbonne Paris Nord, Laboratory for VascularTranslational Science, LVTS, INSERM, UMR 1148, Bobigny, F- 93000, France; 2Service de biochimie, Hôpital Avicenne, hôpitaux universitaires Paris-Seine-Saint-Denis, Assistance publique Hôpitaux de Paris, Bobigny, F-93000, France; 3Service d’hépatologie, Hôpital Avicenne, AP-HP, hôpitaux universitaires Paris-Seine-Saint-Denis, Assistance publique Hôpitaux de Paris, Bondy, F-93143, France; 4Service de radiologie, Hôpital Avicenne, hôpitaux universitaires Paris-Seine-Saint-Denis, Assistance publique Hôpitaux de Paris, Bobigny, F-93000, France; 5Inserm, UMR 1162, Génomique fonctionnelle des tumeUrs solides, Paris, F-75010, France; 6Centre de Ressources Biologiques BB-0033-00027, Hôpital Avicenne, hôpitaux universitaires Paris-Seine-Saint-Denis, Assistance publique Hôpitaux de Paris, Bobigny, F-93000, France; 7Service d’anatomie et cytologie pathologique, Hôpital Avicenne, hôpitaux universitaires Paris-Seine-Saint-Denis, Assistance publique Hôpitaux de Paris, Bobigny, F-93000, France
*These authors contributed equally to this work
Correspondence: Erwan Guyot, Hôpitaux Universitaires Paris Seine-Saint-Denis, Laboratoire Biochimie-Pharmacologie et Biologie Moléculaire, 125 Rue de Stalingrad, Bobigny, 93000, France, Tel +33 1 48 95 56 29, Fax +33 1 48 95 56 27, Email [email protected]
Purpose: Sulfatase 2 (SULF2) is an enzyme related to heparan sulfate modifications. Its expression, as for some heparan sulfate proteoglycans expression, has been linked to hepatocellular carcinoma (HCC) at mRNA level and immunohistochemistry staining on biopsy samples. This study aims to evaluate the prognostic value of serum levels of SULF2 in patients with alcoholic cirrhosis with or without HCC.
Patients and Methods: Two hundred and eighty-seven patients with alcoholic cirrhosis were enrolled in this study: 164 without HCC, 57 with early HCC, and 66 with advanced HCC at inclusion. We analyzed the association between SULF2 serum levels and prognosis using Kaplan–Meier method and univariate and multivariate analysis using a Cox model.
Results: Child-Pugh C Patients have higher serum levels of SULF2 than Child-Pugh A patients. Serum levels of SULF2 were also higher in patients with advanced HCC compared with the other groups. In patients with advanced HCC, high serum levels of SULF2 were associated with less favorable overall survival. Combination of SULF2 with Glypican 3 (GPC3) and Syndecan 1 (SDC1) serum levels enhanced the ability to discriminate worst prognostic in advanced HCC.
Conclusion: SULF2 along with GPC3 and SDC1 serum levels have been shown to be associated with a prognostic value in advanced HCC.
Keywords: sulfatase 2, proteoglycans, prognosis, hepatocellular carcinoma, cirrhosis
Plain Language Summary
Chronic alcohol consumption can lead to liver cirrhosis and then to hepatocellular carcinoma (HCC). Along with diligent clinical monitoring, biomarkers are currently limited tools to help physicians assess prognosis for patients with HCC. Syndecan 1 (SDC1) and glypican 3 (GPC3), previously associated with biomarker characteristics in these patients, and their modifying enzyme sulfatase 2 (SULF2), have already been associated with liver carcinogenesis. The aim of our study was to assess the prognostic value of serum levels of SULF2 in patients with alcohol-related cirrhosis with or without HCC. We demonstrate in these patients that serum levels of SULF2 were associated with the severity of liver disease and with less favorable overall survival in patients with advanced HCC. Combination of SULF2 with SDC1 and GPC3 serum levels enhanced the ability to discriminate worst prognostic in advanced HCC and could be helpful for the choice of adapted therapeutic procedures.
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer related-death worldwide.1 In western countries, 90% of the HCC cases develop within an established chronic liver disease2 including cirrhosis due to non-alcoholic steatohepatitis (NASH),3 chronic infection with hepatitis B virus (HBV), hepatitis C virus (HCV), and alcohol abuse.4 Chronic alcohol abuse represents a public health scourge5 and the incidence of alcoholic cirrhosis is increasing, especially in Europe.6 HCC developed on alcoholic cirrhosis remains poorly studied compared with HBV and HCV associated HCC. In clinical practice, HCC surveillance of cirrhotic patients currently consists of patients’ visits at regular intervals (6 months), including liver ultrasonography and serum level of α-fetoprotein (AFP) assessment.7 Nevertheless, most proportion of HCC is still detected at an advanced stage. Radical treatment such as radiofrequency ablation or surgical resection has improved overall survival of patients with early HCC stage,8,9 but the majority of patients diagnosed with HCC are not eligible for such treatments and have rather a poor prognosis and only palliative treatment is offered to support symptom management.,10,11
Alcoholic Liver Disease (ALD) is associated with excessive alcohol intake and can lead to different types of complications. Serum biomarkers associated with liver fibrosis in patients with ALD could help in predicting liver-related mortality.11 In surveillance and management of HCC, serum biomarkers are helpful at different steps with important limitations. Cirrhotic patients with HCC possess abnormal liver function for whom the severity influences their prognosis as well as tumor characteristics. Among serum biomarkers associated with tumor characteristics, only AFP is used in clinical practice in Europe.12 However, other molecules along with AFP have been widely studied with these aims, mainly Protein Induced by Vitamin K Absence (PIVKA – also known as DCP – Des-γ-carboxy prothrombin) and AFP L3 (subfraction of AFP), alone or included in algorithms and could be useful.13 Underlying aetiology is not a key feature taken into account in the management of HCC to date.
Investigations about serologic biomarkers related to the severity of cirrhosis and tumor progression remain relevant in order to define more adapted patient care and progress in therapeutic strategies.
Among different molecules, heparan sulfate proteoglycans (HSPG) and enzymes linked to their biosynthesis and metabolism could be considered as biomarker candidates with growing evidence body in this field14,15. Many proteoglycans have been studied in liver cancer such as syndecan 1 (SDC1) and glypican 3 (GPC3) that seem to be of particular interest. Over-expression of SDC1, a cell surface HSPG, has been observed by immunohistochemistry in primary liver HCC comparatively to cirrhosis and healthy individuals.16 Such HSPG can be shed by different enzymes and be found in serum samples; we have previously shown that levels of SDC1 increased in serum of patients with advanced HCC comparatively to early HCC and that high levels are associated with poor overall survival.17 SDC1 is also overexpressed in other types of tumors.18
Glypican 3 (GPC3) is an onco-fetoprotein also characterized as a cell membrane HSPG. While not detected in adult organs, its expression reappears in various tumors and is particularly elevated in more than 70% of HCC tissues, characterized by poor differentiation that is related to a poor prognosis and a higher incidence of tumor recurrence.19 Serum GPC3 levels and GPC3 immunohistochemistry are useful as biomarkers and prognostic factors for HCC patients.20,21 A large number of studies have shown that GPC3 is more sensitive than AFP in the diagnosis of HCC. GPC3 can be used not only for the diagnosis of surgically resected samples but also for the diagnosis of biopsy samples.22 In a previous work, we focused on these two proteoglycans, SDC1 and GPC3, because of their well-established relevance in carcinogenesis.
Different enzymes operate in the edition and the remodeling of these HSPG. Regarding the remodeling of HSPG, some enzyme, such as MMPs, target the protein part resulting in the shedding of these membrane associated proteoglycans and thus, the liberation of the shedded part into the extracellular matrix (ECM). The second group of enzymes targets the glycosaminoglycan chains in the ECM and provokes two types of modifications. Heparanase is able to cleave heparan sulfate chains acting as an endoglucuronidase23 and extracellular sulfatases (SULF1 and SULF2) are highly specific and remove sulfate groups from the C-6 position of glucosamine within specific subregions.24 One of the main consequences is the release of heparin binding proteins (HBP), which include growth factors and chemokines, from HSPG. These dissociations either cause facilitation or are a hamper to the activation of signaling pathways triggered by different HBP. Thus, fine structural changes produced by SULFs have been associated with tumor growth, angiogenesis, and metastasis.25–27
Over-expression of sulfatase-2 (SULF2) has been observed in a variety of cancer including HCC,25 lung28 and breast carcinomas.29 High levels of SULF2 have also been found in the serum of cirrhotic patients, suggesting potential use as a serologic biomarker.30 These findings encourage us to further investigate whether SULF2 could be used as a prognostic biomarker in HCC. In this regard, a retrospective cohort of patients with alcoholic cirrhosis has been established in which frozen serum samples are available from the day of inclusion. After a long follow-up, which resulted in the diagnosis of a large number of HCC cases, the cohort was divided into three subgroups, composed of patients with alcoholic cirrhosis without HCC, patients with early HCC, and patients with advanced HCC classified as Barcelona clinic liver cancer (BCLC). The aim of the present study was to assess a potential association between circulating levels of proteoglycans and SULF2 with HCC occurrence and overall survival in patients with alcoholic cirrhosis with or without HCC, as well as to evaluate if these circulating levels are associated with the prognosis of cirrhotic patients with HCC eligible for curative or palliative treatment. Eventually, this study contributes to initiate the investigations about how this biomarker could contribute to patient care.
Materials and Methods
Patient Selection
To evaluate serum levels of sulfatase 2 (SULF2), we retrospectively used collected samples of sera from a cohort of prospectively followed-up cirrhotic patients. All cirrhotic patients referred between January 2007 and December 2009 to Jean Verdier Hospital for the management of their liver disease were considered. In this study, only patients who met the following criteria were selected: (i) a biopsy-proven cirrhosis; (ii) an alcohol-related liver disease defined by a daily alcohol intake over 50 g in the absence of other etiologies; (iii) a serum sample collected and frozen before any treatment; and (iv) the availability of a written consent for the use of blood samples provided by patient. In addition, HIV infection and severe alcoholic hepatitis were considered as exclusion criteria.
The date of the collection of the first serum sample corresponds to the date of inclusion. These samples then served for sulfatase 2 level assessment. Radiological, biological, and clinical features were recorded at inclusion. Patients were classified into three groups at inclusion according to their HCC status as follows:
Patients with alcohol-related cirrhosis “without” HCC as confirmed with normal ultrasonography and a baseline serum level of α-fetoprotein (AFP) <100 ng/mL.
Patients were considered with “early” HCC using the Barcelona clinic liver cancer (BCLC) classification: stage 0 (one nodule <2 cm) or A (one nodule <5 cm or maximum three nodules <3 cm).
Patients were considered with “advanced” HCC when they were classified as BCLC stage B (multinodular form apart from Milan criteria), BCLC stage C (portal invasion or metastasis), or BCLC stage D (poor performance status or Child-Pugh C).
The diagnosis of HCC was established using either guided biopsy or Barcelona non-invasive criteria defined by the European Association for the Study of the Liver (EASL).31
Follow-Up and Endpoints
All cirrhotic patients without HCC were screened for HCC every 6 months using serum AFP levels and liver ultrasonography as recommended.31 In this cohort of patients, the two main endpoints were the occurrence of HCC and the occurrence of liver transplantation or death.
The treatment of patients with early HCC in our liver unit consisted of radiofrequency ablation (RFA) performed by the same unique operator. All these patients were then followed-up using serum AFP and abdominal CT scan at 1 month and then every 3 months. Patients exhibiting a partial response at 1 month defined by persistent enhancement of the lesion on CT scan could then undergo a new RFA. The two main endpoints were recurrence-free survival (RFS) and overall survival for these patients.
Patients with “advanced” HCC were followed-up and treated according to the recommendations of the EASL: arterial chemoembolization for BCLC stage B, sorafenib since 2008 for BCLC stage C, and best supportive care for BCLC stage D.20,29 The main endpoint was overall survival for the latter patients.
Quantification of Circulating Sulfatase-2 and Heparan Sulfate Proteoglycans (Syndecan 1 and Glypican 3)
Blood samples were collected from patients under fasting conditions prior to treatment. Sera were separated after centrifugation at 4000 rpm for 15 min and stored at −80°C for later use. Commercial ELISA kits with high specificity for detection of Sulfatase 2 (SULF2) were purchased from Cloud Clone (SEH107Hu) and used according to the manufacturer’s instructions. The sera were diluted at 1/10. Repeatability and reproducibility for six samples were tested 3 times on one plate and 4 times on four different plates, respectively. The calculated coefficients of variation (CV) were 5.0% for intra-assay and 10.9% for inter-assay. The commercial kits for detection of proteoglycans Glypican 3 and Syndecan 1 were purchased from Cusabio Biotech Co., Ltd. and Gen-Probe Diaclone respectively and used previously.17
Statistical Analysis
Fisher exact tests and Kruskal–Wallis tests were used to analyze variables, respectively, for qualitative and continuous data.
In each subgroup of patients, follow-up ended at the date of liver transplantation or death or at the date of the last recorded visit (or information) during the 6 months before July 2019. Patients free of events were censored at their last visit, recorded until July 31, 2019. For the group of patients without HCC, time to HCC occurrence was defined as the duration between the date of inclusion and date of diagnosis of HCC. For the group of patients with early HCC, recurrence-free survival (RFS) has been defined as the duration between the date of inclusion and the date of HCC recurrence was established or the date of death. For the group of patients with advanced HCC, progression-free survival (PFS) has been defined as the time between the date of inclusion and the date of HCC recurrence or the date of death.
Distribution of time-to-failure endpoints was estimated by the Kaplan–Meier method. Levels of serum SULF2 and circulating Heparan Sulfate Proteoglycans (SDC1 and GPC3) were dichotomized using median values (low levels versus high levels). The ALBI score was calculated based on the total bilirubin and albumin level as previously described.32
Univariate and multivariate data analysis were conducted using Cox models for overall survival and RFS. The statistical tests were two-sided and P values ≤0.05 were considered to be significant. The statistical analysis was performed using Prism (GraphPad) and R (R Development Core Team) software packages.
Results
Patients’ Baseline Characteristics
Two hundred and eighty-seven patients with alcoholic cirrhosis were enrolled in the study. These patients were classified according to absence or presence of HCC at the date of inclusion. Among them, 164 patients had alcohol-related cirrhosis without HCC and were prospectively screened for HCC, 57 patients had “early” HCC, and 66 patients had “advanced” HCC. Their characteristics are displayed in Table 1. Patients with early HCC had a unique nodule in 46 cases (81%), with a main nodule of less than 3 cm in 37 cases (65%). Patients with advanced HCC had multiple nodules in 34 cases (55%), with a tumor portal thrombosis in 24 cases (36%).
The three subgroups of cirrhotic patients were heterogeneous in their underlying stage of liver disease, as reflected by the Child-Pugh classification (Table 1). In this cohort, significantly more elevated levels of PIVKA (but not AFP) were found in patients with advanced HCC (Figure 1A). There was a positive correlation between Child-Pugh classification and ALBI score, and levels of SULF2 serum in patients with alcoholic cirrhosis without HCC (consistently, as for Albumin and Bilirubin levels). Indeed, Child-Pugh C patients possessed significantly higher levels of serum SULF2 (Figure 1B), such a significant difference was also found in patients with HCC (Figure 1C).
Next, we compared the clinical and biological features of patients without HCC dichotomized according to their median serum SULF2 value. The results show significant associations between worse values in parameters linked to liver function (notably ALBI score) and highest values of SULF2 (Table 2) supporting the previous result. These results strongly suggest that serum SULF2 is associated with the stage of liver disease.
Serum SULF2 Levels are Associated to Overall Survival in Patients with Advanced HCC
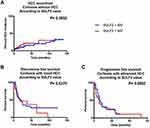
We subsequently evaluated the ability of SULF2 serum levels to predict overall survival in patients with alcohol-related cirrhosis and advanced HCC. Our results indicate that high levels of serum SULF2 were not associated with a higher risk of death in patients with alcohol-related cirrhosis (Figure 2; median survival of 113 months vs 117 months for SULF2 <65.2 ng/mL, p=0.1146).
Regarding tumor occurrence in patients with cirrhosis and without HCC at baseline or recurrence after ablation for early HCC, the analysis performed shows that serum levels of SULF2 were neither predictive of tumor occurrence or recurrence nor of free survival in patients with alcohol-related cirrhosis (Figure 3). We therefore conclude that SULF2 serum levels are not associated with HCC occurrence or recurrence.
Moreover, the same analysis has been performed for patients with early HCC (n = 57), no statistical difference has been found between the subgroups of patients according to their serum levels of SULF2 (Figure 3).
We also focused on patients with advanced HCC. Among this latter group of 66 patients, circulating SULF2 levels allowed a significant stratification regarding the risk of death (Figure 4; median survival of 5 months vs median survival not reached for SULF2 <65.2 ng/mL, p=0.03). In univariate analysis, circulating SULF2 levels, tumor portal vein thrombosis and Child Pugh Score were associated with the risk of death. In a first multivariate analysis, circulating SULF2 levels (HR=2.722, CI95% [1.08–6.489], p=0.033), circulating GPC3 levels (HR=2.81, CI95% [1.17–6.75], p=0.021), circulating SDC1 levels (HR=3.87, CI95% [1.37–10.97], p=0.011), and tumor portal vein thrombosis (HR=4.338, CI95% [1.88–9.976], p=0.000554) remains independently associated with a higher risk of death (data not shown).
Correlations Between SULF2 and Other Biomarkers: HSPG (SDC1 and GPC3) and Alanine Aminotranferase (ALAT)
Many in vitro studies have shown links between the different HSPG and enzymes associated with HS modifications, especially regarding their expression. In our previous study, we found a significant increase in SDC1 and GPC3 levels in sera of patients with advanced hepatocellular carcinoma.15
We subsequently evaluated the correlation between serum levels of SULF2 and SDC1 or GPC3. Our analysis shows that such positive correlations exist in our study for both parameters (Figure 5). Conversely, SULF2 serum levels were not significantly correlated with ALAT (Figure 5). We also performed a Pearson test in order to know if SULF2 and AFP could be correlated, the result does not show a correlation between SULF2 and AFP serum levels in patients with HCC (R = −0.06163 (95% CI: −0.2559–0.1375), p=0.5445).
Prognostic Values of SULF2 in Combination with Heparan Sulfate Proteoglycans (GPC3 and SDC1) Serum Levels in Patients with Advanced HCC
We previously analyzed the prognostic value of two proteoglycans, SDC1 and GPC3, in the serum of the same patients enrolled in this study. High serum levels of SDC1 and GPC3 were strongly associated with a prognostic value in patients with alcoholic cirrhosis.17 To predict the outcome according to levels of serum SULF2 in combination with SDC1 and GPC3, patients were stratified according to the median level of each biomarker, a group of patients whom all markers were increased (serum SULF2 >65.2 ng/mL) in combination with high levels of circulating GPC3 (>2.5 ng/mL) and circulating SDC1 (>50 ng/mL) were compared to the other cases in patients with alcohol-related cirrhosis or advanced HCC (Figure 6A).
The combination of the three biomarkers (highest concentrations for each) was associated with a strong risk of death in patients with advanced HCC (p<0.0001; Figure 6A). Furthermore, SULF2 serum levels remain discriminative if we consider separately the subgroups of patients with elevated serum levels of GPC3 (p=0.0699; Figure 6B) and SDC1 (p=0.0414; Figure 6C) consistent with the fact that SULF2 possesses an additional value as a prognostic biomarker along with GPC3 and SDC1 in advanced HCC. If we consider only the use GPC3 and SDC1 markers, the result obtained suggests that adding SULF2 serum levels to GPC3 and SDC1 could increase the discriminative power considering the overall survival endpoint in these patients even if the limited number of patients included does not allow us to conclude to the superiority of the combination of the three markers vs elevated GPC3 and SDC1 serum levels only (data not shown).
In the multivariate analysis, the combination of elevated circulating biomarkers levels (HR=3.13, CI95% [1.3641–7.182], p=0.007) is strongly associated with a higher risk of death (Table 3).
Discussion
The choice of treatment is currently guided by the BCLC (Barcelona Clinic Liver Cancer) classification.31 Prognosis of patients with alcoholic cirrhosis is related to the results of liver function tests. Several scores involving classical biomarkers such as ALBI score have been built and evaluated in order to contribute to a better classification of cirrhotic patients with HCC.33 Along with these biomarkers and scores, biomarkers more specific to HCC have been assessed. AFP is currently used in Europe according to EASL guidelines despite its relatively weak sensitivity.31 Mainly two other biomarkers have emerged, namely PIVKA-II and AFPL3, and exhibit comparable performances with AFP. Subsequently, different promising algorithms including these three biomarkers aiming at improving early diagnosis and refining patients with HCC prognosis have been developed. Their performances regarding the improvement of HCC diagnosis seem modest.34 Otherwise, among these algorithms, Balad scores exhibit promising results regarding their predictive value of overall survival in patients with HCC.35 These latter scores require further validation, but it would be interesting to compare their performances to the results obtained with HSPG-related biomarkers revealed from our study in patients with advanced HCC.
Heparan sulfate proteoglycans such as syndecans have been shown to play an important role in inflammation in various models.32 In liver disease, several modifications of heparan sulfate-related proteins have been described. For instance, Tatrai et al observed that Sulf2 mRNA was significantly overexpressed (2.8-fold) in fibrotic biopsies.36 Immunohistochemistry studies show that SULF2 is overexpressed in fetal liver and adult liver disease (fatty degeneration, chronic hepatitis, cirrhosis, and HCC) but not in normal tissue, this expression has not been found in the same proportion depending on the type of the lesion.37 Another study shows that SULF2 protein is upregulated in HCC, compared to surrounding nontumoral liver.38 In line with these observations, SULF2 has been shown to be overexpressed in different human HCC derived cell lines.39
Only few studies have evaluated serum levels of SULF2. A first study observed a significant increase of SULF2 serum level in patients with cirrhosis (HCV (n=19) and alcohol related (n=15)) compared to “healthy individuals” (n=37).30 In this study using a “home-made” ELISA kit, Singer et al obtained a difference of approximately 50% between median values of serum SULF2 in the group of cirrhotic patients compared to the group of healthy subjects (1050 pg/mL vs 703 pg/mL; p=0.001).
In this study, we assessed the potential of serum SULF2 as a prognostic biomarker in 287 patients with alcohol-related cirrhosis with or without HCC at inclusion. Even if our study does not include healthy subjects since all the patients enrolled here are cirrhotic, our results (Figure 1) also show that SULF2 serum levels are associated with the Child Pugh score and ALBI score, another reflect of the degree of liver damage/hepatic reserve function.33 Even though SULF2 levels do not seem significantly associated with HCC occurrence in patients with cirrhosis, we found an association with a high level of SULF2 and a poor prognosis in patients with advanced HCC.
Our results displayed significant increase levels in sera of patients with advanced HCC. Consistent with our study, Zaghloul et al measured increased levels of serum SULF2 in patients with HCC.40 Indeed, diagnosis of early HCC is the most clinically relevant setting, as this subgroup of patients may benefit from curative treatment thus far. Nevertheless, our results show that serum levels of this enzyme did not increase in patients with early HCC; therefore, it is hard to envision a usefulness of this marker for diagnostic purposes. However, patients with advanced HCC have a poor but heterogeneous prognostic. It is worth identifying factors associated with prognostics for these patients.
Altogether, our results show that high serum SULF2 level (>65 ng/mL) has a prognostic value in patients with alcohol-related cirrhosis, especially, a shorter survival time in patients with advanced HCC. Circulating SULF2 levels are indeed associated with Child Pugh score C and predict short-term death risk. At this time, only studies looking at SULF2 expression using IHC revealed a prognostic value of this protein in liver cancer and other types of cancer.39,41,42 Besides, about a hypothetic reflect of HCC development, our results seem to characterize SULF2 high serum level rather as a marker of the severity of hepatic lesions (or health status of the cirrhotic patient) than a marker of HCC. In fact, we do not know the main tissue of origin or secreting cells, which supply blood in SULF2. SULF2 serum levels remain to be correlated with the expression in a tissue IHC staining would likely still be more interesting if we consider HCC development as the main endpoint.
Functions of SULF2 as an enzyme and the presence of HSPG on the cell membranes are very interrelated. Few studies have focused investigations on the concomitant regulation of these components. However, this aspect has been particularly well established regarding the impact of modifications of expression of SULF2 on GPC3 expression level.25 In this latter study, the authors demonstrated both on an HCC cell line and in vivo using a xenograft model that induced variations of the expression of SULF2 by molecular tools (plasmid and small hairpin RNA) triggers parallel variations of expression of GPC3. In our study, we observed a modest but consistent positive correlation between SULF2 and both GPC3 and SDC1 serum concentrations. Because of the biological rationale, we found it interesting to analyze if SULF2 were still discriminant when HSPG such as GPC3 and SDC1 were also highly expressed (hypothesizing that serum levels reflect their liver expression). Previous results obtained for Endocan (ESM1) have not been included here because ESM1 is not an HSPG but a proteoglycan with a dermatan sulfate chain (that cannot be modified by SULF2). Our results indicate that serum level of SULF2 maintains a discriminative value when HSPG (such as GPC3 and SDC1) levels are elevated in patients with advanced HCC. We have combined these markers and compared a group of patients with elevated values for all the markers to another group including the other patients. The comparison between Kaplan-Meier curves obtained with a lone marker to the combination of these three elevated (SULF2, SDC1, and GPC3) markers suggests additional but not synergistic effects. Along with the increased values of these markers (SULF2, SDC1, and GPC3) in patients with alcoholic cirrhosis observed in our study, literature provides some clues that such increased levels for these circulating markers would also be observed in patients with chronic HCV. It has been reported for SULF2 serum levels30 and for SDC1 serum levels, which were associated with liver fibrosis stages in patients with hepatitis C.43 Among these biomarkers, Glypican-3 is the most investigated one.44 Like for others, most of the studies involved IHC experiments but few have assessed circulating GPC3. Interestingly, in a recent study, a correlation has been established between plasma and tumor GPC-3 suggesting the potential use of serum or plasma GPC-3 as HCV-related HCC biomarker.45 Nevertheless, the prognostic values of these biomarkers are still unevaluated in various contexts and even if we can suspect that elevated serum levels of these biomarkers found in different studies could be correlated to a prognostic value in HCC associated with other aetiologies, such as NASH or viruses, it remains to be proven. If serum SULF2, GPC3 and SDC1 were validated in other works, it would be interesting to refine a strategy along with other markers such as AFP or PIVKA-II with the possibility to include all these biomarkers in an algorithm.
It is not known whether SULF2 plays a key role in the initiation and promotion of neoplastic process in HCC or if its contribution is ancillary to HCC occurrence. Several studies have shown that SULF2, like some HSPG, is involved in neoangiogenesis and microenvironment remodeling, notably during liver cancer progression.46 The enzymatic action of SULF2 could be associated with liver pathogenesis through different mechanisms. SULF enzymatic activity impacts HS chains in the tumor environment of HCC.47 Demonstration has been done that SULF2 could have some promoting tumor growth and metastasis effects via enhancement of fibroblast growth factor-2 (FGF2) and WNT/β-catenin signaling pathways.25,48 SULF2 has also been shown to activate the transforming growth factor beta (TGFβ) and Hedgehog/GLI1 pathways in HCC.43,49 A SULF2 inhibitor (OKN-007) has been shown to possess an interesting therapeutic perspective in HCC through the inhibition of this latter pathway.50 Effects of SULF2 on lipid metabolism would be associated with steatohepatitis and fibrosis process in an animal model.51 It has been demonstrated in humans that SULF2 genetic variants are associated with triglyceride levels.52,53
Many other questions related to SULF2 in HCC are not addressed here but are of interest. Among them, the importance of SULF2 splice variants should be studied in the context of liver cancer. Other enzymes of HS extracellular modifications, namely SULF1 and Heparanase, have to be investigated in parallel to decipher the way they interact and if/how their serum levels could be used as biomarkers altogether in HCC. Molecular classifications of HCC have defined subsets of tumors according to their genetic alterations that are closely related to pathological features and prognosis.54 It would also be interesting to evaluate if high levels of SULF2 are over-represented in particular subgroups of HCC from this classification. This classification or a surrogate biomarker such as SULF2 could help to identify patients who can benefit from targeted therapies. Yoon et al have already demonstrated that the loss-of-function mutation of SULF2 or its inhibition by a pharmacological agent (OKN-007) enhanced sorafenib sensitivity in liver cancer cells and in vivo mouse models through a deregulation of EGFR signalling. They also report that non-responding patients exhibit higher expression of SULF2 in immunostaining analysis.55
Conclusion
Biomolecules identified both from pathologic liver and carcinoma samples and with clear links to neoplastic processes are fair candidates to become bona fide biomarkers in HCC. This is the case with numerous HS associated proteins. Considering blood samples are much more accessible than biopsy samples and can be used less cautiously, circulating molecules remain of prime interest. Regarding SULF2, our study is the first to show a prognostic value for its serum levels in HCC. This confirms the interesting data obtained elsewhere from immunostaining experiments on SULF2 and reveals the use of serum SULF2 as a potential biomarker. We also combine this feature with previously demonstrated HSPG biomarkers. Along with other new biomarkers, serum SULF2 level appears to be a candidate to integrate algorithm to better classify patients with HCC in patients with alcohol-related cirrhosis.
Institutional Review Board Statement
The liver biobank is registered with the French Ministry of Research and is declared to CNIL (reference 1254760). The protocol obtained approval from the Ethics Committee (CPP, Aulnay-sous-Bois, France).
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author [EG, JCN]. The data are not publicly available due to the fact that it could compromise research participant privacy/consent.
Ethics Statement
A written consent for blood sampling has been provided by every patient (in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki)); local ethics committee (USPN) approval for the protocol was obtained.
Informed Consent Statement
All patients gave written informed consent to participate in the study and all research carried out in participants was in compliance with the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by grants from the Fondation Recherche en Alcoologie (FRA) and Société Nationale Française de Gastro-Entérologie (SNFGE).
Disclosure
Dr Jean-Charles Nault reported grants from Ipsen and personal fees from Bayer, during the conduct of the study. The authors declare no other conflicts of interest.
References
1. Woodrell CD, Hansen L, Schiano TD, Goldstein NE. Palliative care for people with hepatocellular carcinoma, and specific benefits for older adults. Clin Ther. 2018;40(4):512–525. doi:10.1016/j.clinthera.2018.02.017
2. Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70(2):284–293. doi:10.1016/j.jhep.2018.10.008
3. Negro F. Natural history of NASH and HCC. Liver Int. 2020;40(S1):72–76. doi:10.1111/liv.14362
4. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi:10.1056/NEJMra1001683
5. Borie F, Trétarre B, Bouvier A-M, et al. Primitive liver cancers: epidemiology and geographical study in France. Eur J Gastroenterol Hepatol. 2009;21(9):984–989. doi:10.1097/MEG.0b013e3283293783
6. Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735. doi:10.1016/j.jhep.2018.05.011
7. Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157(1):54–64. doi:10.1053/j.gastro.2019.02.049
8. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi:10.1002/hep.24199
9. Qi X, Berzigotti A, Cardenas A, Sarin SK. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3(10):708–719. doi:10.1016/S2468-1253(18)30232-2
10. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
11. Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. doi:10.1038/s41572-018-0014-7
12. Singal AG, Hoshida Y, Pinato DJ, et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160(7):2572–2584. doi:10.1053/j.gastro.2021.01.233
13. Ahn JC, Lee Y-T, Agopian VG, et al. Hepatocellular carcinoma surveillance: current practice and future directions; 2022. Available from: https://hrjournal.net/article/view/4614.
14. Lai J-P, Thompson JR, Sandhu DS, Roberts LR. Heparin-degrading sulfatases in hepatocellular carcinoma: roles in pathogenesis and therapy targets. Future Oncol. 2008;4(6):803–814. doi:10.2217/14796694.4.6.803
15. Baghy K. Proteoglycans in liver cancer. WJG. 2016;22(1):379. doi:10.3748/wjg.v22.i1.379
16. Regős E, Karászi K, Reszegi A, et al. Syndecan-1 in liver diseases. Pathol Oncol Res. 2020;26(2):813–819. doi:10.1007/s12253-019-00617-0
17. Nault J-C, Guyot E, Laguillier C, et al. Serum proteoglycans as prognostic biomarkers of hepatocellular carcinoma in patients with alcoholic cirrhosis. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1343–1352. doi:10.1158/1055-9965.EPI-13-0179
18. Teixeira FCOB, Götte M. Involvement of syndecan-1 and Heparanase in cancer and inflammation. In: Vlodavsky I, Sanderson RD, Ilan N, editors. Heparanase. Cham: Springer International Publishing; 2020.
19. Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125(1):89–97. doi:10.1016/S0016-5085(03)00689-9
20. Haruyama Y, Yorita K, Yamaguchi T, et al. High preoperative levels of serum glypican-3 containing N-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy: high preoperative levels of serum glypican-3. Int J Cancer. 2015;137(7):1643–1651. doi:10.1002/ijc.29518
21. Zhang J, Zhang M, Ma H, et al. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: an updated meta-analysis. Medicine. 2018;97(24):e11130. doi:10.1097/MD.0000000000011130
22. Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: a new target for diagnosis and treatment of hepatocellular carcinoma. J Cancer. 2020;11(8):2008–2021. doi:10.7150/jca.39972
23. Vlodavsky I, Friedmann Y, Elkin M, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5(7):793–802. doi:10.1038/10518
24. Seffouh A, Milz F, Przybylski C, et al. HSulf sulfatases catalyze processive and oriented 6‐ O ‐desulfation of heparan sulfate that differentially regulates fibroblast growth factor activity. FASEB J. 2013;27(6):2431–2439.
25. Lai J-P, Sandhu DS, Yu C, et al. Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology. 2008;47(4):1211–1222. doi:10.1002/hep.22202
26. El Masri R, Crétinon Y, Gout E, Vivès RR. HS and inflammation: a potential playground for the sulfs? Front Immunol. 2020;11:570. doi:10.3389/fimmu.2020.00570
27. Vivès RR, Seffouh A, Lortat-Jacob H. Post-synthetic regulation of hs structure: the Yin and Yang of the sulfs in cancer. Front Oncol. 2014;3:1. doi:10.3389/fonc.2013.00331
28. Lemjabbar-Alaoui H, van Zante A, Singer MS, et al. Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene. 2010;29(5):635–646. doi:10.1038/onc.2009.365
29. Morimoto-Tomita M, Uchimura K, Bistrup A, et al. Sulf-2, a proangiogenic heparan sulfate endosulfatase, is upregulated in breast cancer. Neoplasia. 2005;7(11):1001–1010. doi:10.1593/neo.05496
30. Singer MS, Phillips JJ, Lemjabbar-Alaoui H, et al. SULF2, a heparan sulfate endosulfatase, is present in the blood of healthy individuals and increases in cirrhosis. Clin Chim Acta. 2015;440:72–78. doi:10.1016/j.cca.2014.10.038
31. Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
32. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—The ALBI grade. JCO. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
33. Hiraoka A, Kumada T, Michitaka K, Kudo M. Newly proposed ALBI grade and ALBI-T score as tools for assessment of hepatic function and prognosis in hepatocellular carcinoma patients. Liver Cancer. 2019;8(5):312–325. doi:10.1159/000494844
34. Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): a phase 3 biomarker study in the United States. Clin Gastroenterol Hepatol. 2022;2022:1.
35. Wongjarupong N, Negron-Ocasio GM, Mara KC, et al. BALAD and BALAD-2 predict survival of hepatocellular carcinoma patients: a North American cohort study. HPB. 2021;23(5):762–769. doi:10.1016/j.hpb.2020.09.014
36. Tátrai P, Egedi K, Somorácz Á, et al. Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. J Histochem Cytochem. 2010;58(5):429–441. doi:10.1369/jhc.2010.955161
37. Graham K, Murphy JI, Dhoot GK. SULF1/SULF2 reactivation during liver damage and tumour growth. Histochem Cell Biol. 2016;146(1):85–97. doi:10.1007/s00418-016-1425-8
38. Lai J-P, Sandhu DS, Yu C, et al. Sulfatase 2 protects hepatocellular carcinoma cells against apoptosis induced by the PI3K inhibitor LY294002 and ERK and JNK kinase inhibitors: SULF2 protects against apoptosis in hepatocellular carcinoma. Liver Int. 2010;30(10):1522–1528. doi:10.1111/j.1478-3231.2010.02336.x
39. Lai J-P, Oseini AM, Moser CD, et al. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology. 2010;52(5):1680–1689. doi:10.1002/hep.23848
40. Zaghloul RA, El-Shishtawy MM, El Galil KHA, Ebrahim MA, Metwaly AA, Al-Gayyar MM. Evaluation of antiglypican-3 therapy as a promising target for amelioration of hepatic tissue damage in hepatocellular carcinoma. Eur J Pharmacol. 2015;746:353–362. doi:10.1016/j.ejphar.2014.11.008
41. Alhasan SF, Haugk B, Ogle LF, et al. Sulfatase-2: a prognostic biomarker and candidate therapeutic target in patients with pancreatic ductal adenocarcinoma. Br J Cancer. 2016;115(7):797–804. doi:10.1038/bjc.2016.264
42. Flowers SA, Zhou X, Wu J, et al. Expression of the extracellular sulfatase SULF2 is associated with squamous cell carcinoma of the head and neck. Oncotarget. 2016;7(28):43177–43187. doi:10.18632/oncotarget.9506
43. Zvibel I, Halfon P, Fishman S, et al. Syndecan 1 (CD138) serum levels: a novel biomarker in predicting liver fibrosis stage in patients with hepatitis C. Liver Int. 2009;29(2):208–212. doi:10.1111/j.1478-3231.2008.01830.x
44. Shih T-C, Wang L, Wang H-C, Wan Y-JY. Glypican-3: a molecular marker for the detection and treatment of hepatocellular carcinoma. Liver Res. 2020;4(4):168–172. doi:10.1016/j.livres.2020.11.003
45. Shimizu Y, Mizuno S, Fujinami N, et al. Plasma and tumoral glypican‐3 levels are correlated in patients with hepatitis C virus‐related hepatocellular carcinoma. Cancer Sci. 2020;111(2):334–342. doi:10.1111/cas.14251
46. Chen G, Nakamura I, Dhanasekaran R, et al. Transcriptional induction of periostin by a sulfatase 2–TGFβ1–SMAD signaling axis mediates tumor angiogenesis in hepatocellular carcinoma. Cancer Res. 2017;77(3):632–645. doi:10.1158/0008-5472.CAN-15-2556
47. Yu Y, Li H, Yang Y, Ding Y, Wang Z, Li G. Evaluating tumor-associated activity of extracellular sulfatase by analyzing naturally occurring substrate in tumor microenvironment of hepatocellular carcinoma. Anal Chem. 2016;88(24):12287–12293. doi:10.1021/acs.analchem.6b03469
48. Nakamura I, Fernandez-Barrena MG, Ortiz-Ruiz MC, et al. Activation of the transcription factor GLI1 by WNT signaling underlies the role of SULFATASE 2 as a regulator of tissue regeneration. J Biol Chem. 2013;288(29):21389–21398. doi:10.1074/jbc.M112.443440
49. Carr RM, Romecin Duran PA, Tolosa EJ, et al. The extracellular sulfatase SULF2 promotes liver tumorigenesis by stimulating assembly of a promoter-looping GLI1-STAT3 transcriptional complex. J Biol Chem. 2020;295(9):2698–2712. doi:10.1074/jbc.RA119.011146
50. Zheng X, Gai X, Han S, et al. The human sulfatase 2 inhibitor 2,4-disulfonylphenyl- tert -butylnitrone (OKN-007) has an antitumor effect in hepatocellular carcinoma mediated via suppression of TGFB1/SMAD2 and Hedgehog/GLI1 signaling. Genes Chromosom Cancer. 2013;52(3):225–236. doi:10.1002/gcc.22022
51. Kim TH, Banini BA, Asumda FZ, et al. Knockout of sulfatase 2 is associated with decreased steatohepatitis and fibrosis in a mouse model of nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;319(3):G333–44. doi:10.1152/ajpgi.00150.2019
52. Hassing HC, Surendran RP, Derudas B, et al. SULF2 strongly predisposes to fasting and postprandial triglycerides in patients with obesity and type 2 diabetes mellitus: SULF2 and Triglycerides in Obesity. Obesity. 2014;22(5):1309–1316. doi:10.1002/oby.20682
53. Matikainen N, Burza MA, Romeo S, et al. Genetic variation in SULF2 is associated with postprandial clearance of triglyceride-rich remnant particles and triglyceride levels in healthy subjects. PLoS One. 2013;8(11):e79473. doi:10.1371/journal.pone.0079473
54. Nault J, Martin Y, Caruso S, et al. Clinical impact of genomic diversity from early to advanced hepatocellular carcinoma. Hepatology. 2020;71(1):164–182. doi:10.1002/hep.30811
55. Yoon S, Lee E-J, Choi J-H, et al. Recapitulation of pharmacogenomic data reveals that invalidation of SULF2 enhance sorafenib susceptibility in liver cancer. Oncogene. 2018;37(32):4443–4454. doi:10.1038/s41388-018-0291-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.