Back to Journals » ClinicoEconomics and Outcomes Research » Volume 8
Sugammadex for reversal of neuromuscular blockade: a retrospective analysis of clinical outcomes and cost-effectiveness in a single center
Authors Carron M, Baratto F, Zarantonello F, Ori C
Received 20 November 2015
Accepted for publication 6 January 2016
Published 18 February 2016 Volume 2016:8 Pages 43—52
DOI https://doi.org/10.2147/CEOR.S100921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Michele Carron, Fabio Baratto, Francesco Zarantonello, Carlo Ori
Department of Medicine, Anesthesiology and Intensive Care, University of Padova, Padova, Italy
Objective: The aim of the study is to evaluate the clinical and economic impact of introducing a rocuronium–neostigmine–sugammadex strategy into a cisatracurium–neostigmine regimen for neuromuscular block (NMB) management.
Methods: We conducted a retrospective analysis of clinical outcomes and cost-effectiveness in five operating rooms at University Hospital of Padova. A clinical outcome evaluation after sugammadex administration as first-choice reversal drug in selected patients (rocuronium–sugammadex) and as rescue therapy after neostigmine reversal (rocuronium–neostigmine–sugammadex) compared to control was performed. A cost-analysis of NMB management accompanying the introduction of a rocuronium–neostigmine–sugammadex strategy into a cisatracurium–neostigmine regimen was carried out. To such purpose, two periods were compared: 2011–2012, without sugammadex available; 2013–2014, with sugammadex available. A subsequent analysis was performed to evaluate if sugammadex replacing neostigmine as first choice reversal drug is cost-effective.
Results: The introduction of a rocuronium–neostigmine–sugammadex strategy into a cisatracurium–neostigmine regimen reduced the average cost of NMB management by 36%, from €20.8/case to €13.3/case. Patients receiving sugammadex as a first-choice reversal drug (3%) exhibited significantly better train-of-four ratios at extubation (P<0.001) and were discharged to the surgical ward (P<0.001) more rapidly than controls. The cost-saving of sugammadex as first-choice reversal drug has been estimated to be €2.9/case. Patients receiving sugammadex as rescue therapy after neostigmine reversal (3.2%) showed no difference in time to discharge to the surgical ward (P=0.44) compared to controls. No unplanned intensive care unit (ICU) admissions with rocuronium–neostigmine–sugammadex strategy were observed. The potential economic benefit in avoiding postoperative residual curarization (PORC)-related ICU admission in the 2013–2014 period was estimated at an average value of €13,548 (€9,316–€23,845).
Conclusion: Sugammadex eliminated PORC and associated morbidities. In our center, sugammadex reduced the costs of NMB management and promoted rapid turnover of patients in operating rooms, with total cost-effectiveness that counteracts the disadvantages of its high cost.
Keywords: neuromuscular blockade; neuromuscular blocking agents; rocuronium; sugammadex; postoperative residual curarization; cost-benefit analysis.
Introduction
Neuromuscular blocking agents (NMBAs) are routinely used worldwide as part of a modern concept of balanced anesthesia. Rocuronium, an aminosteroid NMBA, and cisatracurium, a NMBA of the benzylisoquinoline family, are two common intermediate-duration NMBAs whose pharmacokinetic properties make them suitable for administration by either bolus or continuous infusion.1 Rocuronium features a rapid onset of action.2 When a rapid induction of NMB is required, rocuronium 1–1.2 mg/kg may be substitutive of succinylcholine, which is effective, but has a wide range of potentially dangerous adverse effects, including death.3 Cisatracurium has an organ-independent metabolism since it is (at physiological pH and temperature) rapidly degraded by Hoffmann elimination in plasma and tissues.4 This allows to limit the variability in duration of effect of cisatracurium,2 particularly in the presence of kidney or liver disease, and after continuous infusion in case of prolonged surgical procedure.1
Acetylcholinesterase inhibitors, such as neostigmine, are generally administered to hasten recovery from NMB and reduce the likelihood of postoperative residual curarization (PORC)5,6 that may occur with any NMBAs.1,5 PORC can result in potentially fatal adverse respiratory events (AREs), and, therefore, represents a clinically relevant problem.5,6 Administering sugammadex, a modified γ-cyclodextrin that encapsulates and inactivates unbound aminosteroid NMBA, but not benzylisoquinoline NMBA, is emerging as a more favorable approach to achieving full reversal of NMB than neostigmine.3,7 Sugammadex has been approved for a quick and predictable reversal of moderate and profound NMB at doses of 2 and 4 mg/kg, respectively,8,9 and for immediate reversal at a dose of 16 mg/kg after the administration of 1.2 mg/kg rocuronium.10 However, the cost of sugammadex has so far hindered its progress of becoming a widely used alternative to neostigmine.11 The potential advantage for the health system associated with the routine use of sugammadex has been demonstrated through cost-effectiveness analyses.12–14 However, there are no reports of a cost analysis of sugammadex use in clinical practice that considered the outcomes of treated patients, the real costs, and the potential benefits for the health system.3
Therefore, we performed a retrospective analysis of NMB management that examined the clinical and economic impact of introducing a rocuronium plus neostigmine and sugammadex strategy into a regimen based mainly on cisatracurium plus neostigmine.
Materials and methods
The study was approved by the Ethics Committee for Clinical Research of Padova, which waived the requirement to obtain patient’s written informed consent. It was performed in five operating rooms (ORs) at University Hospital of Padova. These ORs have more than 8 hours of cases each workday. Abdominal surgery was the most frequently performed procedure.
Introduction of sugammadex in our center
Given its cost, the Hospital Pharmacy approved (January 1, 2013) the use of sugammadex for reversal of rocuromium-induced NMB under quantitative neuromuscular monitoring only in select patients who were judged to have an increased risk of complications with reversal of NMB by neostigmine (“preventive” use): elderly patients; patients with morbid obesity; patients presenting neurologic impairment, neuromuscular, respiratory, cardiac, kidney (with creatinine clearance [CrCl] >30 mL/min), and liver disease; patients with difficult airway management; and patients with contraindications to neostigmine plus atropine.3,15
Sugammadex was also approved for use as rescue therapy in two situations: “emergency use”, for rapid reversal of high dose rocuronium-induced NMB in case of “cannot ventilate, cannot intubate” (CVCI) situation and “curative” use, for treatment of any PORC-related AREs after reversal of rocuronium-induced NMB with neostigmine,16,17 with PORC defined as a train-of-four (TOF) ratio <0.9.5 PORC-related AREs were defined as difficult weaning from mechanical ventilation after general anesthesia with NMB and, after tracheal extubation, any critical respiratory event during the early postoperative period.6
Outcome evaluation
An evaluation of outcomes of sugammadex use in the 2013–2014 period was carried out. All treated patients in both the “preventive” and “curative” group were matched with controls for comparison. The controls were chosen from patients with similar clinical characteristics in whom rocuronium-induced NMB was reversed with neostigmine and who did not receive sugammadex. Both cases and controls were chosen from patients who underwent surgery, between 8 am and 8 pm, Monday through Friday, from January 1, 2013 to December 31, 2014.
All patients received inhalational or intravenous anesthesia and NMB with quantitative neuromuscular monitoring. At the time of tracheal extubation, the TOF ratio was measured and recorded. Patients were categorized into one of three groups based on the TOF ratio: acceptable neuromuscular recovery, TOF ratio ≥0.9; mild-to-moderate NMB, TOF ratio 0.7 and 0.9; and severe NMB, TOF ratio <0.7.6
In “curative” use patients, sugammadex was administered to treat PORC-related AREs occurring after the reversal of rocuronium-induced NMB with neostigmine and for which muscle relaxant antagonism was judged necessary. All PORC-related AREs were identified by an anesthetist in the OR or a nurse in the recovery room (RR). For those that occurred in the RR, an anesthetist examined the patient to confirm a PORC-related ARE.6
Reports sent to the Hospital Pharmacy to justify sugammadex use, the anesthesia records, and our information system’s computer database were used to retrieve data about patients and controls.
The pharmacoeconomic evaluation of sugammadex use
The pharmacoeconomic evaluation involved two aspects: cost-analysis of NMB management accompanying the introduction of a rocuronium–neostigmine–sugammadex strategy into a cisatracurium–neostigmine regimen and estimation of the economic benefits of using sugammadex as first choice reversal drug.
Costs analysis of NMB management
For the cost-analysis of NMB management, two periods were considered: January 1, 2011 to December 31, 2012 (before the introduction of sugammadex) and January 1, 2013 to December 31, 2014 (after the introduction of sugammadex).
Data about the type of anesthesia were collected from the surgical information system’s computer database and classified as general anesthesia with NMB, general anesthesia without NMB, and regional anesthesia (without NMB).
Data about the cost of drugs used for NMB management were retrieved from the Hospital Pharmacy, whose personnel tracked drug costs electronically. As the ORs at University Hospital of Padova are allocated a specific cost center number, we were able to retrieve data on the costs of drugs used exclusively in the ORs involved in this study. For each drug, the unit price was considered. The total costs of drugs used for NMB management were calculated by multiplying each drug cost by the number of vials used. The total costs were also compared for the two time periods.
Economic benefits of using sugammadex as first-choice reversal drug
Two factors were considered for estimation of economic benefits: the difference in minutes between sugammadex and neostigmine with respect to the time until a complete recovery from NMB (TOF ratio ≥0.9) and the estimated costs of OR, and OR and RR staffs per minute using a validated model.13,14 With regard to the first factor, two previous randomized controlled trials comparing sugammadex to neostigmine for reversing moderate and deep NMB were considered for evaluating the time of complete recovery from NMB.18,19 With regard to the second factor, the estimated OR cost was based on the average cost of an OR (comprehensive of OR staff and material costs) at the University Hospital of Padova. The estimated OR staff cost was calculated assuming that the OR staff comprised two consultant surgeons, a consultant anesthetist, and three nurses. The estimated RR staff cost was calculated by assuming that the RR staff comprised one nurse. All costs were expressed in Euros 2015 (€). The potential gain in using sugammadex instead of neostigmine as first choice reversal drug was obtained by multiplying the time difference in minutes for the costs of OR, and OR and RR staffs. Costs of using sugammadex as rescue therapy and cost of neostigmine avoided were excluded in this analysis. A cost-saving analysis was then performed considering the cost of drugs used and the economic benefits.
Patients transferred from ORs to the intensive care unit (ICU) in the two periods (2011–2012 vs 2013–2014) were screened for the cause of ICU admission. Only patients admitted because of a PORC were considered for the economic assessment of the impact on unplanned ICU admissions by the introduction of sugammadex. The estimated cost of ICU admission was obtained from previously published data20,21 and adjusted to 2015 using the coefficient of conversion produced by ISTAT.22 This value corresponds to the average cost of a 1 day of ICU stay at University Hospital of Padova.23
Statistical analysis
Data regarding surgery and costs are presented as totals and analyzed with chi-square test. For “preventive” and “curative” use of sugammadex, continuous data are reported as means (± standard deviation) and compared using the Student’s t-test. Categorical data are reported as the absolute number with percentages and compared with the chi-square test or Fisher’s exact test. Linear correlation analysis was used to estimate the association between the NMBA administered and presence of PORC-related AREs. Kaplan–Meier estimate-of-survival curves were used to determine the cumulative probability of delayed discharge from the OR to the surgical ward after 1 hour. Curves for the two groups were compared using the log-rank test. A P-value <0.05 was considered statistically significant.
Results
Outcome
Patients undergoing general anesthesia with NMB who received sugammadex for “preventive” use were 3% of the total cases (128 of 4,282 [total cases]) (Table 1). The treated group showed a better TOF ratio at extubation (P<0.001) and a more rapid discharge to the surgical ward than controls (P<0.001) (Table 1).
In eight cases, sugammadex was used for reversal of NMB at the end of surgery because of difficult airway management at induction of anesthesia. In two cases, sugammadex was successfully used intraoperatively as rescue therapy for immediate reversal of a high-dose rocuronium-induced NMB for CVCI situation.
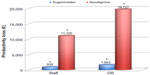
Patients undergoing general anesthesia with rocuronium-induced NMB who received sugammadex for treatment of PORC-related AREs (“curative” use) were 3.2% of the cases (96 of 3,017 [cases treated with rocuronium]) (Table 2). PORC-related AREs included both difficult weaning (2% of cases) and postextubation AREs (1.2% of cases). The treated group showed a worse TOF ratio at the end of surgery than controls (Table 2). In the treated group, the occurrence of an ARE was significantly associated with longer surgical procedures (P<0.001), higher dosage of rocuronium (P<0.001), repeated doses of rocuronium (P<0.001), and shorter time between the last dose of rocuronium and tracheal extubation (P<0.001). The most frequent PORC-related AREs were difficult weaning from mechanical ventilation (60.4%), postextubation severe hypoxemia (19.8%), inability to breathe deeply (9.4%), upper airway obstruction (5.2%), signs of respiratory distress (3.1%), and respiratory failure requiring mask ventilation (2%). In the treated group, the PORC-related AREs did not prolong the RR stay, as the time to discharge from the RR was similar in the treated and control groups (Table 2). Sugammadex administration showed not only reduction of the probability of delayed discharge to the surgical ward after 1 hour (Figure 1A and B) but also reduction in PORC-related ICU admissions. Ten PORC-related unplanned ICU admissions were registered in the 2011–2012 period and one in the 2013–2014 period. No unplanned ICU admissions with rocuronium–neostigmine–sugammadex strategy were observed. No adverse drug reactions related to drugs used for NMB management were registered.
Costs analysis of NMB management
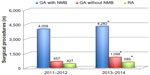
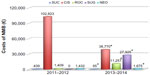
Despite the overall number of procedures involving general anesthesia with NMB increasing significantly (P<0.0001) (Figure 2), the introduction of rocuronium–neostigmine–sugammadex into a regimen based on cisatracurium–neostigmine as the main strategy for NMB management decreased the total costs of NMB management by 25% from 2011–2012 to 2013–2014 (Figure 3). Although the costs per vial remained constant, the total costs for succinylcholine (P=0.0026) and cistracurium (P<0.0001) significantly decreased, whereas the costs for rocuronium (P<0.0001), sugammadex (P<0.0001), and neostigmine (P=0.0164) significantly increased over the two time periods (Figure 2). The increased use of rocuronium (€1.85/vial), which is less expensive than cisatracurium (€6.25/vial), induced by introduction of sugammadex, allowed the reduction of the average cost of NMB management by 36%, from €20.8/case to €13.3/case. The sugammadex cost for “preventive” use was €19,987 (272 vials), for “curative” use was €7,056 (96 vials), and for “emergency use” was €882 (12 total vials). In the last situation, as recommended by the manufacturer, sugammadex 16 mg/kg was administered for immediate reversal of a high-dose rocuronium-induced NMB in two CICV situations.
Economic benefits of using sugammadex as first-choice reversal drug
The economic benefit in speeding the complete recovery from NMB and the discharge to surgical ward was estimated to be €6.6/min based on OR cost, and €3.72/min and €0.35/min based on OR and RR staffs, respectively (Table 3).
Considering that in the University Hospital of Padova the OR cost (€6.6/min) includes also the OR staff cost (€3.72/min), the gain in shorter OR stay based on OR cost was estimated to be €18,064 as obtained by calculating the difference between the loss of gain associated to neostigmine (€19,727) and sugammadex (€1,663) (Table 4 and Figure 4). Based on RR staff cost, the gain in shorter RR stay was estimated to be €2,105.6, considering the mean difference time of RR stay (Table 2). The total gain was then €20,169.6 which is derived from the sum of estimated gains in OR (€18,064) and in RR (€2,105.6). The final analysis, in which the cost of neostigmine for “preventive” use (€94) was excluded, showed a cost of €154.7/case and an estimated gain of €157.6/case, with a net cost-saving of €2.9/case.
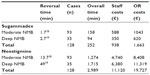  | Table 4 Comparison of productivity loss according to reversal strategy |
The average cost of ICU stay was estimated to be €1,354.8/day (range from €931.6/day to €2384.5/day). Based on estimated cost of ICU stay, the economic benefit in avoiding PORC-related ICU admission was estimated at €13,548 (€9,316–€23,845).
Discussion
An NMB management based on rocuronium–neostigmine–sugammadex strategy is less expensive than that based on cisatracurium–neostigmine regimen. Sugammadex was demonstrated to increase safety in patients receiving a rocuronium-induced NMB, avoiding PORC if given as the first-reversal drug in high-risk patients and allowing prompt treatment of PORC-related AREs occurring after administration of standard reversal drugs. Despite its cost, sugammadex showed resource savings to the hospital by speeding the recovery from NMB in the OR. It was also shown to potentially produce resource savings by reducing the rate of PORC, time spent in the RR, and rate of unplanned ICU admissions.
PORC following intraoperative NMBAs administration is common in the postoperative period, with rates ranging from 26% to 64%.24,25 Residual effects of NMBAs are associated with an increased risk of AREs.6,26,27 The overall incidence of AREs (3.2%) observed in our study is within the range of 1.3%–6.9% reported in the literature.6,26 The incidence of postextubation AREs is greater than the 0.8% reported by Murphy et al,6 but close to the 1.3% that Rose et al26 reported in their study. Postoperative AREs are associated with increased postoperative morbidity and mortality, prolonged postanesthesia care unit (PACU) stay, or unanticipated admissions to the ICU.5,26,28 Strategies to reduce PORC include the use of short- or intermediate-duration NMBAs, neuromuscular monitoring, and reversal agents.5
The use of intermediate-duration NMBAs has been shown to reduce (albeit not eliminate) the risk of AREs.28,29 Cisatracurium is purported to be advantageous over rocuronium because of its organ-independent metabolism.4,30,31 However, a regimen based on cisatracurium for NMB does not completely eliminate the risk of PORC.5 In a randomized, controlled, double-blind, multicenter trial of 338 patients, the incidence of PORC in patients receiving cisatracurium was 57%, which was significantly (P<0.05) higher than the 44% incidence in those receiving rocuronium.4 A wide variability in the rate of spontaneous recovery has been observed after a single dose of an intermediate-duration NMBA, which can be prolonged by such factors as hypothermia, interactions with halogenated agents, and underlying diseases, and which may extend even longer than 2 hours.32 Furthermore, both a continuous infusion and repeated doses of these NMBAs are associated with an increased risk of PORC.1,4,33
The routine use of neuromuscular function monitoring has been previously shown to reduce the incidence of PORC.5,29,34 However, quantitative monitoring of neuromuscular transmission does not entirely abolish the risk of PORC.28 Furthermore, it is not always used in clinical practice.35
The administration of reversal agents plays, then, an important role in reducing the risk of PORC.5,29,34 Traditionally, acetylcholinesterase inhibitors, such as neostigmine, are used to hasten recovery from NMB. However, their efficacy is limited because of a ceiling effect, which limits their effectiveness when the NMB is profound.5,29 Moreover, using higher or repeated doses of neostigmine to improve neuromuscular recovery and reduce the rate of PORC may also be associated with increased postoperative respiratory morbidity.36 Because of its mechanism of action, sugammadex improves the quality and safety of NMB reversal in clinical practice.1,4,14,37 Sugammadex has been shown to reduce the incidence of PORC and AREs, and to shorten the time for discharge from the OR.7,29,38 The usefulness of sugammadex for treatment of AREs after neostigmine reversal has also been reported.17,39 Our data provide evidence supporting the benefit of sugammadex in preventing PORC and associated morbidities,38 particularly in high-risk patients, such as those who are elderly or obese and those who have respiratory, cardiac, kidney, liver, or neuromuscular disease.5,40–43
The benefits associated with sugammadex have a cost, which is primarily related to the direct cost of the drug itself. However, in our economic evaluation, sugammadex may contribute to overall cost-savings for the hospital in several ways. First, it reduced the cost of NMB management by requiring the use of rocuronium, which is three times less expensive than cisatracurium at the current list price in our hospital. Second, sugammadex allowed a rapid, safe, and complete recovery from NMB in high-risk patients.15,40–44 Third, sugammadex as rescue therapy after neostigmine reversal quickly resolved PORC-related AREs. Both these latter two aspects have clinical and economic implications. Prolonged time to extubation at the end of general anesthesia delays OR exit and slows OR workflow.45 Different surgical and anesthesiological factors may concur to a prolonged extubation that is regarded unfavorably by both anesthesiologists and surgeons.45,46 To such purpose, residual curarization showed to increase significantly the risk of delayed OR exit and PACU discharge.46 A proper NMB management may then play an important role in helping to avoid prolonged extubation time and delayed OR exit.42,47 Sugammadex has been shown to reduce the anesthesia time,42 and time for OR exit and PACU discharge.38,48 The economic benefits have been estimated to have a value of €6.6/min gained in OR (€3.72/min considering only the OR staff cost) and €0.35/min gained in the RR, with a net saving of €2.9/case. A recent pharmacoeconomic evaluation estimated that time saved in the OR has a value of £4.44/min, whereas time saved in the PACU has a value of £0.33/min, confirming that sugammadex 2 mg/kg (or 4 mg/kg) is cost-effective for reversal of moderate (or deep) NMB induced by rocuronium13,14 and has the potential to increase the OR productivity with an associated economic benefit.21 However, the effort to reduce extubation time from the end of surgery has an economical advantage only if the OR represents a variable cost and not a fixed cost.45,49 This may be the case in which all (eg, >90%) ORs at a hospital have more than 8 hours of cases each workday. In such situations, there is a rationale to reduce the chance that tracheal extubation times will be prolonged.45,49
Another suggested benefit of sugammadex that was not investigated in this study is that scheduled surgical procedures may not be delayed or suspended because of unanticipated problems with the preceding surgery, such as prolonged reversal of NMB or PORC-related AREs requiring postoperative care or unplanned ICU admission.13,14 The economic benefit may reach an average value of €1,354.8 for each avoided day of ICU stay. Finally, by reducing morbidity and mortality associated with PORC or being unable to quickly reverse profound NMB with standard reversal drugs in a CVCI situation, sugammadex promotes improved quality and safety of anesthetic management that may not be quantifiable from an economic viewpoint, but is nevertheless highly desired.
The study has some limitations. First, it was performed in a single national center. The cost of drug use may be subject to variations among national hospitals. Furthermore, some aspects of the cost-analysis may not apply to other countries and different health-care systems.14 Second, the benefit of reduction in OR time may depend on the day of the week and the hours of cases in the OR in which the intervention is applied and may be lost in case of emergency room or ORs scheduled relatively empty in which ORs represent a fixed cost.45,50 Third, it is possible that sugammadex produces further resource savings than those considered; however, there are no suitable data to provide a basis for such modeling, so these were not considered.14 Finally, an evaluation of the outcomes of patients undergoing general anesthesia with cisatracurium-induced NMB was not performed.14
Conclusion
In conclusion, sugammadex promotes a rapid turnover of patients in the OR, which is cost-effective and limits the disadvantage of its high cost. Through a rapid, predictable, and safe reversal of rocuronium-induced NMB, sugammadex minimizes the risk of PORC and its consequences.
Acknowledgments
The authors would like to thank Dr S Boccella, Head of Nurse of Padova Hospital, for invaluable support in the acquisition of data; Dr C Battistuta, staff member of Pharmacy of Padova Hospital, for important contributions in the acquisition of data; and Dr A Marcolongo and Dr L Furlan, staff members of Administration of Padova Hospital, for indispensable support with the economic assessment.
Author contributions
MC has made contributions to conception and design of the study, acquisition of data, analysis and interpretation of data, and drafting and revising the manuscript.
FB has made contributions to conception and design of the study, acquisition and interpretation of data, and revising the manuscript.
FZ has made contributions to acquisition and interpretation of data, and revising the manuscript.
CO has made contributions to conception and design of the study, analysis and interpretation of data, and revising the manuscript.
All authors agree on all aspects of the work, and read and approved the final paper.
Disclosure
MC, FB, and CO have received payments for lectures from Merck Sharp and Dohme (MSD), Italy. The authors report no other conflicts of interest in this work.
References
Jellish WS, Brody M, Sawicki K, Slogoff S. Recovery from neuromuscular blockade after either bolus and prolonged infusions of cisatracurium or rocuronium using either isoflurane or propofol-based anesthetics. Anesth Analg. 2000;91(5):1250–1255. | |
Lighthall GK, Jamieson MA, Katolik J, Brock-Utne JG. A comparison of the onset and clinical duration of high doses of cisatracurium and rocuronium. J Clin Anesth. 1999;11(3):220–225. | |
Schaller SJ, Fink H. Sugammadex as a reversal agent for neuromuscular block: an evidence-based review. Core Evid. 2013;8:57–67. | |
Maybauer DM, Geldner G, Blobner M, et al. Incidence and duration of residual paralysis at the end of surgery after multiple administrations of cisatracurium and rocuronium. Anaesthesia. 2007;62(1):12–17. | |
Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology. 2010;112(4):1013–1022. | |
Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesthesia and Analgesia. 2008;107(1):130–137. | |
Caldwell JE, Miller RD. Clinical implications of sugammadex. Anaesthesia. 2009;64(Suppl 1):66–72. | |
Sorgenfrei IF, Norrild K, Larsen PB, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology. 2006;104(4):667–674. | |
Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, Sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth Analg. 2007;104(3):555–562. | |
de Boer HD, Driessen JJ, Marcus MA, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose-finding and safety study. Anesthesiology. 2007;107(2):239–244. | |
Ledowski T, Hillyard S, Kozman A, et al. Unrestricted access to sugammadex: impact on neuromuscular blocking agent choice, reversal practice and associated healthcare costs. Anaesth Intensive Care. 2012;40(2):340–343. | |
Chambers D, Paulden M, Paton F, et al. Sugammadex for reversal of neuromuscular block after rapid sequence intubation: a systematic review and economic assessment. Br J Anaesth. 2010;105(5):568–575. | |
Paton F, Paulden M, Chambers D, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth. 2010;105(5):558–567. | |
Chambers D, Paulden M, Paton F, et al. Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment. Health Technol Assess. 2010;14(39):1–211. | |
Craig RG, Hunter JM. Neuromuscular blocking drugs and their antagonists in patients with organ disease. Anaesthesia. 2009;64(Suppl 1):55–65. | |
Lenz A, Hill G, White PF. Emergency use of sugammadex after failure of standard reversal drugs. Anesth Analg. 2007;104(3):585–586. | |
Carron M, Freo U, Ori C. Sugammadex for treatment of postoperative residual curarization in a morbidly obese patient. Can J Anaesth. 2012;59(8):813–814. | |
Illman HL, Laurila P, Antila H, Meretoja OA, Alahuhta S, Olkkola KT. The duration of residual neuromuscular block after administration of neostigmine or sugammadex at two visible twitches during train-of-four monitoring. Anesth Analg. 2011;112(1):63–68. | |
Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109(5):816–824. | |
Cavallo MC, Lazzaro C, Tabacchi M, et al. [Cost of ICU in Italy. Results from an empirical study on a sample of 12 hospitals]. Minerva Anestesiol. 2001;67(1–2):41–53. Italian. | |
Sacchi V, Iannazzo S, Giunta F. Sugammadex nell’antagonismo del blocco neuromuscolare in anestesia: profilo clinico ed economico [Sugammadex in antagonism of neuromuscular block in anesthesia: a clinical and economic profile]. Farmeconomia e percorsi terapeutici. 2009;10(3):103–114 | |
ISTAT (Italian National Institute of Statistics). Price index for monetary reevaluations. Reference period: September 2015. Available from: http://www.istat.it/it/files/2011/06/coefficienti_annuali_1861_2014.pdf. Accessed January 11, 2016. | |
Tan SS, Bakker J, Hoogendoorn ME, et al. Direct cost analysis of intensive care unit stay in four European countries: applying a standardized costing methodology. Value Health. 2012;15(1):81–86. | |
Esteves S, Martins M, Barros F, et al. Incidence of postoperative residual neuromuscular blockade in the postanaesthesia care unit: an observational multicentre study in Portugal. Eur J Anaesthesiol. 2013;30(5):243–249. | |
Hayes AH, Mirakhur RK, Breslin DS, Reid JE, McCourt KC. Postoperative residual block after intermediate-acting neuromuscular blocking drugs. Anaesthesia. 2001;56(4):312–318. | |
Rose DK, Cohen MM, Wigglesworth DF, DeBoer DP. Critical respiratory events in the postanesthesia care unit. Patient, surgical, and anesthetic factors. Anesthesiology. 1994;81(2):410–418. | |
Fortier J, Chung F, Su J. Unanticipated admission after ambulatory surgery – a prospective study. Can J Anaesth. 1998;45(7):612–619. | |
Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012;345:e6329. | |
Brull SJ, Murphy GS. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth Analg. 2010;111(1):129–140. | |
Boyd AH, Eastwood NB, Parker CJ, Hunter JM. Pharmacodynamics of the 1R cis-1 R cis isomer of atracurium (51 W89) in health and chronic renal failure. Br J Anaesth. 1995;74(4):400–404. | |
De Wolf AM, Freeman JA, Scott VL, et al. Pharmacokinetics and pharmacodynamics of cisatracurium in patients with end-stage liver disease undergoing liver transplantation. Br J Anaesth. 1996;76(5):624–628. | |
Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98(5):1042–1048. | |
Cammu G, de Baerdemaeker L, den Blauwen N, de Mey JC, Struys M, Mortier E. Postoperative residual curarization with cisatracurium and rocuronium infusions. Eur J Anaesthesiol. 2002;19(2):129–134. | |
Baillard C, Clec’h C, Catineau J, et al. Postoperative residual neuromuscular block: a survey of management. Br J Anaesth. 2005;95(5):622–626. | |
Naguib M, Kopman AF, Lien CA, Hunter JM, Lopez A, Brull SJ. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111(1):110–119. | |
Sasaki N, Meyer MJ, Malviya SA, et al. Effects of neostigmine reversal of nondepolarizing neuromuscular blocking agents on postoperative respiratory outcomes: a prospective study. Anesthesiology. 2014;121(5):959–968. | |
Mirakhur RK. Sugammadex in clinical practice. Anaesthesia. 2009;64(Suppl 1):45–54. | |
Brueckmann B, Sasaki N, Grobara P, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized, controlled study. Br J Anaesth. 2015;115(5):743–751. | |
de Menezes CC, Peceguini LA, Silva ED, Simões CM. Use of sugammadex after neostigmine incomplete reversal of rocuronium-induced neuromuscular blockade. Rev Bras Anestesiol. 2012;62(4):543–547. | |
McDonagh DL, Benedict PE, Kovac AL, et al. Efficacy, safety, and pharmacokinetics of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in elderly patients. Anesthesiology. 2011;114(2):318–329. | |
Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium-induced muscle relaxation in morbidly obese undergoing general anaesthesia. Br J Anaesth. 2012;108(2):236–239. | |
Carron M, Veronese S, Foletto M, Ori C. Sugammadex allows fast-track bariatric surgery. Obes Surg. 2013;23(10):1558–1563. | |
Amao R, Zornow MH, Cowan RM, Cheng DC, Morte JB, Allard MW. Use of sugammadex in patients with a history of pulmonary disease. J Clin Anesth. 2012;24(4):289–297. | |
Dahl V, Pendeville PE, Hollmann MW, Heier T, Abels EA, Blobner M. Safety and efficacy of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in cardiac patients undergoing noncardiac surgery. Eur J Anaesthesiol. 2009;26(10):874–884. | |
Dexter F, Epstein RH. Increased mean time from end of surgery to operating room exit in a historical cohort of cases with prolonged time to extubation. Anesth Analg. 2013;117(6):1453–1459. | |
Butterly A, Bittner EA, George E, Sandberg WS, Eikermann M, Schmidt U. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth. 2010;105(3):304–309. | |
White PF. Pharmacoeconomic issues related to selection of neuromuscular blocking agents. Am J Health Syst Pharm. 1999;56(11 Suppl 1):S18–S21. | |
Watts RW, London JA, van Wijk RM, Lui YL. The influence of unrestricted use of sugammadex on clinical anaesthetic practice in a tertiary teaching hospital. Anaesth Intensive Care. 2012;40(2):333–339. | |
Epstein RH, Dexter F, Brull SJ. Cohort study of cases with prolonged tracheal extubation times to examine the relationship with duration of workday. Can J Anaesth. 2013;60(11):1070–1076. | |
Dexter F, Dutton RP, Kordylewski H, Epstein RH. Anesthesia workload nationally during regular workdays and weekends. Anesth Analg. 2015;121(6):1600–1603. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.