Back to Journals » Journal of Pain Research » Volume 12
Sufentanil Sublingual Tablet System (SSTS) for the management of postoperative pain after major abdominal and gynecological surgery within an ERAS protocol: an observational study
Authors Turi S , Deni F, Lombardi G , Marmiere M , Nisi FG, Beretta L
Received 6 May 2019
Accepted for publication 8 July 2019
Published 26 July 2019 Volume 2019:12 Pages 2313—2319
DOI https://doi.org/10.2147/JPR.S214600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Stefano Turi, Francesco Deni, Gaetano Lombardi, Marilena Marmiere, Francesco Giuseppe Nisi, Luigi Beretta
Dipartimento di Anestesia e Rianimazione, Ospedale San Raffaele, Milano, Italy
Background: The Sufentanil Sublingual Tablet System (SSTS) is a new, pre-programmed, noninvasive, handheld system for patient-controlled analgesia (PCA) which may allow a faster postoperative recovery compared with standard PCA. The efficacy of SSTS in controlling pain after open abdominal surgery has already been documented. However, to our knowledge SSTS has never been investigated in patients undergoing major surgery within an Enhanced Recovery After Surgery (ERAS) protocol.
Methods: This observational, retrospective analysis included consecutive patients undergoing elective major abdominal and gynecological surgery. All patients received the SSTS device once they were fully awake and had a good control of pain at the end of the surgery. We analyzed changes in pain intensity according to the numerical rating scale (NRS) throughout the treatment as well as its duration, the number of administrations, and possible related adverse events. Patients were also interviewed to assess their quality of sleep and overall satisfaction with the SSTS device.
Results: The study included 308 patients. Compared to the first SSTS administration, pain intensity decreased from a median NRS of 6 to 0 at day 3, for an overall reduction of 79%. Results were already statistically significant at postoperative day 1 (p<0.01). Adverse reactions were observed in 62 patients, with nausea being the most frequent (12%), and in 93% of patients SSTS was discontinued because it was considered no longer necessary. Patient satisfaction was high, with 89% of them judging the device as “easy” or “very easy” to use.
Conclusions: Although the retrospective and observational nature of the study as well as the absence of a comparative group limits the strength of evidence, our results consider SSTS an effective and safe tool for the management of postoperative pain after major abdominal and gynecological surgery within an ERAS protocol.
Keywords: SSTS, ERAS, analgesia, postoperative, PCA
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
The Enhanced Recovery After Surgery (ERAS) protocol includes a series of different multimodal, perioperative interventions aimed at accelerating postoperative recovery.1,2
Elements included in ERAS pathways are goal-directed fluid therapy, prevention of nausea and ileus, thromboembolic prophylaxis, minimally invasive surgical techniques, intraoperative normothermia, early nutrition and mobilization, and the use of short-acting anesthetics and multimodal analgesia.3,4 The common goal of all these interventions is to reduce the stress response to surgery and decrease the risk of postoperative complications, aiding patient’s recovery and reducing health costs.5,6
Adequate pain control in the postoperative period is mandatory in all ERAS protocols.1
However, consensus on the optimal management of pain within ERAS regimens has not been reached.7,8
The Sufentanil Sublingual Tablet System (SSTS; Zalviso; AcelRx Pharmaceuticals, Redwood City, CA, USA) is a new, pre-programmed, noninvasive, handheld system for patient-controlled analgesia (PCA). It enables patients to manage moderate-to-severe acute pain autonomously within the hospital setting. This system allows the sublingual administration of sufentanil 15 μg nanotablets with a fixed, 20-min lockout interval, which cannot be changed by patients or health care professionals. Moreover, the presence of a radiofrequency identification thumb tag allows the patient only to use the device.9
Compared with standard, morphine-based intravenous patient-controlled analgesia (IV-PCA), SSTS is associated with faster onset and a higher rate of success.10
Moreover, the fixed drug dose and the unmodifiable lockout interval account for a lower risk of programming errors. SSTS may also be associated with a lower incidence of infections and analgesic gaps, since the device does not rely on the presence of an intravenous line. Taken together, these properties perfectly fit into the minimally invasive orientation of ERAS protocols and might allow an earlier mobilization.
The efficacy of SSTS in controlling pain after open abdominal surgery has been documented in two randomized studies, which compared this device to morphine.11,12
However, to our knowledge, SSTS has never been investigated in patients undergoing major surgery within an ERAS protocol.
We conducted an observational study to evaluate the feasibility of SSTS in the control of pain in patients subjected to major abdominal and gynecological surgery within an ERAS pathway.
Patients and methods
Study setting and design
Our study was an observational, retrospective analysis conducted at San Raffaele Hospital, Milan, Italy. The study design was approved by the Ethical Committee of San Raffaele Hospital in Milan, and the study was conducted in accordance with the declaration of Helsinki. All patients signed an informed consent to the use of their data for research purposes.
Data from consecutive clinical charts of patients undergoing elective major laparoscopic abdominal and gynecological surgery from June 2016 to October 2017 who received PCA with SSTS were collected by two anesthesiologists (ST and FD). Patients presenting contraindications to the placement of epidural catheterization receiving open surgeries were also included.
Procedures
The ERAS protocol
All patients received a carbohydrate load the night before and the morning of the surgery, and long-acting benzodiazepines were avoided prior to surgery. Normothermia was maintained throughout the intraoperative period and antibiotic and antiemetic prophylaxis were administered. The postoperative period was characterized by a near-zero balance fluid management, prompt removal of nasogastric tube, and resumption of oral intake.
Anesthesia and analgesia procedures
Anesthesia was induced with propofol 2 mg/kg, fentanyl 1–2 μg/kg, and rocuronium 0.6 mg/kg and maintained with inhalation agents and fentanyl as needed.
Intravenous multimodal analgesia with morphine 0.05–0.1 mg/kg and NSAIDs (ketorolac 30 mg) or paracetamol (1 g) was administered to all patients before the end of the surgical procedure to minimize the possible side effects of the drugs and enhance pain control by employing different mechanisms of action. In addition, the administration of NSAIDs or paracetamol was repeated, at fixed intervals, during the postoperative period. Our Acute Pain Service, available 24 hrs a day, monitored all the patients throughout their hospital stay.
Gastric protection and antiemetic prophylaxis were also administered to all patients intraoperatively. The latter was continued in high-risk patients, identified using the Apfel scoring system, or upon patient’s needs.13,14
SSTS
All patients received instructions regarding SSTS use by the anesthesiologist, using adequate educational support, the day before surgery. Every patient received the radiofrequency identification thumb tag after the intervention. Patients were given the SSTS device only when they fulfilled the criteria of being fully awake, scoring 4–5 on the modified Wilson scale, and having a good control of pain.15
The first administration of sublingual sufentanil was directly supervised by the anesthesiologist.
Assessments
Data stored in the internal memory of the device were collected and evaluated: number of SSTS dosing, setup time, mean treatment duration, mean number of required doses and mean interdosing time. Moreover, from the clinical charts, we collected data regarding pain intensity at rest and upon movement using the NRS, the need of concomitant pain therapies, treatment interruption, recovery in terms of number of days required to attain the sitting and standing positions and return of bowel function, and possible adverse events. By interviewing enrolled patients, we also assessed their quality of sleep on a 4-point scale, their opinion about SSTS in terms of efficacy, ease of understating and use, as well as their mobility using a 5-point scale.
Data analysis
Data were analyzed using descriptive statistics, with percentages calculated on available data for each variable. Pain intensity was calculated as the mean NRS value at first administration (baseline, V0), and during the following 24, 48, and 72 hrs (V1, V2, and V3, respectively). Pain intensity at different timepoints was compared using the Student’s t-test. Quality of sleep during the first three postoperative days was compared using the McNemar test.
A p-value <0.05 was considered statistically significant. All analyses were performed by SAS 9.4 software.
Results
Patient population
A total of 308 patients were evaluated (mean age 51±15 years; 38% males). Baseline characteristics are shown in Table 1. Among them, 53 patients (17%) received gynecological surgery and 9 patients (2.9%) received open abdominal surgery. The majority of patients scored 5 on the modified Wilson scale, meaning they were “alert” (n=223; 72%), while the remaining scored 4, indicating a “light” sedation level. Whether the score was 4 or 5 was not reported on 23 charts (8%).
 |
Table 1 Baseline patient and surgical characteristics (n=308) |
Setup and duration of SSTS treatment
The SSTS device was set up either by the anesthesiologist (94%) or by a trained nurse. The mean setup time was 4.1±6.9 mins, but in 50% of cases the setup required less than 2 mins.
Mean duration of SSTS use was 46±25 hrs (Figure 1). The mean number of required doses per patient was 19.4±17.3. The number of required doses decreased over time, from 9±6 at V1 to 3.4±5.3 at V3. The mean interval from the delivery of SSTS to first dosing was 2.1±3 hrs; the time between the first four consecutive doses was 2.5±4.4, 3.4±6.4 and 3.3±3.9 hrs, respectively.
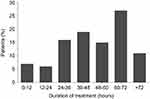 |
Figure 1 Duration of Sufentanil Sublingual Tablet System (SSTS) treatment. |
Pain intensity
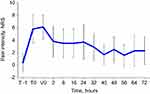
Pain intensity progressively decreased throughout the observation period, and effective control of pain was maintained over time (Figure 2). Specifically, pain intensity on the NRS scale decreased from a median of 6 at V0 to 0 at V3, with an overall decrease of 79%. Pain reduction was already statistically significant at V1 (p<0.01, for all visits compared to V0). Both pain at rest and upon movement followed a similar pattern, with progressively decreasing NRS values (Figure 3). In addition, pain therapy with paracetamol (n=199, 64%) and ketorolac (n=141, 46%) was administered, sometimes in combination, to the majority of patients.
 |
Figure 3 Mean (SD) pain intensity at rest and upon movement throughout SSTS treatment. *p<0.01 compared to first administration (V0). |
Treatment interruption
SSTS device was removed because it was considered no longer necessary in the majority of cases (n=285; 93%). A precautionary interruption of treatment was done in 14 patients (4.5%) for adverse events, and only one patient (0.3%) requested the removal of the device due to poor control of pain.
Recovery
A substantial proportion of subjects could sit (n=54; 27%) or even stand up (n=38; 20%, data available for 191 patients) on the very same day of the intervention. The majority of patients was able to tolerate the sitting position (n=171; 87%, data available for 197 patients) and the standing position (n=132; 70% data available for 191 patients) by the day after the surgery, and almost all of them were able to stand 2 days after surgery (n=189, 96%). Recovery of bowel function was possible in less than 1 day in 22% of patients (n=34; data available for 157 patients) and in less than 2 days for another 27% (n=42).
The proportion of patients experiencing restful sleep the night after the intervention was 51% (n=78, data available for 152 patients); this number increased to 76% at V3 (p<0.01).
Adverse events
A total of 73 adverse reactions were reported in 62 patients (20%), with nausea being the most frequent (n=37, 12%) (Table 2). The intensity of adverse reactions was mild-to-moderate in the majority of cases, and only 12 severe reactions were rereported (15%, all episodes of nausea and vomiting). An explicit correlation between the adverse event and SSTS treatment was observed in 2 cases (ie, respiratory depression and nausea/vomiting).
 |
Table 2 Reported adverse events, n (%) |
Patient’s opinion
Information on patients’ evaluation of SSTS is reported in Table 3. Overall, 89% of patients judged this device as “easy” or “very easy” to use, and similar results were observed for the understanding of use, ease of administration, ease of mobilization, and analgesic efficacy.
 |
Table 3 Patient’s opinion on Sufentanil Sublingual Tablet System (SSTS) |
Discussion
SSTS is a new, pre-programmed, noninvasive, handheld system for PCA, which can potentially present some advantages over standard opioid-based IV-PCA. In comparison, SSTS is associated with faster onset and a higher rate of successful analgesia.10
Moreover, the fixed drug dose and the unmodifiable lockout interval guarantee a lower risk of programming errors and facilitate the setup of the device. Properly trained nurses can also perform SSTS configuration, which has been shown in our study to require less than 4 mins, with potential benefits for the management of the Unit.
In line with the ERAS principle of minimal invasiveness, SSTS does not rely on the presence of an intravenous line. This is associated with several advantages, among which are the low incidence of infections or analgesic gaps due to flow interruptions and the decreased limitations in patient mobility resulting in higher satisfaction. Moreover, the absence of a venous access and the minimal necessity of supervision make the SSTS device really appreciated by nurses. In addition, its internal memory stores information about the number of self-administered doses and the interval between consecutive ones, allowing for improved management of patients.
According to recent studies, PCA allows to limit the use of opioids without increasing complications or prolonging the length of hospital stay.16,17
In particular, PCA with opioids has shown some advantages over thoracic epidural analgesia in the context of laparoscopic procedures within ERAS protocols.18,19
Our observational study evaluated the feasibility of SSTS for pain control in patients undergoing major abdominal and gynecological surgery within an ERAS pathway. Results showed that SSTS was associated with a rapid and long-lasting control of pain, with an NRS<3 starting from postoperative day 1 in most patients. Compared to our clinical experience with standard IV-PCA, a lower number of administrations were sufficient for a good analgesic effect, likely due to the sublingual formulation of sufentanil which confers a prolonged half-life. SSTS use was interrupted since it was no longer necessary in more than 90% of cases, indicating a satisfactory analgesic efficacy, and in only 1 case interruption was due to poor pain control.
A good analgesia resulted in a fast recovery, with almost all patients able to stand by the day after surgery and a substantial proportion of subjects that could sit or even stand up on the very same day of the intervention. Moreover, complete recovery of bowel function was possible in less than 2 days for about half of the subjects. These effects were paralleled by an improvement in the quality of sleep. The length of hospital stay was comparable to that observed with IV-PCA in our Acute Pain Service experience.20
SSTS was well accepted by patients, who provided a favorable feedback on its ease of use and analgesic efficacy.
Incidence of adverse events was limited (20%) and not directly attributable to the use of SSTS in the majority of cases. Ringold et al described 26% of complications potentially associated with the surgical procedure in the control arm of their randomized controlled study, with a 22% incidence of nausea.11
Our study reported a lower incidence of nausea (12%), which did not determine the interruption of the SSTS device use in the majority of patients.
It is important to notice that the population studied in this analysis is known to be at risk of developing adverse events with opioid therapy, since it included several overweight patients (mean BMI 29.6 kg/m2, with some cases of grade III obesity) and patients with renal failure.21,22
A specific correlation between the adverse event and SSTS therapy was observed in only 2 cases, the first being an episode of severe nausea/vomiting and the second a case of respiratory depression in a patient already on heavy opioid therapy, antipsychotic therapy, and only 1 SSTS administration. To manage these events, no further action but the interruption of treatment was required.
According to our experience, we would recommend the use of SSTS into an ERAS protocol, given its prompt analgesic action and the limited incidence of adverse events. In addition, patients on SSTS therapy may theoretically present less hypotension, thus requiring less fluid supplementation and paresthesia compared with those on epidural analgesia; these characteristics, together with the limited invasiveness, further enhance the possibility of patient’s mobilization.18
Our data are consistent with a recent study by Meijer et al on postoperative SSTS use in a large cohort of patients.23
Similar to our study, it was retrospective and without a control group; however, our study was monocentric, assuring a consistent patient management, and our surgical population was more homogeneous, with patients undergoing abdominal and gynecological surgery only. In our study, the main difference with Meijer’s results was the lower incidence of nausea (34% vs 12%); however, they did not describe the intraoperative use of opioids, and a higher use of these drugs might have contributed to the difference.
Our study presents several limitations, including its retrospective observational nature and the absence of a comparative group, with respect to similar published randomized trials.11,12 Furthermore, a small proportion of missing data was not available for the final evaluation.
Conclusions
Although the retrospective and observational nature of the study as well as the lack of a comparative group limits the strength of evidence, our results deem SSTS an effective and safe tool for the management of postoperative pain after major abdominal and gynecological surgery within an ERAS protocol. Further large randomized controlled trials are necessary to confirm these positive preliminary results and to explore the involvement of different health care providers in the management of SSTS therapy.
Acknowledgments
Medical writing was performed by Luca Giacomelli, PhD; this assistance and editorial support was provided by Grunenthal, who did not have any part in the development of the study rationale, the carrying out of the study, nor writing of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bugada D, Bellini V, Fanelli A, et al. Future perspectives of ERAS: a narrative review on the new applications of an established approach. Surg Res Pract. 2016;2016:3561249.
2. Liungqqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi:10.1001/jamasurg.2016.4952
3. Debarros M, Steele SR. Perioperative protocols in colorectal surgery. Clin Colon Rectal Surg. 2013;26(3):139–145. doi:10.1055/s-0033-1351128
4. Zhuang CL, Huang DD, Chen F, et al. Laparoscopic versus open colorectal surgery within enhanced recovery after surgery programs: a systematic review and meta-analysis of randomized controlled trials. Surg Endosc. 2015;29(8):2091–2100. doi:10.1007/s00464-014-3922-y
5. Li P, Fang F, Cai JX, et al. Fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for colorectal malignancy: a meta-analysis. World J Gastroenterol. 2013;19(47):9119–9126. doi:10.3748/wjg.v19.i47.9119
6. Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(6):1531–1541. doi:10.1007/s00268-013-2416-8
7. Fawcett WJ, Baldini G. Optimal analgesia during major open and laparoscopic abdominal surgery. Anesthesiol Clin. 2015;33(1):65–78. doi:10.1016/j.anclin.2014.11.005
8. Caesar Y, Sidlovskaja I, Lindqvist A, Gislason H, Hedenbro JL. Intraabdominal pressure and postoperative discomfort in laparoscopic Roux-en-Y Gastric Bypass (RYGB) surgery: a randomized study. Obes Surg. 2016;26(9):2168–2172. doi:10.1007/s11695-016-2091-6
9. Sacerdote P, Coluzzi F, Fanelli A. Sublingual sufentanil, a new opportunity for the improvement of postoperative pain management in Italy. Eur Rev Med Pharmacol Sci. 2016;20:1411–1422.
10. Frampton JE. Sublingual sufentanil: a review in acute postoperative pain. Drugs. 2016;76:719–729. doi:10.1007/s40265-016-0625-9
11. Ringold FG, Minkowitz HS, Gan TJ, et al. Sufentanil sublingual tablet system for the management of postoperative pain following open abdominal surgery: a randomized, placebo-controlled study. Reg Anesth Pain Med. 2015;40(1):22–30. doi:10.1097/AAP.0000000000000152
12. Melson TI, Boyer DL, Minkowitz HS, et al. Sufentanil sublingual tablet system vs. intravenous patient-controlled analgesia with morphine for postoperative pain control: a randomized, active-comparator trial. Pain Pract. 2014;14(8):679–688. doi:10.1111/papr.12238
13. Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anesthesia practice. Acta Anaesthesiol Scand. 2016;60:289–334.
14. Pierre S, Benais H, Pouymayou J. Apfel’s simplified score may favourably predict the risk of postoperative nausea and vomiting. Am J Anesth. 2002;49(3):237–242. doi:10.1007/BF03020521
15. Höhener D
16. Palmer PP, Miller RD. Current and developing methods of patient-controlled analgesia. Anesthesiol Clin. 2010;28(4):587–599. doi:10.1016/j.anclin.2010.08.010
17. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17:131–157. doi:10.1016/j.jpain.2015.12.008
18. Hubner M, Blanc C, Roulin D, Winiker M, Gander S, Demartines N. Randomized clinical trial on epidural versus patient-controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg. 2015;261(4):648–653. doi:10.1097/SLA.0000000000000838
19. Turi S, Gemma M, Braga M, Monzani R, Radrizzani D, Beretta L. Epidural analgesia vs systemic opioids in patients undergoing laparoscopic colorectal surgery. Int J Col Dis. 2019:34(5):915–921. Epub 2019 Mar 29. doi:10.1007/s00384-019-03284-4
20. Deni F, Greco M, Turi S, et al. Acute pain service: a 10-year experience. Pain Pract. 2019. doi:10.1111/papr.12777
21. Budiansky AS, Magarson MP, Eipe N. Acute pain management in morbid obesity- an evidence based clinical update. Surg Obese Relat Dis. 2017;13(3):523–532. doi:10.1016/j.soard.2016.09.013
22. Overdyk F, Dahan A, Roozekrans M, et al. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317–325. doi:10.2217/pmt.14.19
23. Mejier F, Cornelissen P, Sie C, et al. Sublingual sufentanil for postoperative pain relief: first clinical experiences. J Pain Res. 2018;11:987–992. eCollection 2018. doi:10.2147/JPR.S160091
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.