Back to Journals » OncoTargets and Therapy » Volume 15
Successful Treatment with Short-Term Steroid Against Severe Hepatitis Confirmed by Liver Biopsy in a Patient with Advanced Squamous-Cell Lung Cancer Receiving a Combination of Pembrolizumab, Carboplatin, and Nab-Paclitaxel: A Case Report
Authors Hayashi A, Nakamichi S, Nakayama Y, Nagano A, Mikami E, Takano N , Tozuka T , Matsumoto M, Miyanaga A , Noro R, Terasaki Y , Kubota K, Seike M, Gemma A
Received 9 February 2022
Accepted for publication 17 May 2022
Published 7 June 2022 Volume 2022:15 Pages 637—642
DOI https://doi.org/10.2147/OTT.S361467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Anna Hayashi,1 Shinji Nakamichi,1 Yukako Nakayama,1 Atsuhiro Nagano,1 Erika Mikami,1 Natsuki Takano,1 Takehiro Tozuka,1 Masaru Matsumoto,1 Akihiko Miyanaga,1 Rintaro Noro,1 Yasuhiro Terasaki,2 Kaoru Kubota,1 Masahiro Seike,1 Akihiko Gemma1
1Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, Bunkyo-ku, Japan; 2Department of Analytic Human Pathology, Nippon Medical School, Tokyo, Bunkyo-ku, Japan
Correspondence: Anna Hayashi, Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, 1-1-5, Sendagi, Tokyo, Bunkyo-ku, 113-8603, Japan, Tel +81-3-3822-2131, Fax +81-3-5685-3075, Email [email protected]
Abstract: Pembrolizumab is an immune checkpoint inhibitor (ICI) that targets programmed death-1. Although ICIs have shown efficacy in the treatment of lung cancer, they have also been reported to cause a variety of immune-related adverse events (irAEs). Hepatotoxicity is a known irAEs, but currently, there is not enough information on its pathological characteristics and treatment. We report the case of a 70-year-old man with advanced squamous-cell lung cancer who developed severe grade 4 hepatitis on day 8 after receiving carboplatin, nab-paclitaxel, and pembrolizumab as fourth-line therapy. We treated him with steroid therapy the day after a liver biopsy was performed to investigate his pathological features, which led to a rapid and remarkable improvement. Confirmation of immune-related hepatotoxicity by pathological findings allowed the early tapering and discontinuation of steroid therapy. Performing a liver biopsy and verifying histological characteristics are needed for successful treatment with short-term steroids when drug-induced hepatitis caused by anti-cancer therapy including pembrolizumab is considered.
Keywords: combination immunotherapy, immune checkpoint inhibitor, immune-related adverse events, irAEs, hepatotoxicity, liver biopsy, short-term steroid
Introduction
Immune checkpoint inhibitors (ICIs), such as pembrolizumab, have been reported to be effective in the treatment of lung cancer.1–4 In recent years, combination immunotherapy, which aims to achieve synergistic effects by combining therapies with different mechanisms of action, has become widely used.5,6 Although ICIs have demonstrated immune responses against cancer, they can also cause adverse events.1–6 Because high doses of steroids are generally administered for a long time to treat immune-related adverse events (irAEs), complications such as infection, diabetes, and osteoporosis can occur. In addition, it takes a long time to taper off the steroids, and lung cancer treatment cannot be resumed early. A precise diagnosis of the irAE and the dosage and duration of steroid administration still need to be investigated. Hepatotoxicity is a known irAE, but its histopathology and treatment are unclear. We report the case of a patient with lung cancer who developed severe liver injury after treatment with pembrolizumab, carboplatin, and nanoparticle albumin-bound paclitaxel (nab-paclitaxel), which improved with short-term steroid treatment after liver biopsy.
Case Report
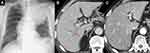
A 70-year-old Japanese man with a 46-year history of smoking was referred to our hospital because of left chest pain and an abnormal shadow in the left lung. He was diagnosed with squamous-cell lung carcinoma in the left upper lobe (cT4N3M0, Stage IIIC) with idiopathic interstitial pneumonia four years ago. Although no histological diagnosis was decided, there was a reticular shadow on the basal segment of the light lung, and specific diagnostic high-resolution computed tomography (HRCT) criteria was determined to be indeterminate for UIP. Immunohistochemistry revealed the high expression of programmed death ligand 1 (PD-L1) with a tumor proportion score of 70–80% on the tumor cell membrane (≥50%, by 22C3 pharmDx assay). The patient entered a clinical trial for non-small cell lung cancer with interstitial pneumonia, and received chemotherapy consisting of carboplatin plus nab-paclitaxel as first-line treatment. This chemotherapy was terminated due to anemia and thrombocytopenia after 3 cycles. Six months later, he received nab-paclitaxel alone as second-line treatment because of disease progression. Best response of the second-line nab-paclitaxel was stable disease. One year later, atezolizumab as third-line treatment was started. Although the best response of the third-line atezolizumab was stable disease, long-term tumor shrinkage for about one year was observed. Interstitial pneumonia was regularly followed up with HRCT, but no worsening of interstitial pneumonia was observed. Eighteen months after the start of atezolizumab, the patient was treated with carboplatin, nab-paclitaxel, and pembrolizumab as fourth-line therapy. Nab-paclitaxel alone as second-line treatment was rechallenged with the expect of same tumor shrinkage as the first-line treatment and lower toxicity than combination therapy with carboplatin. Four courses of nab-paclitaxel alone caused the best response of stable disease and long-term duration with tolerable toxicities. Therefore, we determined carboplatin and nab-paclitaxel with pembrolizumab as fourth-line treatment, adjusting the amount of carboplatin and nab-paclitaxel to reduce adverse events. Based on the good results of previous treatments, a combination of chemotherapy (carboplatin plus nab-paclitaxel) and immune checkpoint inhibitor (pembrolizumab) was performed with expectation of further improving response and progression-free survival. On day 8, he had a marked elevation in liver enzyme levels and was urgently hospitalized. His consciousness was clear without jaundice. Laboratory examinations revealed the following: aspartate aminotransferase (AST) 3803 U/L, alanine transaminase (ALT) 2385 U/L, alkaline phosphatase (ALP) 652 U/L, gamma-glutamyl transpeptidase (γ-GTP) 242 U/L, total bilirubin 0.6 mg/mL, and international normalized ratio of prothrombin time (PT-INR) 1.56. These values were within the normal range before fourth-line therapy. Infectious etiologies (including hepatitis B, hepatitis C, cytomegalovirus, Epstein-Barr virus, Herpes simplex virus, Varicella Zoster virus) and autoantibody screening (including antinuclear, antimitochondrial) were negative. He had not consumed alcohol and there were no recent medication changes except for pembrolizumab, carboplatin, and nab-paclitaxel. Chest X-ray showed a mass shadow in the left upper lung field (Figure 1A). Enhanced abdominal computed tomography (CT) showed periportal collar signs and a heterogeneous contrast effect on the liver in the early contrast phase (Figure 1B and C). These findings were consistent with acute drug-induced hepatitis caused by the combination of pembrolizumab, carboplatin, and nab-paclitaxel. A liver biopsy was immediately performed on the day of admission to confirm the pathological features of hepatitis. The chemotherapy was discontinued and intravenous prednisolone 40 mg daily (0.6 mg/kg) was initiated. Histopathology of the liver revealed focal necrosis in the liver parenchyma and lymphocytic infiltration around the central vein. Immunohistopathological staining showed that the number of CD3 or CD8 positive lymphocytes was markedly higher than that of CD20 positive lymphocytes (Figure 2). These pathological findings suggested hepatocellular liver injury, and we diagnosed him with grade 4 hepatotoxicity according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0). Based on these results, steroids were planned to be tapered and terminated as soon as possible. After starting steroids, the patient improved to AST 34 U/L and ALT 224 on the tenth day. The amount of steroids was gradually tapered to 30, 20, 10, 5, and 0 mg daily every 7–10 days without any recurrence of liver injury (Figure 3). The best response of fourth-line combination immunotherapy was stable disease. Four months after steroid treatment, his lung cancer showed no growth. There was no sudden increase in KL-6 or SP-D and HRCT showed no worsening of interstitial pneumonia before or after the fourth-line treatment. No irAEs other than liver injury were observed during the treatment of hepatotoxicity.
 |
Figure 3 Clinical course of the patient. Decreases in AST and ALT were observed after the administration of steroids. |
Discussion and Conclusions
We experienced a case of grade 4 liver injury caused by pembrolizumab plus chemotherapy as fourth-line treatment for squamous-cell lung carcinoma, which was diagnosed by liver biopsy and improved with the short-term administration of steroids.
In the KEYNOTE-407 Clinical Trials, hepatitis was observed in 5 of 278 patients (1.8%), and grade 3 or higher was observed in 5 patients.6 For a diagnosis of drug-induced hepatitis including irAEs, it is necessary to exclude viral, other autoimmune diseases, and other drug-induced causes.7
Drug-induced hepatitis including irAEs is often clinically diagnosed without liver biopsy, and the histological features are not well known.8 Liver biopsy is not often performed but it is important to allow the exclusion of other causes of liver diseases, confirming the diagnosis, and evaluating the histologic severity.9 Drug-induced liver injury can be classified as a hepatocellular type or cholestatic type injury.10 Hepatotoxicity caused by PD-1 inhibitors was reported in hepatocellular and cholestatic type injury.11–13 There is often a higher infiltration of CD3 or CD8 positive cells and a significantly lower infiltration of CD20 and CD4 positive cells than in autoimmune hepatocellular hepatitis or other drug-induced liver disorders.11 Cholestatic liver injury is more resistant to immunosuppressive therapy containing corticosteroids than hepatocellular injury and a prolonged clinical course has been reported.12,13 We judged this patient to have a hepatocellular type injury because of the presence of lobular hepatitis, which was mainly due to the infiltration of CD3 and CD8 positive lymphocytes. The high infiltration of CD3 and CD8 positive cells was consistent with the characteristics of hepatic irAEs. However, to the best of our knowledge, no case reports of hepatotoxicity caused by pembrolizumab, carboplatin, and nab-paclitaxel have been reported in PubMed, and the pathological features of the combination therapy are unknown. Hepatotoxicity due to chemotherapy (carboplatin and nab-paclitaxel) is considered to be rare and mild. We determined severe liver dysfunction in this case was unlikely to be chemotherapy-induced hepatotoxicity based on the clinical course and pathological findings of the low numbers of CD20 positive lymphocytes infiltration. It was not considered to be viral hepatitis or alcoholic liver disease because infectious etiologies and autoantibody screening were negative and he had not consumed alcohol. Furthermore, from the pathological findings, autoimmune hepatitis and acute ischemic hepatitis were unlikely. Therefore, this case could be considered as hepatotoxicity caused by pembrolizumab alone or hepatotoxicity caused by the combination of pembrolizumab, carboplatin, and nab-paclitaxel.
Clinical guidelines recommend the discontinuation of immunotherapy and administration of corticosteroids (1–2 mg/kg) for high-grade liver injury.14,15 However, this patient was administered a lower dose of steroids than that recommended and showed an improvement in liver damage. De Martin et al reported that six patients with grade 3 or higher hepatotoxicity who did not meet the severity criteria of elevated bilirubin, coagulation disorders, and histological findings were followed without corticosteroids and all showed an improvement in liver enzymes.7 In another report on the characteristics of drug-induced liver injury caused by immune checkpoint inhibitors, several grade 3–4 cases were mildly relieved without steroid treatment, or even improved with prednisolone less than 1.0 mg/kg (0.6 mg/kg).16 This suggests it may be possible to avoid corticosteroid use or to treat patients with corticosteroids at doses lower than those recommended in the guidelines for hepatocellular injury, even with high-grade AEs. Based on these reports, we diagnosed this patient with hepatocellular liver injury by liver biopsy, and were able to reduce the steroid dose at an early stage.
In summary, we experienced a case of severe hepatocellular liver injury induced by pembrolizumab, carboplatin, and nab-paclitaxel in a patient with advanced squamous-cell lung carcinoma that was successfully treated with short-term steroids at a dose lower than that recommended on the basis of our pathological findings. This case suggests that the accurate identification of liver injury type by biopsy is important for the decisions of initial dose and the successful early discontinuation of steroid therapy.
Ethical Approval
Institutional approval was not required to publish the case details.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report including the patient’s details and accompanying images.
Disclosure
Prof. Dr. Kaoru Kubota reports personal fees from Chugai, personal fees from Taiho, personal fees from MSD, grants, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Bristol Myers Squibb, personal fees from Eli-Lilly, personal fees from Daiichi Sankyo, grants, personal fees from Ono, personal fees from Kirin, personal fees from Eisai, personal fees from Shionogi, personal fees from Nippon Kayaku, outside the submitted work. Dr Masahiro Seike reports grants, personal fees from MSD K.K, grants, personal fees from Taiho Pharmaceutical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(373):1627–1639. doi:10.1056/NEJMoa1507643
2. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(375):1823–1833. doi:10.1056/NEJMoa1606774
3. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X
4. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(377):1919–1929. doi:10.1056/NEJMoa1709937
5. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa1801005
6. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi:10.1056/NEJMoa1810865
7. De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. doi:10.1016/j.jhep.2018.01.033
8. Nadeau B, Fecher L, Owens S, et al. Liver toxicity with cancer checkpoint inhibitor therapy. Semin Liver Dis. 2018;38:366–378. doi:10.1055/s-0038-1667358
9. Peeraphatdit T, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72(1):315–329. doi:10.1002/hep.31227
10. Mosedale M, Watkins PB. Drug-induced liver injury: advances in mechanistic understanding that will inform risk management. Clin Pharmacol Ther. 2017;101(4):469–480. doi:10.1002/cpt.564
11. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31(6):965–973. doi:10.1038/s41379-018-0013-y
12. Doherty GJ, Duckworth AM, Davies SE, et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open. 2017;2(4):e000268. doi:10.1136/esmoopen-2017-000268
13. Kurokawa K, Hara M, Iwakami S, et al. Cholestatic injury induced by pembrolizumab in a patient with lung adenocarcinoma. Intern Med. 2019;58:3283–3287. doi:10.2169/internalmedicine.2591-18
14. Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:119–142. doi:10.1093/annonc/mdx225
15. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(36):1714–1768. doi:10.1200/JCO.2017.77.6385
16. Imoto K, Kohjima M, Hioki T, et al. Clinical features of liver injury induced by immune checkpoint inhibitors in Japanese patients. Can J Gastroenterol Hepatol. 2019;2019:6391712. doi:10.1155/2019/6391712
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.