Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Successful Treatment of Refractory Synovitis, Acne, Pustulosis, Hyperostosis, and Osteitis (SAPHO) Syndrome with Baricitinib, a Janus Kinase Inhibitor
Authors Yang J, Yuan C, Zhou S , Teng Z, Li M
Received 25 October 2023
Accepted for publication 6 February 2024
Published 4 March 2024 Volume 2024:17 Pages 529—537
DOI https://doi.org/10.2147/CCID.S446468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jianqiu Yang,1,2 Chunyu Yuan,1 Shengru Zhou,1 Zhicheng Teng,2 Min Li1
1Department of Dermatology, Dushu Lake Hospital Affiliated to Soochow University (Medical Center of Soochow University), Suzhou, People’s Republic of China; 2Suzhou Medical College of Soochow University, Suzhou, People’s Republic of China
Correspondence: Min Li, Department of Dermatology, Dushu Lake Hospital Affiliated to Soochow University (Medical Center of Soochow University), No. 9, Chongwen Road, Suzhou, 215100, People’s Republic of China, Tel +86-18936140383, Email [email protected]
Abstract: Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome, a rare immune-mediated inflammatory disease, poses diagnostic and therapeutic challenges owing to its multi-system involvement, high heterogeneity, and lack of specific laboratory tests. Additionally, lacking evidence-based treatment recommendations, with the primary approach focusing on symptomatic relief. Herein, we report the case of a 32-year-old Chinese woman who presented with recurrent, generalized multiple osteoarticular pain lasting over one year and skin erythema pustulosis for 11 months. Traditional treatments, including non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying antirheumatic drugs (DMARDs), and other traditional approaches, yielded no significant effects. Despite the prior use of adalimumab and acitretin capsules, the treatment remained unsatisfying, especially regarding the skin lesions. Considering the complex pathogenesis of SAPHO syndrome, the patient was orally administered baricitinib (2 mg), a Janus kinase (JAK) inhibitor, twice daily. A notable improvement in both skin lesions and osteoarticular pain was observed within two weeks of treatment initiation. Subsequently, the dosage of baricitinib was halved and continued for an additional three months, during which regular follow-ups revealed neither disease recurrence nor adverse effects. Collectively, the successful treatment of refractory SAPHO syndrome with baricitinib presents a promising implication for addressing the therapeutic challenges of this rare autoimmune condition, offering a potential breakthrough in managing its complex manifestations.
Keywords: SAPHO syndrome, autoinflammatory conditions, therapeutic challenges, baricitinib
Introduction
Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome is a rare autoimmune disease and can be considered as an autoinflammatory condition.1 This syndrome is primarily characterized by chronic aseptic inflammation affecting the skin, bones, and joints, presenting clinical rarity with no precise data on its prevalence, especially among different ethnic groups.2 Although previous studies explored the genetic susceptibility, infection, and immune dysfunction associated with SAPHO, the exact etiology and pathogenesis of this disease remain incompletely elucidated.1,3–5 Whole exome sequencing(WES) and Variant Enrichment Analysis(VEA) have been used to investigate the complex pathogenic landscape of SAPHO syndrome and other related autoinflammatory syndromes. WES was used to identify polygenic variants in patients with overlapping pyoderma gangrenosum, acne, and hidradenitis suppurativa (PASH)/SAPHO, and VEA was used to explore molecular mechanisms and etiopathogenic pathways involved in its onset. This approach revealed impaired macroautophagy pathways in patients with PASH/SAPHO. Autophagy deficiency in endothelial cells (EC) results in unregulated leukocyte transendothelial migration, increasing neutrophil infiltration and tissue damage. Moreover, enriched exclusive pathways (eEP) are associated with extracellular matrix organization in SAPHO patients.6,7 Due to the involvement of multiple systems, high heterogeneity, and a lack of specificity in laboratory tests, diagnosing and treating this disease pose significant challenges. Currently, standardized treatment guidelines for SAPHO syndrome are lacking, and the primary therapeutic strategy involves symptomatic relief to alleviate pain, control inflammation, and enhance the overall quality of life of patients.8,9 In recent years, the therapeutic strategies consisting of a combination of biologics and small molecules have brought new promising options for patients.
In this study, we present a case of SAPHO syndrome unresponsive to conventional therapy that was effectively treated with an oral Janus kinase (JAK) inhibitor, baricitinib.
Case Report
A 32-year-old woman presented with sternal pain one year ago, followed by pain in the left articulatio humeri, spine, and right ankle with limited activity. A month later, erythema and pustules appeared on the surfaces of the soles, and the lesions gradually expanded. Diffuse psoriasis-like lesions appeared on the trunk and limbs, accompanied by rice grain-sized pustules on the anterior chest, causing mild itching. Imaging examinations, including computed tomography (CT) and magnetic resonance imaging (MRI), were conducted at the local hospital to establish a definite diagnosis. All tests revealed an abnormal signal in the sternum. Positron emission tomography (PET)/CT indicated bone destruction, osteogenic changes, and bone marrow edema. Partial sternotomy and bone histopathology were subsequently performed, which revealed a large number of neutrophil-dominated inflammatory infiltrates and fibrous tissue hyperplasia. After treatment with non-steroidal anti-inflammatory drugs (NSAIDs), high-dose corticosteroids, and disease-modifying antirheumatic drugs (DMARDs), the osteoarticular pain improved slightly; however, the treatments were insufficient to control the disease. Notably, the skin lesions exhibited no observable improvement.
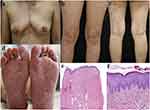
The patient was admitted to our hospital for diagnostic and therapeutic purposes. Physical examination revealed swelling, tenderness, and limited range of motion in the sternum, left articulatio humeri, and right ankle; reduced lumbar motion; tenderness between the 4–5 lumbar vertebrae and sacral vertebrae (+); and percussion pain (+). The visual analog scale (VAS) indicated a severity score of 9. Dermatology examination revealed erythema and scales of varying sizes scattered on the trunk and limbs, multiple red papules accompanied by pustules ranging from needle tip-to-rice grain size visible on the anterior chest. Both soles exhibited diffuse erythema with lamellar peeling and with needle tip-to-rice grain-sized pustules (Figure 1a-d). Laboratory results revealed a C-reactive protein (CRP) level of 57.82 mg/L (normal range: 0–10 mg/L) and an erythrocyte sedimentation rate (ESR) level of 39 mm/h (normal range: < 20 mm/h). The serum leukocyte count was within the normal range. Tests for serum autoantibody, antinuclear antibody (ANA), rheumatoid factor (RF), human leukocyte antigen B27 (HLA-B27), CMV-DNA, EBV-DNA, HBV-DNA, tuberculosis infection T cell, HIV antibody, streptococcus pneumoniae antigen, and cytokine detection [including interleukin (IL)-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, tumor necrosis factor (TNF)-α, interferon (IFN), IL-17], and microorganism cultivation from the pustular secretion of the soles, were all negative. Skin biopsy pathology of the sole revealed mild hyperkeratosis accompanied by parakeratosis and infiltration of neutrophils and lymphocytes in the superficial and intermediate dermis (Figure 1e and f).
The patient initially presented with pustules on the soles and psoriasis-like lesions on her trunk and limbs. No signs of acne were observed. The patient experienced recurrent pain and swelling in the affected osteoarticular areas, including the sternum, spine, and limbs, which was consistent with the diagnostic criteria for SAPHO syndrome, as proposed by Kahn and Khan.10 Considering the extensive skin lesions, osteoarthrosis, and inadequate response to previous medical treatment, adalimumab was subcutaneously injected at 40 mg once weekly. After five weeks, her joint symptoms improved. The skin lesions showed no improvement. The patient voluntarily requested discontinuation of adalimumab therapy. Subsequently, acitretin capsules (10 mg twice daily) were introduced; however, the skin lesions still did not improve significantly after four weeks, leading to the discontinuation of acitretin. Ultimately, we recommended oral JAK inhibitors; baricitinib (2 mg twice daily) was prescribed. Two weeks after initiation, the skin lesions disappeared across the entire body, and the symptoms of osteoarticular pain were significantly relieved. Laboratory review indices returned to normal, with the VAS of pain decreasing from 9 to 2. Following discharge, the dose of baricitinib was halved and continued for three months, with no recurrence or adverse effects observed during the follow-up period (Figure 2a-d).
 |
Figure 2 SAPHO syndrome patient with after-treatment skin lesions. (a-d) Erythema, papules, and pustules gradually subsided, leaving slight pigmentation. |
Discussion
SAPHO syndrome is a heterogeneous disease characterized by chronic inflammation affecting osteoarticular and cutaneous manifestations, presenting with a broad spectrum of clinical features and a highly diverse combination. The disease can manifest at all ages, with the most common occurrence between 30–50 years, showing a slightly higher prevalence in women than in men.11 The main skin lesions consist of multiple sterile pustulosis in the palmoplantar region, accompanied by keratosis and scaling. Severe pustulosis may extend to the chest, back, or even the entire body. Subsequently, acne and psoriasis-like lesions may develop.12 Hidradenitis suppurativa is also considered a dermatological manifestation of SAPHO syndrome; however, data from the literature remain controversial.13 Women are reportedly more likely than men to exhibit pustulosis, whereas men are more prone to develop acne than women, with varying degrees of skin damage.14 The location of osteoarticular damage is usually related to age; in adults, it primarily occurs in the anterior chest wall, especially at the sternal end of the sternoclavicular joint and the front end of the ribs of the upper chest wall. It then extends to the spine, sacroiliac joint, and limb joints, showcasing chronic inflammatory changes such as bone destruction, hyperostosis, sclerosis, and osteogenic changes. The later the age of onset, the more likely the initial symptom of osteoarthrosis is to manifest. Laboratory tests lack specificity, with approximately 50% of patients exhibiting elevated ESR and CRP levels, and 20% of patients exhibiting elevated cytokine levels during the active stage of the disease.15,16 Imaging examination can help identify the lesion site, osteolysis, osteitis, hyperplasia, osteosclerosis, and bone marrow edema.17 In this patient, the age of onset, skin and osteoarticular damage, and laboratory tests were consistent with the characteristics of the disease.
NSAIDs, intra-articular injection, or systemic use of corticosteroids, DMARDs, and bisphosphonates continue to serve as the main methods of traditional treatment.18,19 However, NSAIDs, often prove insufficient in controlling the widespread involvement observed in patients with SAPHO when used as an initial treatment strategy.11 Corticosteroids, generally requiring large doses for effectiveness, are not recommended for long-term use owing to potential side effects. The efficacy of conventional DMARDs and bisphosphonates in treating skin lesions remains unsatisfactory.20,21 In our case, the disease did not control well after undergoing various conventional treatments, especially the skin lesions. In recent years, biologics (including TNF-α antagonists such as adalimumab, infliximab, and etanercept; IL-1, IL-17, IL-12/IL-23 receptor antagonists, such as anakinra, secukinumab, ustekinumab) and small-molecule targeted drugs have garnered interest as an alternative approach when traditional drug therapy exhibits partial effectiveness or is ineffective. These options provide more diverse programs for disease management.22 The combination of biologics and conventional treatments as valid therapeutic option.23 In a systematic review of cases from 2019, the response rates of TNF-α inhibitors for osteoarthritis and skin lesions were 93.3% and 72.4%, respectively, demonstrating positive therapeutic effects.24 Furthermore, the TNF-α antagonist adalimumab is the most widely used; therefore, we initially considered the use of adalimumab. However, while biologics have proven effective in treating some patients, they have also demonstrated ineffectiveness in others and may even cause new rashes or aggravate existing ones. Additionally, adverse reactions may occur, potentially leading to treatment discontinuation.24,25 This patient was treated with adalimumab for five weeks, which improved the bone and joint pain; however, the rash did not subside, and the patient refused to continue adalimumab treatment. Subsequently, the patient was treated with acretin capsules to alleviate pustules and psoriasis-like lesions; however, the skin lesions did not respond well after four weeks of treatment. Additionally, there have been reports suggesting that retinoids could act as a trigger for osteoarthritis, leading to its discontinuation.26 This indicates the refractory of this patient.
The use of the phosphodiesterase-4 inhibitor apremilast and the JAK inhibitors opens a new era for pan-cytokines and cells.27 JAK inhibitors are key targets in signaling pathways for inflammatory diseases, effectively blocking the upstream components of various cytokines and their pathway effects. These inhibitors exhibit more significant anti-inflammatory activity compared with that of biologics that target a single cytokine.28 Simultaneously, they contribute to regulating the expression of inflammatory factors, proving beneficial for autoimmune diseases. Additionally, baricitinib inhibits osteoclast-mediated structural damage in arthritis by blocking the receptor activator for the nuclear factor kB ligand (RANKL) pathway. Based on these findings, we decided to administer baricitinib, a JAK inhibitor. Currently, there are over 10 reported cases worldwide of SAPHO syndrome treated with JAK inhibitors (Table 1). In these patients, the time required to improve osteoarticular pain symptoms and cutaneous damage is typically around three months, whereas the duration needed for bone marrow edema to subside was longer. Among these reports, tofacitinib was more frequently used, with only two cases involving baricitinib. In one case series with five patients, four exhibited varying degrees of remission in clinical symptoms and laboratory indices after 12 weeks of treatment, whereas one patient showed no significant improvement.29–32 Our patient experienced a significant and rapid response after two weeks of oral administration of baricitinib, a selective JAK inhibitor targeting JAK1 and JAK2. Common adverse reactions, including infection, anemia, platelet elevation, leukopenia, and cholesterol elevation, were considered during the course of use.30 The relevant indicators were closely monitored, and regular follow-up was conducted. No disease recurrence, abnormalities in serological indicators, or adverse effects were observed. This suggests that baricitinib may be a beneficial treatment for patients with refractory SAPHO syndrome; however, individual differences exist in treatment, and our findings from a single case cannot be extrapolated to the entire patient population. Thereby, further investigations on a larger scale are warranted to validate and generalize these findings. Our findings hold the potential for reshaping the paradigm for treating similar inflammatory diseases with JAK inhibitors. Additionally, the patient had a short follow-up period and would be reevaluated at six months and one year, highlighting the need for validating the optimal treatment duration and indications for drug withdrawal.
 |
Table 1 A Summary of Cases with SAPHO Syndrome That Were Treated Using JAK Inhibitor |
Conclusion
Our findings suggest that JAK inhibitors hold great breakthrough promise as a therapeutic approach for SAPHO syndrome, particularly for treating inflammatory diseases, and exhibit favorable efficacy with minimal side effects. When conventional drugs or biologics are not well tolerated or fail to produce adequate results, JAK inhibitors offer a viable alternative. These insights not only open avenues for personalized treatment but also prompt a reevaluation of the conventional therapeutic landscape for this challenging condition.
Ethics statement
The Ethics Committee at Dushu Lake Hospital Affiliated with Soochow University approved the study. This study complied with the Declaration of Helsinki. The participant provided her written informed consent to participate in this study and was allowed to publish images and clinical details.
Consent for publication
The authors certify that they have obtained the consent of the patient for publication.
Acknowledgments
We are thankful to the patient who participated in this study.
Disclosure
The authors declare no potential conflicts of interest in this work.
References
1. Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39(2):401–418. doi:10.1016/j.rdc.2013.02.009
2. Liu S, Tang M, Cao Y, et al.. Synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: review and update. Ther adv Musculoskelet Dis. 2020:12. DOI:10.1177/1759720X20912865
3. Cai R, Dong Y, Fang M, et al. Genome-Wide Association Identifies Risk Pathways for SAPHO Syndrome. Front Cell Dev Biol. 2021;9:643644. doi:10.3389/fcell.2021.643644
4. Hurtado-Nedelec M, Chollet-Martin S, Nicaise-Roland P, et al. Characterization of the immune response in the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Rheumatology. 2008;47(8):1160–1167. doi:10.1093/rheumatology/ken185
5. Berthelot JM, Corvec S, Hayem G. SAPHO, autophagy, IL-1, FoxO1, and Propionibacterium (Cutibacterium) acnes. Joint Bone Spine. 2018;85(2):171–176. doi:10.1016/j.jbspin.2017.04.010
6. Marzano AV, Genovese G, Moltrasio C, et al. Whole-Exome Sequencing in 10 Unrelated Patients with Syndromic Hidradenitis Suppurativa: a Preliminary Step for a Genotype-Phenotype Correlation. Dermatology. 2022;238(5):860–869. doi:10.1159/000521263
7. Brandão LAC, Moura RR, Marzano AV, et al. Variant Enrichment Analysis to Explore Pathways Functionality in Complex Autoinflammatory Skin Disorders through Whole Exome Sequencing Analysis. IntJ Mol Sci. 2022;23(4):2278. doi:10.3390/ijms23042278
8. Wang L, Sun B, Li C. Clinical and Radiological Remission of Osteoarticular andCutaneous Lesions in SAPHO Patients Treated With Secukinumab: a Case Series. J Rheumatol. 2021;48(6):953–955. doi:10.3899/jrheum.201260
9. Cianci F, Zoli A, Gremese E, et al. Clinical heterogeneity of SAPHO syndrome: challenging diagnose and treatment. Clin Rheumatol. 2017;36(9):2151–2158. doi:10.1007/s10067-017-3751-1
10. Kahn MF, Khan MA. The SAPHO syndrome. Baillieres Clin Rheumatol. 1994;8(2):333–362. doi:10.1016/s0950-3579(94)80022-7
11. Hussain A, Gondal M, Abdallah N, et al. Synovitis, Acne, Pustulosis, Hyperostosis, Osteitis (SAPHO): an Interesting Clinical Syndrome. Cureus. 2020;12(9):e10184. doi:10.7759/cureus.10184
12. Furer V, Kishimoto M, Tsuji S, et al. The Diagnosis and Treatment of Adult Patients with SAPHO Syndrome: controversies Revealed in a Multidisciplinary International Survey of Physicians. Rheumatol Ther. 2020;7(4):883–891. doi:10.1007/s40744-020-00235-2
13. Li C, Xu H, Wang B. Is SAPHO Syndrome Linked to PASH Syndrome and Hidradenitis Suppurativa by Nicastrin Mutation? A Case Report. J Rheumatol. 2018;45(11):1605–1607. doi:10.3899/jrheum.171007
14. Przepiera-Będzak H, Brzosko M. SAPHO syndrome: pathogenesis, clinical presentation, imaging, comorbidities and treatment: a review. Postepy Dermatol Alergol. 2021;38(6):937–942. doi:10.5114/ada.2020.97394
15. Colina M, Govoni M, Orzincolo C, et al. Clinical and radiologic evolution of synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: a single center study of a cohort of 71 subjects. Arthritis Rheum. 2009;61(6):813–821. doi:10.1002/art.24540
16. Li C, Xiang Y, Wu X, et al. Serum IgG4 elevation in SAPHO syndrome: does it unmask a disease activity marker?. Clin Exp Rheumatol. 2020;38(1):35–41.
17. Jinag CZ, Zhao YJ, Li XH, et al. Whole-body Bone Scan in the Diagnosis and Treatment of SAPHO Syndrome. J Coll Physicians Surg Pak. 2022;32(4):S64–S66. doi:10.29271/jcpsp.2022.Supp1.S64
18. Nguyen MT, Borchers A, Selmi C, et al. The SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):254–265. doi:10.1016/j.semarthrit.2012.05.006
19. Demirci YT, Sari İ. SAPHO syndrome: current clinical, diagnostic and treatment approaches. Rheumatol Int. 2023. doi:10.1007/s00296-023-05491-3
20. Huang H, Zhang Z, Zhao J, et al. The effectiveness of treatments for patients with SAPHO syndrome: a follow-up study of 24 cases from a single center and review of literature. Clin Rheumato l. 2021;40(3):1131–1139. doi:10.1007/s10067-020-05322-x
21. Colina M, La Corte R, Trotta F. Sustained remission of SAPHO syndrome with pamidronate: a follow-up of fourteen cases and a review of the literature. Clin Exp Rheumato l. 2009;27(1):112–115.
22. Cheng W, Li F, Tian J, et al. New Insights in the Treatment of SAPHO Syndrome and Medication Recommendations. J Inflamm Res. 2022;15:2365–2380. doi:10.2147/JIR.S353539
23. Genovese G, Caorsi R, Moltrasio C, et al. Successful treatment of co-existent SAPHO syndrome and hidradenitis suppurativa with Adalimumab and methotrexate. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):40–41. doi:10.1111/jdv.15849
24. Daoussis D, Konstantopoulou G, Kraniotis P, et al. Biologics in SAPHO syndrome: a systematic review. Semin Arthritis Rheum. 2019;48(4):618–625. doi:10.1016/j.semarthrit.2018.04.003
25. Firinu D. A promising new treatment for SAPHO syndrome that deserves further studies. Br J Dermatol. 2018;179(4):823. doi:10.1111/bjd.16771
26. Luzzati M, Simonini G, Filippeschi C, et al. SAPHO syndrome: the supposed trigger by isotretinoin, the efficacy of Adalimumab and the specter of depressive disorder: a case report. Ital J Pediatr. 2020;46(1):169. doi:10.1186/s13052-020-00933-1
27. Adamo S, Nilsson J, Krebs A, et al. Successful treatment of SAPHO syndrome with apremilast. Br J Dermatol. 2018;179(4):959–962. doi:10.1111/bjd.16071
28. Yue Q, Ma M, Liu S, et al. JAKi: can it be used to treat SAPHO syndrome?. Int J Rheum Dis. 2023;27(1). doi:10.1111/1756-185X.14930
29. Yang Q, Zhao Y, Li C, et al. Case report: successful treatment of refractory SAPHO syndrome with the JAK inhibitor tofacitinib. Medicine. 2018;97(25):e11149. doi:10.1097/MD.0000000000011149
30. Li B, Li GW, Xue L, et al. Rapid remission of refractory synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome in response to the Janus kinase inhibitor tofacitinib: a case report. World J Clin Cases. 2020;8(19):4527–4534.10.12998/wjcc.v8.i19.4527. doi:10.12998/wjcc.v8.i19.4527
31. Li C, Li Z, Cao Y, et al. Tofacitinib for the Treatment of Nail Lesions and Palmoplantar Pustulosis in Synovitis, Acne, Pustulosis, Hyperostosis, and Osteitis Syndrome. JAMA Dermatol. 2021;157(1):74–78. doi:10.1001/jamadermatol.2020.3095
32. Wuriliga WX, Cao Y, Cao Y, et al. Successful treatment of Hip involvement in SAPHO syndrome with baricitinib. Rheumatol Adv Pract. 2022;6(3):rkac084. doi:10.1093/rap/rkac084
33. Liu S, Li C, Tang MW, et al. Improvement of lymphangioleiomyomatosis followingSuccessful tofacitinib treatment for refractory synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome. Chin Med J. 2019;132(19):2378–2379.10.1097/CM9.0000000000000441.
34. Li Y, Huo J, Cao Y, et al. Efficacy of tofacitinib in synovitis, acne, pustulosis, hyperostosis and osteitis syndrome: a pilot study with clinical and MRI evaluation. Ann Rheum Dis. 2020;79(9):1255–1257. doi:10.1136/annrheumdis-2020-217250
35. Yuan F, Luo J, Yang Q. SAPHO Syndrome Complicated by Ankylosing Spondylitis Successfully Treated With Tofacitinib: a Case Report. Front Immunol. 2022;13:911922. doi:10.3389/fimmu.2022.911922
36. Baisya R, Gavali M, Tyagi M, et al. A Case of SAPHO Syndrome Complicated by Uveitis with Good Response to Both TNF Inhibitor and JAKinib. Case Rep Rheumatol. 2023;2023:6201887. doi:10.1155/2023/6201887
37. Ma M, Lu S, Hou X, et al.. Novel JAK-1 inhibitor upadacitinib as a possible treatment for refractory SAPHO syndrome: a case report. Int J Rheum Dis. 2023:10.1111/1756–185X.14774. DOI:10.1111/1756-185X.14774
38. Liu S, Yu Y, Liu Y, et al. Efficacy of baricitinib in synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: a case series. Joint Bone Spine. 2023;90(5):105587. doi:10.1016/j.jbspin.2023.105587
39. Ru C, Qian T, Liu X, et al. SAPHO syndrome with Takayasu arteritis successfully treated with tofacitinib. Int J Rheum Dis. 2023;26(7):1381–1383. doi:10.1111/1756-185X.14628
40. Cao F, Hou X, Kang T, et al. SAPHO syndrome complicated with relapsing polychondritis: a case report. Int J Rheum Dis. 2023;26(10):2060–2063. doi:10.1111/1756-185X.14693
41. Wang R, Li Y, Liu Y, et al. Synovitis, Acne, Pustulosis, Hyperostosis and Osteitis (SAPHO) Syndrome with Henoch-Schönlein Purpura: a Case Report. Clin Cosmet Invest Dermatol. 2023;16:1089–1094. doi:10.2147/CCID.S392909
42. Liu Y, Wu X, Cao Y, et al. Synovitis, acne, pustulosis, hyperostosis and osteitis syndrome with mandibular involvement: would surgical operation help?. Int J Rheum Dis. 2023;26(3):563–567. doi:10.1111/1756-185X.14542185X.14628
43. Luan L, Lv C. Secukinumab-induced paradoxical skin lesions, but successful treatment with tofacitinib in SAPHO syndrome: a case report. J DermatolTreat. 2023;1–16.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Relapse of Low Back Pain After Internal Lumbar Fixation Was Diagnosed with SAPHO Syndrome: A Case Report
Zhang Y, Luo Y, Ruan Y, Qian X, Feng Z
International Medical Case Reports Journal 2023, 16:591-598
Published Date: 25 September 2023