Back to Journals » International Medical Case Reports Journal » Volume 16
Relapse of Low Back Pain After Internal Lumbar Fixation Was Diagnosed with SAPHO Syndrome: A Case Report
Authors Zhang Y, Luo Y , Ruan Y, Qian X, Feng Z
Received 19 January 2023
Accepted for publication 27 April 2023
Published 25 September 2023 Volume 2023:16 Pages 591—598
DOI https://doi.org/10.2147/IMCRJ.S402752
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Yanfeng Zhang,1 Yujia Luo,1 Yachao Ruan,2 Xiang Qian,3 Zhiying Feng1
1Department of Pain Medicine, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, 310003, People’s Republic of China; 2Department of Radiology, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, 310003, People’s Republic of China; 3Stanford University School of Medicine Pain Management Center, Redwood City, CA, 94063, USA
Correspondence: Zhiying Feng, Email [email protected]
Introduction: Early diagnosis of SAPHO syndrome is easily confused with other common spine-related diseases and infections. There is currently no consensus regarding the diagnosis of SAPHO syndrome, and specific treatments are empirical because of its rarity.
Case Presentation: A 62-year-old woman was referred to our department with complaints of low back and lower extremity pain for 2 years, 1.5 years after lumbar spine surgery, and recurrent low back pain for 1 year. Laboratory test results revealed elevated hs-CRP levels and erythrocyte sedimentation rate. Combined with her surgical history and lumbar CT results, adjacent segment degeneration (ASD) was first considered. NSAIDs, analgesics, and supplemental therapies were also administered. However, the patient’s symptoms were not significantly relieved. During re-examination, hyperkeratosis with active pustulosis was observed on the patient’s palms. Osteitis of the left sacroiliac joint was revealed on imaging. Skeletal ECT revealed a typical “horn sign”. The patient was diagnosed with SAPHO syndrome. Based on the original treatment, sulfasalazine enteric-coated tablets, adalimumab (a biological agent of TNF-α), pregabalin, and tramadol sustained-release tablets were administered. The patient reported that her pain was significantly relieved. He was discharged from the hospital and received adalimumab treatment (40 mg once per fortnight in the first 6 months and 40 mg once per month after month 6) in the outpatient clinic. The hyperkeratosis with active pustulosis on both palms fully recovered after 12 months of treatment. The patient was followed up 6 months after full recovery, and no recurrence was found in the symptoms of low back and lower extremity pain and palmar hyperkeratosis with active pustulosis.
Conclusion: SAPHO syndrome should be suspected in patients present with osteoarticular and/or dermatological manifestations. Biological agents can be used to treat patients with refractory SAPHO syndrome.
Keywords: SAPHO syndrome, low back pain, osteitis, pustulosis, adalimumab
Introduction
SAPHO is a specific type of syndrome encompassing a variety of inflammatory osteoarticular disorders with osteoarticular manifestations and dermatoses characterized by neutrophilic pseudo-abscesses.1,2 It is a rare clinical condition first described by Chamot et al in 1987.2 The “SAPHO” acronym was proposed as the alphabet of the five letters: synovitis, acne, pustulosis, hyperostosis, and osteomyelitis.1,2 The ‘S’ was originally proposed as “syndrome” but was changed to ‘synovitis’ in the following year.1,2 The diagnosis of SAPHO syndrome is based on medical history, characteristic imaging findings, and skin manifestations, whereas infective osteomyelitis and bone tumors need to be ruled out.3 Bone hyperplasia and osteitis are the two most common osteoarticular manifestations. Skin lesions are a key diagnostic clue for SAPHO syndrome, but they may occur several years earlier or later.1,2 In addition, the diagnosis of SAPHO syndrome may be further challenging when typical skin lesions are absent or atypical area sites are involved. Early recognition, diagnosis, and timely treatment of SAPHO syndrome can quickly relieve pain in most patients and prevent unnecessary invasive procedures and long-term antibiotic use. Here, we reported a case of SAPHO syndrome not sensitive to conventional nonsteroidal anti-inflammatory drugs (NSAIDs) treatment.
Case Presentation
A 62-year-old woman was referred to our department with complaints of low back and lower extremity pain for 2 years, 1.5 years after lumbar spine surgery, and recurrent low back pain for 1 year. The pain was dull and soreness, located in the lower back, accompanied by difficulty in turning over and radiating to the lower extremities. No numbness in the lower limbs has been previously reported. No apparent cause of the pain has been reported. The patient was treated at a local hospital and underwent L5/S1 discectomy with internal fixation in August 2019. Symptoms were relieved after surgery. However, low back pain recurred four months after the surgery. It was more severe on the left side, with soreness and cramp-like pain that worsened with changes in position and was insensitive to ibuprofen and codeine phosphate tablets. Non-contrast magnetic resonance imaging (MRI) of the lumbar spine was performed three months ago and showed a bulged lumbar intervertebral disc at the L3-L5 level.
Her medical history included hypertension and diabetes. The blood pressure and glucose levels were well controlled, but the specific values did not contribute. She had undergone thyroid surgery eight years ago and continued to take levothyroxine sodium tablets (Euthyrox) after the operation. The patient had no family history of bone tumors.
Examination revealed decreased lumbar mobility, straight leg raise (SLR) test (-), hip test (-), bilateral femoral nerve traction test (+), bilateral sacroiliac joint tenderness (+), bilateral T12-L3 paravertebral tenderness (+). Several questionnaires were used to assess patient status. The scoring results were as follows: numerical rating scale (NRS) 4 points in the resting state and 7 points when moving, ID-pain score 1 point, patient health questionnaire-9 (PHQ-9) 13 points, generalized anxiety disorder 7-item scale (GAD-7) 5 points, Patient Health questionnaire-15 (PHQ-15) 5 points.
The laboratory test results revealed elevated hs-CRP levels (28.3 mg/dL; normal range 0–8 mg/dL), and elevated erythrocyte sedimentation rate (49, 53, 67, 73, and 62 mm/h; normal range 0–20 mm/h). Results for serum autoantibodies, rheumatoid factor, and HLA-B27 were negative. All the other results were within the normal range. Lumbar computed tomography (CT) with three-dimensional reconstruction revealed postoperative changes in the L5-S1 vertebrae and intervertebral disc, narrowing of the intervertebral space, increased vertebral body density, and compressive changes in the L2 vertebrae (Figure 1A). Lumbar noncontrast MRI revealed L5-S1 vertebrae fixation. The L3/4 intervertebral disc slightly bulged, L2 vertebral body compression changes and abnormal signal, and T11-L2 vertebrae abnormal signal (Figure 1B). Sacroiliac joint CT showed high intensity in the left sacroiliac joint (Figure 2A), which indicated osteitis of the left sacroiliac joint. Sacroiliac joint magnetic resonance imaging (MRI) revealed postoperative changes in the L5-S1 vertebrae (Figure 2B).
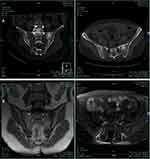 |
Figure 2 Sacroiliac joint imaging examination results revealed osteitis of the left sacroiliac joint. Notes: (A) Sacroiliac joint CT. (B) Sacroiliac joint MRI. |
Adjacent segment degeneration (ASD) is a condition that often occurs after spinal fusion or when another back surgery is performed. Combined with the patient’s back surgery history and lumbar CT results, we first considered the possibility that the patient may have ASD. Symptomatic and supportive NSAIDs and nerve nutritional therapy were administered. Loxoprofen 60 mg TID, cobamamide 1.5 mg QD, calcium carbonate and vitamin D3 600 mg QD, and tromethamine 30 mg BID were given to the patient. However, the patient’s symptoms did not improve significantly. Therefore, we attempted to identify the other diagnoses.
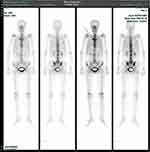
During re-examination, we found that her palms showed hyperkeratosis with active pustulosis (Figure 3A). Combined with the previously elevated hs-CRP and erythrocyte sedimentation rate, we suspected that the patient had SAPHO syndrome and performed skeletal emission computed tomography (ECT). Skeletal ECT found a typical “horn sign” change on the sternum stem and revealed active bone metabolism of T8, T10, T11, L3, S1 vertebrae, bilateral sternoclavicular joints, bilateral sacroiliac joints, and bilateral knee joints, as well as increased bone density in some thoracic vertebrae (Figure 4).
The patient met the diagnostic criteria for SAPHO syndrome and was diagnosed with SAPHO syndrome.1,4 Based on the original treatment, we added sulfasalazine enteric-coated tablets 1 g BID, adalimumab 40 mg once a fortnight, pregabalin 75 mg BID, and tramadol sustained-release tablets 100 mg BID. The patient reported that her pain symptoms were significantly relieved. He was discharged from the hospital and received adalimumab treatment (40 mg once per fortnight in the first 6 months and 40 mg once per month after month 6) in the outpatient clinic. Patient compliance was good during the treatment, and no adverse reactions were reported. Hyperkeratosis with active pustulosis on both palms fully resolved after 12 months of treatment (Figure 3B). The patient was followed up for 6 months after full recovery, and no recurrence was found in the symptoms of low back and lower extremity pain and palmar hyperkeratosis with active pustulosis (Figure 3C).
Discussion and Conclusions
The reported incidence of SAPHO syndrome is 0.00144/100,000 among Japanese people and 1/10,000 among Caucasians.4–6 Currently, there are no accurate statistics regarding its incidence in China. The etiology of SAPHO syndrome remains unclear. It is considered that the SAPHO syndrome may be closely related to autoimmune disorders, infection, and gene mutations.7 The diagnosis of SAPHO syndrome is based on patient history, characteristic imaging test results, and skin manifestations. SAPHO syndrome is not an independent ailment but a combination of symptoms. SAPHO syndrome should be suspected in patients who present with osteoarticular and/or dermatological clinical manifestations.7
Osteoarticular manifestations include osteitis, hyperostosis, synovitis, arthropathy, and enthesopathy, which present with recurrent pain, tenderness, and limited mobility of the bone and joint in the affected areas, with or without swelling of the surrounding soft tissues and fever.7 Two common osteoarticular manifestations are hyperostosis and osteitis. Common imaging findings include narrowing of the joint space, bone loss, and bone destruction, which is like seronegative spondyloarthropathy. The most common bone involvement, from common to uncommon, is the sternocostal joint (65–90%), spine (33%), pelvis (13–52%), long bones (30%), and flat bones (12%).8–11 Osteoarticular involvement is gradual at the onset. There may be no obvious abnormal changes in imaging examinations in the early stages of the disease.12 Osteolytic or sclerotic changes may appear in affected areas as the disease progresses. Typical skin lesions seen in SAPHO patients include palmoplantar pustulosis, severe acne, psoriasis vulgaris, pyoderma gangrenosum, Sweet syndrome, and Sneddon-Wilkinson disease.13,14 Sonozaki et al showed that the time interval between the onset of skin and osteoarticular manifestations in approximately 70% of patients is within two years,15 but 38 years have been reported in the literature.16 However, owing to the small sample size of the study, there is still controversy regarding the temporal correlation between dermatological and osteoarticular symptoms. Recently, Xu et al summarized the clinical characteristics of 69 patients with SAPHO syndrome, with an average course of the disease was 3.9 years.17 The study revealed that dermatological manifestations appeared earlier than osteoarticular manifestations in 55.1% of the patients, whereas 23.2% of the patients had dermatological and osteoarticular symptoms simultaneously, and 21.7% of the patients had dermatological symptoms later than the osteoarticular symptoms.17
To date, there is still no consensus regarding the diagnosis of SAPHO syndrome. Early diagnosis of SAPHO syndrome was based on the 1994 Kahn and Khan criteria.4 In 2012, Nguyen et al1 proposed that one of the following four conditions can be diagnosed as SAPHO syndrome: ① Osteoarticular and joint manifestations with acne conglobata and explosive acne or hidradenitis; ② Osteoarticular and joint manifestations with palmoplantar pustulosis; ③ Osteoarticular hypertrophy with or without typical skin lesions; ④ Chronic multifocal recurrent osteomyelitis (cMR0) with or without typical skin lesions.
We reported a case of SAPHO syndrome that is highly like ASD. The elevated hs-CRP and erythrocyte sedimentation rate were revealed. Hyperkeratosis with active pustulosis was observed on her palms during re-examination, and typical “horn sign” was found via skeletal ECT examination. The diagnosis of SAPHO syndrome is based on history, characteristic imaging, and osteoarticular and dermatological manifestations.7–11,13,14,16–18 There is currently no standardized treatment available.19,20 The NSAIDs are usually considered to be the first-line treatment.21 This patient was not sensitive to conventional NSAIDs treatment, and his symptoms significantly improved after treatment with the adalimumab (a TNF-α biological agent). Overall, SAPHO syndrome should be suspected in patients present with osteoarticular and/or dermatological manifestations. Biological agents can be used to treat patients with refractory SAPHO syndrome.
In clinical practice, the most considered causes of recurrent low back pain after lumbar internal fixation include ASD and other infections, sacroiliac joint pain, muscle strain, chronic postoperative pain syndrome, and psychological disorders. Rare conditions, such as SAPHO syndrome, are easily ignored. When this patient was first admitted to the hospital, both lumbar CT and MRI pointed to bulged lumbar disc and changes after lumbar disc internal fixation. The patient developed recurrent fever, but her white blood cell count was within the normal range, and the effects of conventional anti-inflammatory treatments were not satisfactory. During re-examination, hyperkeratosis with active pustulosis was observed on her palms, and SAPHO syndrome was considered. Therefore, skeletal ECT examination was performed, and the typical “horn sign” was found on her sternal stem. SAPHO syndrome was diagnosed based on the laboratory findings of elevated hs-CRP and erythrocyte sedimentation rate. However, due to the rarity of SAPHO syndrome, we were unable to collect more cases for the time being.
There are no standardized treatment protocols available because of their rarity and the lack of large-scale clinical trials.19 Current treatments for SAPHO syndrome are empirical.20 First-line treatments include nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesics. As first-line drugs for this disease, NSAIDs can quickly alleviate the symptoms of joint swelling and pain. Analgesics are typically used to relieve pain. Systemic corticosteroids and disease-modifying antirheumatic drugs (DMARDs) are typically second-line treatments for SAPHO syndrome.21 The pathogenesis of SAPHO syndrome is suggested to be multifactorial, but this aspect remains poorly explored, although bacterial and immunological dysfunction are hypothesized to play a role. Biological agents can be used to treat patients with refractory SAPHO syndrome. Biological agents target tumor necrosis factor (TNF)-α, interleukin-1 (IL-1), and interleukin-6 (IL-6).
In summary, the clinical manifestations of SAPHO syndrome at an early stage are often atypical and easily confused with other common spine-related diseases and infections. We reported a 62-year-old woman with SAPHO syndrome manifested ASD in a similar way. This patient was not sensitive to conventional NSAIDs treatment, and his symptoms significantly improved after treatment with the TNF-α biological agent, adalimumab. The patient’s symptoms fully resolved after 12 months of adalimumab treatment. No relapse was observed after six months of follow-up. This finding is worthy of further study and discussion. In the future, clinical research on SAPHO syndrome should be conducted to determine diagnostic consensus and therapeutic strategies.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Ethic Research of the First Affiliated Hospital of Zhejiang University. The patient and her family members were informed of and agreed to the therapeutic strategy.
Consent for Publication
The patient and her family members were informed and agreed that this case and related image could be published. Written consent to publish this information was obtained from all the study participants.
Acknowledgments
This study was funded by the Science and Technology Program of Zhejiang Province (2022C03081). The funding body partially funded the cost of follow-up examination and data collection to help us obtain follow-up data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
References
1. Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME. The SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):254–265. doi:10.1016/j.semarthrit.2012.05.006
2. Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. [Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases] Le syndrome acné pustulose hyperostose ostéite (SAPHO). Résultats d’une enquête nationale. 85 observations. Rev Rhum Mal Osteoartic. 1987;54(3):187–196. French.
3. Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol. 1988;6(2):109–112.
4. Kahn MF, Khan MA. The SAPHO syndrome. Baillières Clin Rheumatol. 1994;8(2):333–362. doi:10.1016/s0950-3579(94)80022-7
5. Cao Y, Li C, Xu W, et al. Spinal and sacroiliac involvement in SAPHO syndrome: a single center study of a cohort of 354 patients. Semin Arthritis Rheum. 2019;48(6):990–996. doi:10.1016/j.semarthrit.2018.09.004
6. Rukavina I. SAPHO syndrome: a review. J Child Orthop. 2015;9(1):19–27. doi:10.1007/s11832-014-0627-7
7. Liu S, Tang M, Cao Y, Li C. Synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: review and update. Ther Adv Musculoskelet Dis. 2020;12:1759720x20912865. doi:10.1177/1759720x20912865
8. Boutin RD, Resnick D. The SAPHO syndrome: an evolving concept for unifying several idiopathic disorders of bone and skin. AJR Am J Roentgenol. 1998;170(3):585–591. doi:10.2214/ajr.170.3.9490935
9. Earwaker JW, Cotten A. SAPHO: syndrome or concept? Imaging findings. Skeletal Radiol. 2003;32(6):311–327. doi:10.1007/s00256-003-0629-x
10. Depasquale R, Kumar N, Lalam RK, et al. SAPHO: what radiologists should know. Clin Radiol. 2012;67(3):195–206. doi:10.1016/j.crad.2011.08.014
11. Dihlmann W, Dihlmann SW. Acquired hyperostosis syndrome: spectrum of manifestations at the sternocostoclavicular region. Radiologic evaluation of 34 cases. Clin Rheumatol. 1991;10(3):250–263. doi:10.1007/bf02208686
12. Cianci F, Zoli A, Gremese E, Ferraccioli G. Clinical heterogeneity of SAPHO syndrome: challenging diagnose and treatment. Clin Rheumatol. 2017;36(9):2151–2158. doi:10.1007/s10067-017-3751-1
13. Monsour PA, Dalton JB. Chronic recurrent multifocal osteomyelitis involving the mandible: case reports and review of the literature. Dentomaxillofac Radiol. 2010;39(3):184–190. doi:10.1259/dmfr/23060413
14. Govoni M, Colina M, Massara A, Trotta F. ”SAPHO syndrome and infections”. Autoimmun Rev. 2009;8(3):256–259. doi:10.1016/j.autrev.2008.07.030
15. Sonozaki H, Mitsui H, Miyanaga Y, et al. Clinical features of 53 cases with pustulotic arthro-osteitis. Ann Rheum Dis. 1981;40(6):547–553. doi:10.1136/ard.40.6.547
16. Sugimoto H, Tamura K, Fujii T. The SAPHO syndrome: defining the radiologic spectrum of diseases comprising the syndrome. Eur Radiol. 1998;8(5):800–806. doi:10.1007/s003300050475
17. Xu P, Yi G, Li J. SAPHO syndrome. Rheumatology. 2021. doi:10.1093/rheumatology/keab760
18. Fruehauf J, Cierny-Modrè B, Caelen Lel S, Schwarz T, Weinke R, Aberer E. Response to infliximab in SAPHO syndrome. BMJ Case Rep. 2009;2009. doi:10.1136/bcr.10.2008.1145
19. Zemann W, Pau M, Feichtinger M, Ferra-Matschy B, Kaercher H. SAPHO syndrome with affection of the mandible: diagnosis, treatment, and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):190–195. doi:10.1016/j.tripleo.2010.04.037
20. Firinu D, Garcia-Larsen V, Manconi PE, Del Giacco SR. SAPHO syndrome: current developments and approaches to clinical treatment. Curr Rheumatol Rep. 2016;18(6):35. doi:10.1007/s11926-016-0583-y
21. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349–358. doi:10.1007/s40257-016-0191-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.