Back to Journals » Infection and Drug Resistance » Volume 16
Successful Treatment of Rare Pulmonary Coprinopsis cinerea Infection in a 17-Year-Old Female After Hematopoietic Stem Cell Transplantation: A Case Report
Authors Yu U , Cao K, Yang C, Wang C, Li Y, Zhou X, Zhang Q, Wang Y, Wen F, Liu S, Wang X
Received 11 November 2022
Accepted for publication 16 March 2023
Published 18 March 2023 Volume 2023:16 Pages 1567—1572
DOI https://doi.org/10.2147/IDR.S397233
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Uet Yu,1 Ke Cao,2 Chunlan Yang,1 Chunjing Wang,1 Yue Li,1 Xiaohui Zhou,1 Qian Zhang,1 Yuanxiang Wang,3 Feiqiu Wen,1 Sixi Liu,1 Xiaodong Wang1
1Department of Hematology and Oncology, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China; 2Department of Laboratory Medicine, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China; 3Department of Cardiothoracic Surgery, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China
Correspondence: Xiaodong Wang; Sixi Liu, Email [email protected]; [email protected]
Abstract: Invasive fungal infections (IFIs) are among the most severe complications in recipients of hematopoietic stem cell transplantation (HSCT) recipients and in patients with hematological malignancies. An increasing number of uncommon fungal infections have been reported in this era of antifungal prophylaxis. Coprinopsis cinerea is a rare pathogen that causes opportunistic infections in the immunocompromised patients, including HSCT recipients and is associated with very high mortality rates. Herein, we present a successfully treated pediatric HSCT patient with breakthrough pulmonary IFI caused by Coprinopsis cinerea despite posaconazole, prophylaxis using multidisciplinary approaches.
Keywords: Coprinopsis cinerea, Hormographiella aspergillata, invasive fungal infection, hematopoietic stem cell transplantation, leukemia, case report
Introduction
Invasive fungal infections (IFIs) are among the most severe complications and contribute to high morbidity and mortality in hematopoietic stem cell transplantation (HSCT) recipients and patients with hematological malignancies.1 Administration of high-dose chemotherapy, immunosuppressive agents, steroids, and broad-spectrum antibiotics increases the incidence of IFIs in HSCT recipients and patients with hematological disorders.2,3 In addition, IFIs often exhibit rapid disease progression and a slow response to treatment, leading to limitations in diagnosing them and treatment delays.3
Although antifungal prophylaxis is recommended in most cases of HSCT, the risk of developing an IFI remains high, particularly in patients with co-existing with other complications, such as prolonged neutropenia, severe viral and bacterial infections, and graft-versus-host diseases (GVHDs).1,2 In addition, IFIs on antifungal prophylaxis in HSCT patients often have an insidious onset, which may increase the risk of misdiagnosis and delay treatment.
Candida albicans and Aspergillus spp. are among the most common causative organisms of fungal infections in HSCT recipients and patients with hematological malignancies in China.4,5 However, there is an increasing number of uncommon fungal infections, which may be associated with a shift in the etiology spectrum of IFIs due to antifungal prophylaxis.6–8 In this paper, we report a rare case of pulmonary Coprinopsis cinerea (C. cinerea) infection in a 17-year-old patient with B-cell acute lymphoblastic leukemia (B-ALL) after HSCT.
Case Description
A 17-year-old female with the second relapse of B-ALL received chimeric antigen T-cell (CART) salvage therapy, followed by co-transplantation of haploidentical stem cells and unrelated cord blood in September 2021. The patient had a previous disseminated pulmonary tuberculosis infection during the first relapse in July 2020. However, she recovered well after treatment with anti-tuberculosis drugs, including isoniazid (10 mg/kg/day), rifampicin (10 mg/kg/day), and levofloxacin (10 mg/kg/day) from July 2020 to April 2021, and continued to receive oral isoniazid prophylaxis until 3 days prior to HSCT. Chest computed tomography (CT) revealed no residual tuberculosis lesions and interferon-gamma (IFN-γ) release assays was negative prior to transplantation. Furthermore, there was no evidence of fungal infection during chemotherapy or prior to HSCT.
The patient received a myeloablative conditioning regimen consisting of cyclophosphamide, busulfan, fludarabine, and thiotepa without total body irradiation. The post-transplant immunosuppressants included of post-transplant cyclophosphamide, cyclosporine, and mycophenolate mofetil. In addition, oral posaconazole (200 mg every 8h) was commenced 3 days after HSCT as an antifungal prophylaxis. Additionally, neutrophils and platelets were engrafted on 15 and 24 days after transplantation, respectively. Engraftment syndrome with fever, skin rashes, and elevated bilirubin levels was observed during cell engraftment from day 14 after transplantation. However, these symptoms resolved after intravenous administration of methylprednisolone (1 mg/kg/day for 4 days from day 15 to day 18, after which the dose was tapered and discontinued on 27 days after HSCT). No other severe complications were noted.
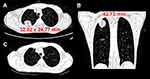
The patient began to cough slightly from 23 days after HSCT. Since this patient had a history of tuberculosis infection, rifapentine (600 mg per week) was administered in combination with isoniazid (10 mg/kg/day) for tuberculosis prophylaxis. However, the patient showed no signs of respiratory distress, including dyspnea, tachypnea, and hypoxemia. The cough lasted five days, and the patient developed a throbbing, dull pain in the back. A chest CT was performed, and a large nodular lesion (32.02 mm × 24.77 mm × 42.73 mm) with a faint surrounding vitreous subpleural of the right upper lobe was observed (Figure 1A and B). Therefore the patient was suspected of having tuberculosis or an IFI, and a bronchoscopy was performed. Caspofungin (loading dose at 70 mg/m2 for 1 day and then 50 mg/m2/day) was commenced on day 27 after HSCT, and posaconazole was switched to intravenous voriconazole (loading dose at 9mg/kg, every 12h, for 1 day and then 8mg/kg, every 12h) because the patient developed a fever 4 days later. The bronchoalveolar (BAL) fluid was positive for cytomegalovirus (CMV), human herpes virus 6 (HHV-6), and Actinomyces odontolyticus using next-generation sequencing. However, in contrast, using the PCR-based analysis, the BAL fluid was negative for fungi and tuberculosis. In addition, bacterial and fungal cultures of the BAL fluid were negative.
The antibiotics, imipenem (15mg/kg, every 6h) and high-dose penicillin (100,000 U/kg, every 6 h) were administered to treat Actinomyces infections for 2 months. Ganciclovir (5 mg/kg, every 12h) was also commenced for antiviral treatment. Despite the above treatment, the patient began to have hemoptysis, and a reassessment of the chest CT showed that the lung lesion was unresolved. Therefore, a wedge resection of the lung lesion was performed because the lesion was still localized. The next-generation sequencing analysis of the lung tissue revealed C. cinerea infection (NCBI accession PRJNA938261). This was later confirmed when septate hyphae were observed by both Wright-Giemsa and hexamine-silver staining of lung sections, indicative of C. cinerea infection (Figure 2). Therefore, antifungal treatment was switched to amphotericin B (starting from 0.1 mg/kg/day then gradually increased to 0.7 mg/kg/day) for 2 weeks, followed by oral voriconazole as follow-up therapy. Chest CT scans showed no new lung lesions 5 months after the wedge resection surgery (Figure 1C).
Discussion
C. cinerea, or normally seen in its asexual form Hormographiella aspergillata (H. aspergillata), is an environmental mold that rarely causes human infection.9 In recent years, the administration of antifungal prophylaxis has reduced the incidence of IFIs in HSCT patients or those with hematological malignancies. However, this may cause a shift in the spectrum of IFI etiology and increase the risk of occasional fungal infections, including C. cinerea.3,10,11 This paper reports a rare pediatric/adolescent case of a patient with recurrent B-ALL who developed a breakthrough C. cinerea infection after HSCT, despite antifungal prophylaxis with posaconazole.
The diagnosis of C. cinerea infection is challenging because patients often lack the typical symptoms. C. cinerea infection is primarily responsible for pulmonary infections. However, C. cinerea infections involving the skin, heart, intestine, central nervous system, and eyes have also been reported. In a very few cases, systemic C. cinerea infection involving multiple organs have been reported, with very high mortality rates despite intensive treatment.12–16 C. cinerea infections are more frequently observed in the immunocompromised patients. However, a few cases of C. cinerea infection have also been observed in the immunocompetent patients, particularly those who undergoing invasive procedures, such as cardiovascular and ophthalmic surgeries.17,18
Fungal cultures to identify the filamentous basidiomycetes directly under a microscope remains the key finding for the diagnosing C. cinerea infections. C. cinerea grows in various media without cycloheximide at 25 °C or 35 °C.14 However, the sensitivity of fungal cultures is low and requires experienced technicians to differentiate them from other fungal species and avoid misdiagnosis.19 Conventional serological methods, including galactomannan and β-D-glucan, cannot be detected in most patients infected with C. cinerea. However, a recent study from France suggested that galactomannan and β-D-glucan could be detected in the culture media of H. aspergillata isolated from two patients, suggesting that these assays may provide additional clues in patients suspected of having an IFI.6 A more reliable method for diagnosing C. cinerea infections is to use molecular techniques such as PCR-based sequencing and other next-generation sequencing methods.16,20,21 These techniques can efficiently analyze a broad spectrum of pathogens, but their sensitivity varies between sample types. In line with previously reported cases in adult patients, we observed that molecular diagnostic methods might have higher sensitivity in lung tissue than in the BAL fluid.6 Thus, a biopsy of the lung lesions may be considered for early diagnosis in future patients as has been recommended in some centers for adult patients.
The efficacy of antiviral drugs varies from person to person. Susceptibility breakpoints may provide a rationale for the use of specific antifungal medications. However, there are no current standard guidelines for interpreting minimum inhibitory concentration (MIC) breakpoints for C. cinerea or H. aspergillata from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or the Clinical and Laboratory Standards Institute (CLSI) guidelines.19 In addition, strains of C. cinerea resistant to various antifungal agents, including azoles, echinocandins, and amphotericin B, have been reported in different institutions.9,13,22,23 Echinocandins, such as caspofungin and micafungin, may not be recommended as a treatment options, probably because of the high MICs in many of the reported cases.10,14,24,25 Fluconazole, flucytosine, and itraconazole have also been reported to have high MICs. Although low MIC values of posaconazole have been reported in some cases, in the present case the patient developed a breakthrough infection despite posaconazole prophylaxis.26 Hence, amphotericin B and voriconazole may be the treatment of choice because of their low MICs in most reported cases. In the present case, amphotericin B was administered after for 2 weeks after surgical resection of the lung lesion, followed by oral maintenance with voriconazole. No relapse of the fungal infection was observed during the follow-up period. Unfortunately, susceptibility tests were not performed on this patient because we could not isolate the mold from the culture.
Due to the uncertainty of the outcomes of antifungal therapy, surgery may be used as a complementary tool to remove localized lesions, particularly in patients with pulmonary IFI.27 In addition, surgical resection in combination with antifungal medications may contribute to a more favorable outcome as this strategy has successfully treated a few cases of invasive C. cinerea infection.28 However, it is difficult to conclude the effectiveness of this strategy at this time because such cases have only been reported in a small series.
In summary, administering antifungal drugs for prophylaxis has greatly reduced the incidence of common fungal infections in HSCT recipients and patients with hematologic malignancies. However, there has been a simultaneous increase in the incidence of uncommon fungal infections that have made the management of these patients challenging in recent years. Molecular techniques play key roles in diagnosing these rare fungal infections; however, the sensitivities of different sample types should be considered. Therefore, performing direct biopsies of infected lesions is ideal for better sensitivity. Furthermore, multiple lines of antifungal drugs should be considered when looking medication with optimal efficacy, as most of these cases have been reported in a small number of patients. Hence, combined antifungal medication and surgical approach may be considered to increase patient survival in patients with localized pulmonary lesions. Nevertheless, prompt diagnosis and intervention are key to optimal prognosis for patients with rare fungal infections including C. cinerea infections.
Ethical Approval and Consent for Participate
Written consents in approval for publication of this manuscript were obtained from both the patients and parents. The publication of this case report has been approved by Shenzhen Children’s Hospital Ethics Committee (approval number 2021102).
Consent for Publication
Written consent was obtained from the parents of the patient for the participation of this study.
Acknowledgments
We thank the patient and parents for giving the consent for the publication of this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This publication fee of this paper is funded by Shenzhen Science and Technology Innovation Commission (RCBS20200714114858018), Shenzhen Key Medical Discipline Construction Fund (SZXK034), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP012), Shenzhen High-level Hospital Construction Fund, Shenzhen Children’s Hospital Research fund (ynkt2021-zz26, ynkt2020-zz01).
Disclosure
The authors have no conflict of interests to declare for this work.
References
1. Ahn H, Lee R, Cho SY, Lee DG. Advances in prophylaxis and treatment of invasive fungal infections: perspectives on hematologic diseases. Blood Res. 2022;57(S1):101–111. doi:10.5045/br.2022.2022036
2. Perez P, Patiño J, Franco AA, et al. Prophylaxis for invasive fungal infection in pediatric patients with allogeneic hematopoietic stem cell transplantation. Blood Res. 2022;57(1):34–40. doi:10.5045/br.2021.2021127
3. Corzo-León DE, Satlin MJ, Soave R, et al. Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-centre study with focus on emerging pathogens. Mycoses. 2015;58(6):325–336. doi:10.1111/myc.12318
4. Sun Y, Hu J, Huang H, et al. Clinical risk score for predicting invasive fungal disease after allogeneic hematopoietic stem cell transplantation: analysis of the China Assessment of Antifungal Therapy in Hematological Diseases (CAESAR) study. Transplant Infect Dis. 2021;23(4):e13611. doi:10.1111/tid.13611
5. Wang L, Wang Y, Hu J, et al. Clinical risk score for invasive fungal diseases in patients with hematological malignancies undergoing chemotherapy: China Assessment of Antifungal Therapy in Hematological Diseases (CAESAR) study. Front Med. 2019;13(3):365–377. doi:10.1007/s11684-018-0641-0
6. Lee SY, Yeo CL, Lee WH, Kwa AL, Koh LP, Hsu LY. Prevalence of invasive fungal disease in hematological patients at a tertiary university hospital in Singapore. BMC Res Notes. 2011;4:42. doi:10.1186/1756-0500-4-42
7. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. doi:10.1086/651263
8. Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–1075.
9. Conen A, Weisser M, Hohler D, Frei R, Stern M. Hormographiella aspergillata: an emerging mould in acute leukaemia patients? Clin Microbiol Infect. 2011;17(2):273–277. doi:10.1111/j.1469-0691.2010.03266.x
10. Pang KA, Godet C, Fekkar A, et al. Breakthrough invasive mould infections in patients treated with caspofungin. J Infect. 2012;64(4):424–429. doi:10.1016/j.jinf.2011.12.015
11. Isabel Cristina RS, Diana A, Karen A. Breakthrough hormographiella aspergillata infection in a patient with acute myeloid leukemia receiving posaconazole prophylaxis: a case report and review. Mycopathologia. 2020;185(6):1069–1076. doi:10.1007/s11046-020-00488-z
12. Chauhan A, Gruenberg J, Arbefeville S, Mettler T, Brent CH, Ferrieri P. Disseminated hormographiella aspergillata infection with lung and brain involvement after allogenic hematopoietic stem-cell transplantation in a 54-year-old man. Lab Med. 2019;50(4):426–431. doi:10.1093/labmed/lmz018
13. Nanno S, Nakane T, Okamura H, et al. Disseminated Hormographiella aspergillata infection with involvement of the lung, brain, and small intestine following allogeneic hematopoietic stem cell transplantation: case report and literature review. Transplant Infect Dis. 2016;18(4):611–616. doi:10.1111/tid.12561
14. Lagrou K, Massonet C, Theunissen K, et al. Fatal pulmonary infection in a leukaemic patient caused by Hormographiella aspergillata. J Med Microbiol. 2005;54(Pt7):685–688. doi:10.1099/jmm.0.46016-0
15. Surmont I, Van Aelst F, Verbanck J, De Hoog GS. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med Mycol. 2002;40(2):217–219. doi:10.1080/mmy.40.2.217.219
16. Verweij PE, van Kasteren M, van de Nes J, de Hoog GS, de Pauw BE, Meis JF. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J Clin Microbiol. 1997;35(10):2675–2678. doi:10.1128/jcm.35.10.2675-2678.1997
17. Jain N, Jinagal J, Kaur H, et al. Ocular infection caused by Hormographiella aspergillata: a case report and review of literature. J Mycol Med. 2019;29(1):71–74. doi:10.1016/j.mycmed.2018.12.002
18. Lamas-Francis D, Llovo-Taboada J, Navarro D, Touriño R, Rodríguez-Ares T. Necrotizing scleritis due to Hormographiella aspergillata. Eur J Ophthalmol. 2022;11206721221118209. doi:10.1177/11206721221118209
19. Chakrabarti A, Mohamed N, Capparella MR, et al. The role of diagnostic tests in antifungal stewardship for treating invasive fungal infections: a plain language summary. Future Microbiol. 2022. doi:10.2217/fmb-2022-0182
20. Chowdhary A, Kathuria S, Agarwal K, Meis JF. Recognizing filamentous basidiomycetes as agents of human disease: a review. Med Mycol. 2014;52(8):782–797. doi:10.1093/mmy/myu047
21. Chong E, Yu HJ, Kim TY, et al. Invasive hormographiella aspergillata infection identified using DNA sequencing. Ann Lab Med. 2022;42(3):370–372. doi:10.3343/alm.2022.42.3.370
22. Godet C, Cateau E, Rammaert B, et al. Nebulized liposomal amphotericin B for treatment of pulmonary infection caused by hormographiella aspergillata: case report and literature review. Mycopathologia. 2017;182(7–8):709–713. doi:10.1007/s11046-017-0117-9
23. Moniot M, Lavergne RA, Morel T, et al. Hormographiella aspergillata: an emerging basidiomycete in the clinical setting? A case report and literature review. BMC Infect Dis. 2020;20(1):945. doi:10.1186/s12879-020-05679-z
24. Suarez F, Olivier G, Garcia-Hermoso D, et al. Breakthrough Hormographiella aspergillata infections arising in neutropenic patients treated empirically with caspofungin. J Clin Microbiol. 2011;49(1):461–465. doi:10.1128/jcm.01213-10
25. Abuali MM, Posada R, Del Toro G, et al. Rhizomucor variabilis var. regularior and Hormographiella aspergillata infections in a leukemic bone marrow transplant recipient with refractory neutropenia. J Clin Microbiol. 2009;47(12):4176–4179. doi:10.1128/jcm.00305-09
26. Bojic M, Willinger B, Rath T, et al. Fatal skin and pulmonary infection caused by Hormographiella aspergillata in a leukaemic patient: case report and literature overview. Mycoses. 2013;56(6):687–689. doi:10.1111/myc.12087
27. Lieber J, Hauch H, Lang P, et al. Surgical management of stem cell transplantation-related complications in children. Pediatr Transplant. 2012;16(5):471–479. doi:10.1111/j.1399-3046.2012.01708.x
28. Heiblig M, Bozzoli V, Saison J, et al. Combined medico-surgical strategy for invasive sino-orbito-cerebral breakthrough fungal infection with Hormographiella aspergillata in an acute leukaemia patient. Mycoses. 2015;58(5):308–312. doi:10.1111/myc.12305
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.