Back to Journals » Infection and Drug Resistance » Volume 17
Successful Treatment of an AML Patient Infected with Hypervirulent ST463 Pseudomonas Aeruginosa Harboring Rare Carbapenem-Resistant Genes blaAFM-1 and blaKPC-2 Following Allogeneic Hematopoietic Stem Cell Transplantation
Authors Shen Y , Cao J, Hu T, Yang X, Zhao Y, Shen Y, Ye B , Yu Y, Wu D
Received 19 December 2023
Accepted for publication 25 March 2024
Published 6 April 2024 Volume 2024:17 Pages 1357—1365
DOI https://doi.org/10.2147/IDR.S455746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Yingying Shen,1,2,* Junmin Cao,3,* Tonglin Hu,1,2 Xiawan Yang,1,2 Yuechao Zhao,1,2 Yiping Shen,1,2 Baodong Ye,1,2 Yunsong Yu,4 Dijiong Wu1,2
1Department of Hematology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, People’s Republic of China; 2National Traditional Chinese Medicine Clinical Research Base (Hematology), Hangzhou, Zhejiang, People’s Republic of China; 3Department of Clinical Laboratory, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, People’s Republic of China; 4Department of Infectious Diseases, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dijiong Wu, Department of Hematology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, 310006, People’s Republic of China, Tel +86-0571-86620325, Email [email protected] Yunsong Yu, Department of Infectious Diseases, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang, 310024, People’s Republic of China, Tel +8613805790432, Email [email protected]
Background: Carbapenem-resistant P. aeruginosa (CRPA) is a common hospital-acquired bacterium. It exhibits high resistance to many antibiotics, including ceftazidime/avibactam and cefteolozane/tazobactam. The presence of carbapenem-resistant genes and co-existence Klebsiella pneumoniae carbapenemase (KPC) and metallo-β-lactamases (MBLs) further inactivated all β-lactams. Understanding the resistance genes of CRPA can help in uncovering the resistance mechanism and guiding anti-infective treatment. Herein, we reported a case of perianal infection with hypervirulent ST463 Pseudomonas aeruginosa.
Case Presentation: The case is a 32-year-old acute myeloid leukemia (AML) patient with fever and septic shock during hematopoietic stem cell transplantation (HSCT), and the pathogen was finally identified as a highly virulent sequence type 463 (ST463) P. aeruginosa harboring carbapenem-resistant genes blaAFM-1 and blaKPC-2, which was detected in the bloodstream and originated from a perianal infection. The strain was resistant to ceftazidime/avibactam but successfully treated with polymyxin B, surgical debridement, and granulocyte engraftment after HSCT. The AML was cured during the 19-month follow-up.
Conclusion: This case emphasizes the importance of metagenomic next-generation sequencing (mNGS) and whole-genome sequencing (WGS) in identifying microbes with rare resistant genes, and managing CRPA, especially in immunocompromised patients. Polymyxin B may be the least resistant option.
Keywords: carbapenem-resistant pseudomonas aeruginosa, blaAFM, blaKPC, ST463
Background
As one of the most common nosocomial bacteria, Pseudomonas aeruginosa often causes infections associated with blood, urinary tract, skin and soft tissue.1 In the treatment of multidrug-resistant P. aeruginosa (MDR-PA), carbapenems have proven to be the most effective antibiotics. However, the extensive use of carbapenems has resulted in a significant increase in carbapenem-resistant P. aeruginosa (CRPA) strains.2 The increasing prevalence of CRPA has further elevated the mortality rate, with a 30% 30-day mortality rate even after appropriate treatment,2 which was higher in hematological disorder patients with granulocytopenia.
Hematopoietic stem cell transplantation (HSCT) is a crucial treatment for curing hematologic malignancies. Infections often occur during the neutropenic phase of HSCT, which may lead to graft failure or life-threatening complications. In particularly, early-onset severe CRPA infection during HSCT will negatively impact the long-term prognosis because it poses a significant constraint on therapeutic strategies. Therefore, it is important to further understand the drug-resistance mechanisms of P. aeruginosa and make rational choices for anti-infection strategies. Herein, we isolated a strain of blaKPC-2 ST463 CRPA, resistant to ceftazidime/avibactam, from the bloodstream of a HSCT patient. The final result of whole-genome sequencing (WGS) analysis indicated that this strain simultaneously produces Klebsiella pneumoniae carbapenemase (KPC) and Alcaligenes faecalis metallo-β-lactamase-1(AFM1) enzymes, exhibiting a high level of drug resistance. A literature review retrieved only a few papers describing a novel type of plasmid with multidrug-resistant (MDR) regions, and no case had been reported in HSCT with severe immune deficiency. To further understand these strains, we described the process of CRPA strain isolated from blood and the patient’s treatment course against CRPA to provide resources for clinicians.We also reviewed the available studies on CRPA carrying the blaAFM gene to further study the resistance mechanism of this bacterium.
Case Presentation
A 32-year-old Asian man with hyperleukocytosis was diagnosed with acute myeloid leukemia (AML) in March 2022 (FAB M1), accompanied with CREBBP and ALK mutations, positive fusion gene ETO and t (8;21) (q22; q22.1) cytogenetic abnormality. He achieved partial remission after the first round of induction chemotherapy (cytarabine 100 mg/m2 days 1–7 and idarubicin 10 mg/m2 days 1, 2 and 3), and achieved complete remission after an additional round of chemotherapy with HAD (homoharringtonine 2 mg days 1–7, idarubicin 10 mg/m2 days 1, 2 and 3, and cytarabine 100 mg/m2 days 1–7). Subsequently, consolidation therapy was administered with HAD and intermediate-dose cytarabine, however, the patient did not reach genetic minimal residual disease (MRD) negativity throughout the treatment course (Figure 1). Owing to hyperleukocytic AML and insufficient depth of remission, the patient was classified as “high-risk AML”, and allogeneic stem cell transplantation was indicated. A fully-matched unrelated donor was found in the China Bone Marrow Donor Registry for allogeneic-HSCT (allo-HSCT), and the patient underwent conditioning regimen with BUCY (Busulfan-Cyclophosphamide). On Day −5, the patient developed febrile neutropenia during the anti-thymocyte globulin (ATG) application. Laboratory tests revealed an elevated C-reactive protein (CRP) level of 81.45 mg/L (0–8 mg/L) and an increased procalcitonin (PCT) level of 11 μg/L (0–0.046 μg/L), which might also be observed after ATG administration.3 Considering the patient’s neutropenic state, we could not rule out that the fever was caused by an infection, and he was treated with meropenem for 2 days but showed inadequate response. Subsequently, tigecycline was added to cover a wider range of bacterial sources. Finally, the patient’s temperature, CRP and PCT returned to normal.
On the evening of stem cell infusion, the patient developed fever again, with a peak temperature of 40.4°C, accompanied by mild perianal pain. Blood culture and metagenomic next-generation sequencing (mNGS) of peripheral blood specimen were performed immediately. The blood culture results were negative, but mNGS indicated the presence of P. aeruginosa carrying blaKPC-2 gene. The antibiotics were adjusted to polymyxin B and ceftazidime/avibactam, considering the presence of KPC-producing P. aeruginosa. Additionally, hematopoietic growth factors and transfusion support were provided. The patient’s experienced persistent agranulocytosis and the condition deteriorated with persistent fever ranging from 39–40°C, despite aforementioned antibiotic treatment (Figure 2A and B). The CRP levels progressively increased to over 200 mg/L, and PCT reached a peak of 26 μg/L on Day +3. The perianal pain became increasingly severe, accompanied by local redness and swelling. Moreover, the perianal skin started rupturing and showed signs of suppuration (pus formation) (Figure 3). Due to uncontrolled infection, the patient developed signs of septic shock.
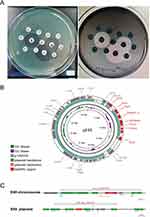
Since there were no antibiotic susceptibility results to guide antibiotic regimen adjustments, we invited a colorectal surgeon to perform surgical debridement and provided aggressive life support treatment to stabilize the patient’s condition. On Day +9 after transplantation, a CRPA strain was isolated from a blood culture specimen, with enzyme testing indicating the production of carbapenemase (KPC). CRPA was also identified in the subsequent cultures of perianal secretions. The susceptibility testing (Table 1) showed that the CRPA was resistant to multi-drug including ceftazidime/avibactam and piperacillin-tazobactam, but was sensitive to polymyxin B. Antimicrobial disk combined susceptibility tests (Figure 4A) indicated that when combined with polymyxin B, there was a slight zone of inhibition observed, suggesting some degree of effectiveness against the strain. Therefore, the antimicrobial treatment was adjusted to a combination of polymyxin B, based on the susceptibility results. Ultimately, with aggressive surgical debridement, effective antimicrobial therapy and successful neutrophil engraftment, the infection was brought under control.
 |
Table 1 Susceptibility to Antimicrobial Agents |
To further elucidate the resistance mechanism of this KPC-producing P. aeruginosa which exhibited resistance to ceftazidime/avibactam, we performed whole-genome sequencing (WGS) (designated as E45). Results showed that the particular strain belonged to ST463 P. aeruginosa, and carried both the blaAFM-1 and blaKPC-2 genes (Figure 4B and C). Antimicrobial resistance genes blaAFM-1 was detected on the chromosome of E45 located within a conserved region of ISCR29 unit, and blaKPC-2 was detected on the plasmid located within a conserved region of IS26 unit. This result explained the reason for the extensive drug-resistance exhibited by this strain.
Discussion
P. aeruginosa is one of the most common nosocomial bacteria and often causes infections associated with blood, urinary tract, skin and soft tissue. Carbapenems have been the most efficient antibiotics for treating multidrug-resistant P. aeruginosa. However, with the extensive use of carbapenems, the incidence of CRPA has increased significantly. In recent years, CRPA have been identified as refractory pathogens because of their increased transmissibility and limited treatment options.2,4
The main mechanisms of resistance to carbapenems in P. aeruginosa involve various factors, including the loss of OprD porin, overexpression of the effector pump and chromosomal β-lactamase AmpC, and acquisition of carbapenemases (such as KPC-2, VIM, NDM, and IMP) through mobile genetic elements like plasmids.5,6 KPC is a serine protease of the molecular class A and is frequently found in K. pneumoniae.7 KPC-producing P. aeruginosa (KPC-PA) was initially reported in 2007, and identified for the first time in Zhejiang in 2011.8 Reports showed that KPC-PA has a prevalence of around 40% in the clinical CRPA population in East China, and ST463 is the predominant KPC-producing clone.9 ST463 is the predominant strain in cases of bloodstream infection caused by CRPA, accounting for 48.0% of the cases. A studyutilizing10 multivariate analysis identified three independent risk factors for a fatal outcome: KPC carriage (odds ratio [OR] 4.8; 95% CI 1.0–23.7; P = 0.05), Pitt bacteraemia score (OR 1.3; 95% CI 1.0–1.6; P = 0.02), and underlying hematological disease (OR 8.5; 95% CI 1.6–46.4; P = 0.01). Mortality associated with ST463 CRPA is significantly higher compared to non-ST463 CRPA.11
BlaAFM-1 CRPA strains are relatively rare. In 2018, a subclass B1b (Metallo-b-lactamase) (MBL) was recognized in an Alcaligenes faecalis strain and named AFM-1. Subsequently, AFM-1 has been found in Comamonas testosteroni, Stenotrophomonas maltophilia and Bordetella trematum. As a novel MBL, AFM alleles have not been widely disseminated in P. aeruginosa (Table 2).12–17 Piaopiao Zhang16 first reported the identification of blaAFM-1 in the chromosome of P. aeruginosa. In a study conducted by Xuefei Zhang,17,605 consecutive non-duplicate P. aeruginosa isolates were examined, and only three blaAFM-positive strains were identified. BlaAFM-1 is located within a conserved region of groEL-ΔfloR-blaAFM-1-ble-ΔtrpF-ΔISCR27n2-ΔISPme1 -msrB2- msrA- yhgU-corA.17 An ISCR27-like element, named ISCR29 by Li,12 inserted into the chromosome of P. aeruginosa is a prerequisite for the insertion of the blaAFM-1-carrying mobile unit, which would enable it to be intrinsically resistant to β-lactam antibiotics, including carbapenems without being subjected to selective pressures. These ISCR29-blaAFM units are highly similar in most plasmids carrying blaAFM1-3, indicating that ISCR29 plays a crucial role in the dissemination of blaAFM. Studies also reported that the co-existence of KPC and MBL in P. aeruginosa can inactivate all b-lactams, making clinical treatment extremely difficult.
 |
Table 2 Related Literatures of PA Strains Isolated Carrying blaAFM |
Based on the succeeded treatment process of this patient, the following three points that contributed to his survival are summarized, to help clinicians in the future: a. patients with hematological disorders, especially those with granulocytopenia, have a low positive rate of blood culture after infection. Therefore, it is recommended to perform pathogenic mNGS detection immediately, and adjust antibiotics promptly after obtaining positive results, which will be beneficial for controlling infection; b. for severe infections caused by CRPA, early, adequate, and combination antibiotic therapy is recommended; c. if there is a local soft tissue infection, surgical debridement can be performed if conditions permit, combined with effective anti-infective treatment, which can achieve better results. In addition, we should also keep an eye on side effect of the long-term polymyxin B application. In our case, the patient developed acute renal failure on Day +24 ascribe to the combination of nephrotoxic medications (cyclosporine A, etc.), but fortunately recovered completely with a timely withdraw of polymyxin B and initiate dialysis, without any recurrence of infection. Nevertheless, in cases where patients exhibit renal impairment and are unable to tolerate polymyxin B therapy, a study18 has shown that cefiderocol exhibits promising efficacy against isolates harboring acquired carbapenemases or PDC variants. It may be considered for use in the treatment thus infections. Ultimately, the patient attained profound molecular remission following negative ETO fusion gene testing (0.00%) 19 months after HSCT.
Conclusion
This rare case highlights the importance of infection prevention in transplant recipients, and strengthening the monitoring of multidrug-resistant bacteria to reduce the incidence and mortality of infections. In conclusion, it is particularly important to identify microbes with rare resistant genes and manage CRPA, especially in immunocompromised patients, using mNGS or WGS. Notably, for CRPA, Polymyxin B is one of the few remaining options for the treatment.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author (Dijiong Wu) upon a reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethical committee of First Affiliated Hospital of Zhejiang Chinese Medical University.
Patient Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and the images. Details of the case can be published without institutional approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Zhejiang Provincial Natural Science Foundation (NO.LY21H290003), Zhejiang Traditional Chinese Medicine Scientific Research Foundation (NO. 2020ZB085), Science and Technology Department of Zhejiang Research Foundation (NO.2019C03047), Project of Academic Inheritance Studio of Famous and Aged Chinese Medicine Experts in Zhejiang Province (NO.GZS2021022), Specific Program of Scientific Research of Zhejiang Chinese Medicine University for Affiliated Hospital (NO. 2023FSYYZZ04), Science and Technological Innovation Project for College Students in Zhejiang Province (Xinmiao Talent Plan) (NO. 2023R410003), Zhejiang Medical and Health Science and Technology Plan Project (NO. 2023RC193) and National Natural Science Foundation of China (NO.82304937).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582–610. doi:10.1128/CMR.00040-09
2. Buehrle DJ, Shields RK, Clarke LG, Potoski BA, Clancy CJ, Nguyen MH. Carbapenem-resistant pseudomonas aeruginosa bacteremia: risk factors for mortality and microbiologic treatment failure. Antimicrob Agents Chemother. 2017;61(1). doi:10.1128/AAC.01243-16
3. Brodska H, Drabek T, Malickova K, et al. Marked increase of procalcitonin after the administration of anti-thymocyte globulin in patients before hematopoietic stem cell transplantation does not indicate sepsis: a prospective study. Crit Care. 2009;13(2):R37. doi:10.1186/cc7749
4. Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15–21. doi:10.1177/2049936115621709
5. Tu Y, Wang D, Zhu Y, et al. Emergence of a KPC-90 variant that confers resistance to ceftazidime-avibactam in an ST463 carbapenem-resistant pseudomonas aeruginosa strain. Microbiol Spectr. 2022;10(1):e0186921. doi:10.1128/spectrum.01869-21
6. Gomez-Martinez J, Rocha-Gracia RDC, Bello-Lopez E, et al. A plasmid carrying bla(IMP-56) in pseudomonas aeruginosa belonging to a novel resistance plasmid family. Microorganisms. 2022;10. doi:10.3390/microorganisms10091863
7. Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother. 2011;66:307–312. doi:10.1093/jac/dkq431
8. Hagemann JB, Pfennigwerth N, Gatermann SG, von Baum H, Essig A. KPC-2 carbapenemase-producing pseudomonas aeruginosa reaching Germany. J Antimicrob Chemother. 2018;73(7):1812–1814. doi:10.1093/jac/dky105
9. Hu Y, Liu C, Wang Q, et al. Emergence and expansion of a carbapenem-resistant pseudomonas aeruginosa clone are associated with plasmid-borne bla KPC-2 and virulence-related genes. mSystems. 2021;6(3). doi:10.1128/mSystems.00154-21
10. Hu H, Zhang Y, Zhang P, et al. Bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing p. aeruginosa sequence type 463, associated with high mortality rates in China: a retrospective cohort study. Front Cell Infect Microbiol. 2021;11:756782. doi:10.3389/fcimb.2021.756782
11. Zhang X, Tang M, Xu Y, et al. Characteristics of rare ST463 carbapenem-resistant pseudomonas aeruginosa clinical isolates from blood. J Glob Antimicrob Resist. 2023;32:122–130. doi:10.1016/j.jgar.2023.01.011
12. Li Y, Zhu Y, Zhou W, et al. Alcaligenes faecalis metallo-beta-lactamase in extensively drug-resistant Pseudomonas aeruginosa isolates. Clin Microbiol Infect. 2022;28(6):880 e1–880 e8. doi:10.1016/j.cmi.2021.11.012
13. Zhang X, Wang L, Li D, et al. An IncP-2 plasmid sublineage associated with dissemination of bla IMP-45 among carbapenem-resistant pseudomonas aeruginosa. Emerg Microbes Infect. 2021;10(1):442–449. doi:10.1080/22221751.2021.1894903
14. Fang Y, Wang N, Wu Z, et al. An XDR pseudomonas aeruginosa ST463 strain with an IncP-2 plasmid containing a novel transposon Tn 6485f encoding bla IMP-45 and bla AFM-1 and a second plasmid with two copies of bla KPC-2. Microbiol Spectr. 2023;11(1):e0446222. doi:10.1128/spectrum.04462-22
15. Chen M, Cai H, Li Y, et al. Plasmid-borne AFM alleles in pseudomonas aeruginosa clinical isolates from China. Microbiol Spectr. 2022;10(5):e0203522. doi:10.1128/spectrum.02035-22
16. Zhang P, Wang J, Shi W, et al. In vivo acquisition of bla(KPC-2) with low biological cost in bla(AFM-1)-harboring ST463 hypervirulent pseudomonas aeruginosa from a patient with hematologic malignancy. J Glob Antimicrob Resist. 2022;31:189–195. doi:10.1016/j.jgar.2022.09.004
17. Zhang X, Wang L, Li D, Wang C, Guo Q, Wang M. Characterization of the novel plasmid-encoded MBL gene bla AFM-1, integrated into a bla IMP-45-bearing transposon Tn 6485e in a carbapenem-resistant pseudomonas aeruginosa clinical isolate. J Antimicrob Chemother. 2021;77(1):83–88. doi:10.1093/jac/dkab342
18. Satlin MJ, Simner PJ, Slover CM, Yamano Y, Nagata TD, Portsmouth S. Cefiderocol treatment for patients with multidrug- and carbapenem-resistant pseudomonas aeruginosa infections in the compassionate use program. Antimicrob Agents Chemother. 2023;67(7):e0019423. doi:10.1128/aac.00194-23
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.