Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Successful switch from bilateral brief pulse to right unilateral ultrabrief pulse electroconvulsive therapy after failure to induce seizures
Authors Kawashima H , Kobayashi Y, Suwa T, Murai T, Yoshioka R
Received 17 December 2017
Accepted for publication 17 January 2018
Published 21 February 2018 Volume 2018:14 Pages 607—610
DOI https://doi.org/10.2147/NDT.S160093
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Hirotsugu Kawashima,1 Yuko Kobayashi,1 Taro Suwa,2 Toshiya Murai,2 Ryuichi Yoshioka1
1Department of Psychiatry, Toyooka Hospital, Toyooka, Hyogo, Japan; 2Department of Neuropsychiatry, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Abstract: Inducing adequate therapeutic seizures during electroconvulsive therapy (ECT) is sometimes difficult due to a high seizure threshold, even at the maximum stimulus charge. Previous studies have demonstrated that seizure threshold is lower in patients treated with right unilateral ultrabrief pulse (RUL-UBP) ECT than in those treated with bilateral or brief pulse (BL-BP) ECT. Therefore, switching to RUL-UBP ECT may be beneficial for patients in whom seizure induction is difficult with conventional ECT. In the present report, we discuss the case of a patient suffering from catatonic schizophrenia in whom BL-BP ECT failed to induce seizures at the maximum charge. However, RUL-UBP ECT successfully elicited therapeutic seizures and enabled the patient to achieve complete remission. This case illustrates that, along with other augmentation strategies, RUL-UBP ECT represents an alternative for seizure induction in clinical practice.
Keywords: electroconvulsive therapy, augmentation, ultrabrief pulse, electrode placement, seizure threshold
Introduction
Electroconvulsive therapy (ECT) is a highly effective treatment for mood disorders, schizophrenia, and other psychiatric disorders.1–3 To ensure good clinical outcomes following ECT, adequate therapeutic seizures must be induced using a stimulus intensity that appropriately exceeds seizure threshold (ST).4–6 However, failure to elicit adequate therapeutic seizures is not uncommon, even at the maximum stimulus charge of the device, due to a high ST. In such cases, augmentation strategies including hyperventilation and reducing or changing anesthetic agents are often applied in clinical settings.7 However, there is a compelling need for further strategies.
One such strategy may involve the adjustment of stimulus parameters. Indeed, ST varies depending on stimulus parameters such as pulse width and electrode placement. Previous studies have demonstrated that ST is lower in patients treated via right unilateral ultrabrief pulse (RUL-UBP) ECT than in those treated via bilateral or brief pulse (BL-BP) ECT.8 Therefore, switching to RUL-UBP ECT may be beneficial for patients in whom seizure induction is difficult with conventional ECT.
In this report, we discuss the case of a patient suffering from catatonic schizophrenia in whom BL-BP ECT failed to induce therapeutic seizures at the maximum charge, while RUL-UBP ECT successfully elicited therapeutic seizures. Following the switch to RUL-UBP ECT, the patient achieved complete remission.
Case report
A 41-year-old man was admitted to our institution with acute exacerbation of schizophrenia, which was accompanied by disorganized behavior and psychomotor excitation. Shortly after admission, he developed catatonic stupor: his extremities were severely rigid, and he was verbally unresponsive. He had a history of two catatonic episodes that had occurred 1 and 3 years prior to admission, respectively. On both occasions, diazepam (40–60 mg/d) was ineffective, although he had attained remission after four sessions of BL-BP ECT using a Thymatron System IV (Somatics LLC, Lake Bluff, IL, USA). Therefore, we chose to start an ECT course shortly after completing a pre-ECT workup. Informed consent for treatment was obtained from his mother. He had taken quetiapine (600 mg/d) for 3 years, and the medication was continued orally or through a tube during admission. ECT was administered twice weekly using the same ECT device, with bitemporal electrode placement.
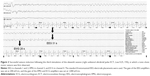
In the first session, anesthesia was induced using 125 mg of thiamylal (2 mg/kg), just as in the previous successful course of ECT, although we were unable to induce a seizure with stimulus settings of 55% (277.2 mC) and 90% (453.6 mC) using bitemporal electrode placement and the preset Low 0.5 program (0.5 ms pulse width). In the following session, we reduced the dose of thiamylal to 50 mg (0.8 mg/kg) and added 100 μg (1.6 μg/kg) of remifentanil. However, stimulus levels of 90%–100% (504 mC) were unable to elicit therapeutic generalized seizures. Although such stimulus levels produced mid-to-high-amplitude slow waves and apparent postictal suppression in the ictal electroencephalogram (EEG), neither motor seizures nor cardiovascular responses were observed (Figure 1). Following the first session, the patient developed aspiration pneumonia because of persistent catatonic dysphagia. The patient was switched from quetiapine to olanzapine (gradually increased from 5 to 20 mg) after the fifth session and received intravenous diazepam (60–80 mg/d) for 4 days after the seventh session, both of which failed to alleviate his catatonic symptoms. In the eighth session, flumazenil (0.5 mg) was administered at the induction of anesthesia to antagonize the anticonvulsant effect of diazepam. However, the seizure responses remained inadequate, and he exhibited no clinical improvement.
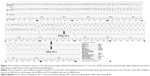
In the eleventh session, we switched to RUL-UBP ECT using the Low 0.25 program (0.25 ms pulse width) and titrated the stimulus dose from 5% (in increments of 5%) to determine the ST. A stimulus level of 15% enabled the induction of seizure activity, which involved tonic-clonic motor responses and was associated with low-to-mid-amplitude slow waves on EEG (Figure 2). In the subsequent sessions, the stimulus was increased to 100% (~6.7 times the ST), which produced adequate therapeutic seizures associated with high-amplitude slow waves and postictal suppression on the ictal EEGs (Figure 3). The patient exhibited clinical improvement: after the 14th session, he regained the ability to speak (albeit haltingly), which revealed the existence of hallucinations and delusions, and after the 15th session, he recovered the ability to eat. He eventually achieved complete remission, and the ECT course was terminated at the twenty-first session. He experienced muscle weakness in the lower extremities and went to a rehabilitation clinic following discharge. He remained in remission with 10 mg of olanzapine. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
To the best of our knowledge, this report is the first to show that switching from conventional to RUL-UBP ECT is effective for patients in whom seizure induction is difficult. While RUL-UBP ECT is an emerging treatment option with evidence of reduced cognitive side effects, it is assumed to be less effective and to require more treatment sessions than conventional ECT.9 It is noteworthy that, in the present case, this “weaker” form of ECT surpassed BL-BP ECT in clinical efficacy, taking advantage of the lower ST.
Sackeim et al8 demonstrated that initial ST is approximately three times higher in patients treated with BP (1.5 ms pulse width) than in those treated with UBP (0.3 ms pulse width), and approximately two times higher in patients treated with BL than in those treated with RUL ECT. In fact, the ST of RUL-UBP ECT is roughly one-sixth of that of BL-BP ECT. Therefore, these findings indicate that seizure induction should be possible in most patients treated using RUL-UBP, even if the ST of conventional ECT exceeds the maximum output of ECT devices.
However, an adequate suprathreshold stimulus is important for ensuring clinical efficacy, not merely seizure induction. The commonly recommended stimulus intensity is 1.5–2.5 times the ST for BL-BP ECT, and 2.5–6 times the ST for right unilateral brief pulse ECT.3 Although the optimal stimulus intensity for RUL-UBP ECT has not been specified, recent studies have generally used an intensity five to eight times the ST.9 Fortunately, in the present case, we were able to elicit a seizure using a 15% stimulus charge by switching to RUL-UBP ECT, following which we administered more than six times the ST. If the ST during RUL-UBP ECT had been more than 20% stimulus charge, even the maximum stimulus charge would have been less than five times the ST, which may have been inadequate. Although this was our concern when considering this strategy, we believed that this result would be preferable to a missed (ie, nongeneralized) seizure, even if the stimulus charge was lower than the recommended dose. Although low-dose RUL ECT is less effective than high-dose treatment, there are indeed some responders.8 Therefore, our findings indicate that RUL-UBP ECT represents a promising strategy when practitioners cannot induce seizures using conventional ECT, even if the initial ST is relatively high.
The patient of the present case required 10 treatment sessions to achieve complete remission after switching to RUL-UBP ECT, although he required only four sessions in the previous courses when BL-BP ECT was used. This finding seems in accordance with the results of a recent meta-analysis, which concluded that RUL-UBP ECT required more treatment sessions than RUL-BP in depressed patients.9 When applying this strategy, practitioners should remain aware of the possibility of a relatively slow response.
Conclusion
Switching to RUL-UBP ECT played a critical role in the present case, as successful treatment could not have been achieved without the adoption of this strategy. While RUL-UBP ECT has gained recent attention solely because of its cognitive advantages, the present case demonstrates that its low ST is also beneficial for patients in whom seizure induction has proven difficult. Our findings illustrate that, along with other augmentation strategies, RUL-UBP ECT represents an alternative for seizure induction that is worthy of further investigation.
Disclosure
The authors report no conflicts of interest in this work.
References
Abrams R. Electroconvulsive Therapy. 4th ed. Oxford, NY: Oxford University Press; 2002. | ||
American Psychiatric Association. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association. American Psychiatric Pub; 2001. Available from: https://www.amazon.com/Practice-Electroconvulsive-Therapy-Recommendations-Privileging/dp/0890422060. Accessed February 15, 2018. | ||
Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical Manual of Electroconvulsive Therapy. Washington, DC: American Psychiatric Publishing; 2010. | ||
Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328(12):839–846. | ||
Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425–434. | ||
McCall W, Reboussin DM, Weiner RD, et al. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch Gen Psychiatry. 2000;57(5):438–444. | ||
Loo C, Simpson B, MacPherson R. Augmentation strategies in electroconvulsive therapy. J ECT. 2010;26(3):202–207. | ||
Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71–83. | ||
Tor PC, Bautovich A, Wang MJ, et al. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression. J Clin Psychiatry. 2015;76(9):e1092–e1098. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.