Back to Journals » Infection and Drug Resistance » Volume 16
Successful Interventional Treatment of Pyopneumothorax Caused by Streptococcus constellatus Associated with Hashimoto’s Thyroiditis: A Case Report and Literature Review
Authors Wang H , Zhou F, Li Z, Ding Y, Wen Q, Tang Q
Received 16 August 2023
Accepted for publication 15 November 2023
Published 11 December 2023 Volume 2023:16 Pages 7581—7586
DOI https://doi.org/10.2147/IDR.S435645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Hongxia Wang,1,* Fating Zhou,2,* Zhilin Li,1 Yulan Ding,1 Qian Wen,1 Quanxing Tang1
1Department of General Practice, People’s Hospital of Deyang City, Deyang, Sichuan, People’s Republic of China; 2Emergency Department, Chongqing Emergency Medical Center, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongxia Wang, Department of General Practice, People’s Hospital of Deyang City, Deyang, Sichuan, People’s Republic of China, Email [email protected]
Background: Streptococcus constellatus rarely causes pyopneumothorax, which is a serious state and requires a surgery. However, not every patient can tolerate surgery and individualized solutions are needed. Furthermore, many known situations are risk factors of S. constellatus infection, but S. constellatus pyopneumothorax associated with Hashimoto’s thyroiditis has not been reported.
Case Presentation: We present the case of a 74-year-old male with multiple encapsulated pyopneumothorax caused by S. constellatus. Given his respiratory failure, we provided two-stage percutaneous right empyema radiography for catheter drainage in the radiology interventional department instead of surgery. Moreover, an occult Hashimoto’s thyroiditis was discovered in the patient, which was possibly associated with S. constellatus pyopneumothorax. Levothyroxine was administered to improve his situation.
Conclusion: To our knowledge, it is the first case described in this context. We provided an alternative treatment for S. constellatus encapsulated pyopneumothorax in patient who might not tolerate surgery. We also revealed the possible relationship between S. constellatus pyopneumothorax and Hashimoto’s thyroiditis.
Keywords: radiology interventional, Streptococcus constellatus, pyopneumothorax, Hashimoto’s thyroiditis
Introduction
Streptococcus constellatus (S. constellatus), a gram-positive and catalase-negative coccus,1 together with Streptococcus intermedius and Streptococcus anginosus, belongs to the Streptococcus anginosus group.2 Although previously thought to be commensal in human body, S. constellatus has been recently considered as a pathogenic bacterium because it can cause bacteremia and serious suppurative infection.3 Pyopneumothorax refers to a collection of pus and air in the pleural cavity, and it is a rare manifestation resulting from S. constellatus, partially presenting as an encapsulated form with separation.1,4 Most of this situation required video-assisted thoracoscopic surgery (VATS) or thoracotomy,5–7 but individualized treatment still needs to be considered.
S. constellatus infection often occurs in patients with underlying diseases such as periodontal disease, diabetes and cancer.3 Hashimoto’s thyroiditis is an autoimmune thyroid disease and manifests as hypothyroidism or hyperthyroidism.8 S. constellatus infection is rare in thyroid diseases especially never found in Hashimoto’s thyroiditis.
Here, we describe a case of multiple encapsulated pyopneumothorax caused by S. constellatus associated with an occult Hashimoto’s thyroiditis. Due to the drainage difficulty and the patient’s weak condition, we chose two-stage interventional treatment instead of surgery and administered levothyroxine for his hypothyroidism.
Case Presentation
A 74-year-old male was admitted to hospital for cough, sputum, and right-sided chest pain for 4 days without fever, hemoptysis, or dyspnea. He had been in good health in the past decades, and he did not receive any treatment before admission. His vital signs showed the following: temperature, 36.8°C; pulse rate, 98 beats per minute; respiratory rate, 20 breaths per minute; blood pressure, 110/59 mmHg; and peripheral oxygen saturation (breathing the atmospheric air), 71%. Physical examination reported decreased breath sounds in the right lung and wet rales in both lungs. There was no abnormality in heart, abdomen and nervous system.
Laboratory results demonstrated the following findings: white blood cell count, 18.64×109/L (normal: 3.5–9.5×109/L); percentage of neutrophils, 80.1% (normal: 40–75%); high-sensitivity C-reactive protein level, 148.80 mg/L (normal: 0.5–10 mg/L). There was no obvious abnormal finding in routine urine and stool examination, liver and kidney function tests, myocardial injury markers, coagulation tests and autoimmune markers. Computed tomography (CT) of the chest demonstrated multiple encapsulated pyopneumothorax in the right pleural cavity and both lungs had scattering pneumonia (Figure 1a and b). After admission, the patient developed slightly apathy but it was not easy to detect. As some patients complicated by thyroid disease might have an apathy manifestation, thyroid function tests were arranged and revealed the following results: thyroid-stimulating hormone (TSH), 23.85 mU/L (normal: 0.27–4.2 mU/L); free thyroxine (FT4), <0.50 pmol/L (normal: 12–22 pmol/L); free triiodothyronine (FT3), <0.40 pmol/L (normal: 3.1–6.8pmol/L); thyroglobulin (TG), <0.04 ng/mL (normal: 3.5–77 ng/mL); thyroid peroxidase antibody (TPOAB), 388.4 IU/mL (normal: <34 IU/mL); anti-thyroglobulin antibodies (TG-AB), >4000 IU/mL (normal: <115 IU/mL).
 |
Figure 1 (a and b) Images of chest-computed tomography scan of the multiple-encapsulated pyopneumothorax in the right hemithorax and pulmonary inflammation at admission. |
On day 1 after admission, he immediately received cefoperazone–sulbactam 2.0 g every 8 hours combined with ornidazole 0.5 g every 12 hours. Continuous oxygen was administered to ameliorate his hypoxemia. Consultation opinion from the cardiothoracic team indicated that a surgery was not suitable due to the patient’s poor condition. Thus, the radiology interventional department performed two-stage percutaneous right empyema radiography on day 2 and day 6 after admission, and two drainage catheters were placed in his right thoracic cavity separately (Figure 2a and b). A large amount of yellow pus and gas accumulation were drained. Bacterial culture of the pus demonstrated the growth of S. constellatus, which was sensitive to penicillin and cephalosporin. The culture results of other bacteria, tuberculosis, or fungi were negative. Meanwhile, Hashimoto’s thyroiditis was clinically considered by the Endocrinologist department, and levothyroxine 25 μg once a day was initially recommended. On day 16 after admission, CT scan showed that the chest image improved significantly (Figure 3a and b) and the patient was finally discharged. After 16 days of intravenous antibiotic treatment, the patient continued to take cefdinir for 4 weeks. During 9 months of follow-up, the patient was in good condition and continued levothyroxine 50 μg once a day since discharge.
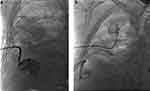 |
Figure 2 (a) Image of interventional therapy for pyopneumothorax on day 2 after admission. (b) Image of interventional therapy for pyopneumothorax on day 6 after admission. |
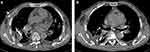 |
Figure 3 (a and b) Images of chest computed tomography scan of the significant resolution of the empyema cavity and pulmonary inflammation. |
Discussion and Conclusion
Pyopneumothorax is defined as an accumulation of pus and gas in the pleural cavity,4 and it is a rare condition.9 Pleural cavity pus is a serious manifestation of lower respiratory tract infection with high morbidity and mortality,2,10 and up to 15% of such patients were in a critical state.11 The main pathogenic microorganisms of pyopneumothorax include Mycobacterium tuberculosis, Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Streptococcus sp.12 Most patients need the combination of antibiotics and surgery, especially VATS or a full thoracotomy with decortication.1,2,13
In recent study, pyopneumothorax caused by S. Constellatus is still a very rare manifestation, and pus is mostly encapsulated, right-sided, accompanied with bilateral pneumonia.1 It is reported that S. constellatus secreted streptolysin S to cause systemic cytotoxicity, induced mitochondrial dysfunction, and it also generated hydrogen sulfide from 1-cysteine through the function of β-cystathionine synthase, which inhibited the killing ability of phagocytes to promote pus and gas formation.14
Pleural cavity drainage of pus needs to be performed on the basis of antibiotic treatment,15 however, it is more difficult to drain encapsulated pus via common chest tube drainage because of septations or loculations.1,15 Therefore, 36–65% of patients with pleural pus frequently need a surgical approach of VATS or thoracotomy.10 In the literature, two published cases of encapsulated pyopneumothorax with septations caused by S. constellatus were treated with thoracotomy and decortication (Table 1).12,13 A pleural tube placement, guided by an imaging technique such as ultrasonography, has been recommended,10,11 providing the best location for drainage, the presence of septations and quantitative echogenicity index on ultrasound.11 But ultrasound images can be deeply affected by gas. Therefore, strong evidence comparing medical and surgical approaches is still lacking now.16,17 In our case of multiple encapsulated pyopneumothorax, two-stage interventional therapy was more accurate to deal with septations, more minimally invasive for adjacent tissue, and it also had real-time monitor function during the whole procedure. This plan was more suitable for this weak patient.
 |
Table 1 Pyopneumothorax Caused by Streptococcus Constellatus in the Literature |
Patients with major underlying diseases, especially leading to a decrease in immune function, were considered to be more susceptible to S. constellatus infection.3 But these common risk factors did not exist in our patient. The patient’s imperceptible apathy, hypothyroidism and endocrine consultation made a diagnosis of Hashimoto’s thyroiditis. Studies have found that the immune system and the hypothalamic-pituitary-thyroid (HPT) axis share a variety of neuropeptides and neurotransmitters, moreover, it is believed that the HPT axis has bidirectional endocrine–immune interactions.18 It has demonstrated that immune cells are the target cells of thyroid hormones, and low thyroid hormone levels have a negative impact on many kinds of immune cells.19 In the literature, only one case has mentioned that a hyperthyroidism woman was in hypothyroidism after thyroidectomy, and she developed S. constellatus pyopneumothorax, but she finally received thoracotomy.12 Hypothyroidism caused by Hashimoto’s thyroiditis reduced the immune function of body. Therefore, we should consider the possible relationship between S. constellatus pyopneumothorax and Hashimoto’s thyroiditis in our case. However, whether there was a causal relationship between the two diseases still needed to be further studied and the immune function changes of patients required dynamic monitoring.
S. constellatus pleural infection is primarily treated with penicillin or cephalosporin, and it is reported that 70% of pleural infection had obligate anaerobic co-infection.11 Obligate anaerobic can inhibit host bactericidal activity, stimulate S. constellatus growth and increase the inflammation virulence of S. constellatus.1,3 Therefore, a plan combined cefoperazone– sulbactam with ornidazole was recommended in our patient.
In conclusion, we recommend to consider the individualized interventional therapy as an alternative to VATS or thoracotomy for S. constellatus encapsulated pyopneumothorax in some weak patients. Moreover, thyroid function should be taken into account in S. constellatus infection.
Abbreviations
CT, computed tomography; FT3, free triiodothyronine; FT4, free thyroxine; HPT, hypothalamic–pituitary–thyroid; S. constellatus, Streptococcus constellatus; TG, thyroglobulin; TG-AB, anti-thyroglobulin antibodies; TPOAB, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone; VATS, video-assisted thoracoscopic surgery.
Data Sharing Statement
All available information is contained within the manuscript.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the People’s Hospital of Deyang City (Date 2021-12-8 /No.2021-04-159-K01).
Consent for Publication
We confirm that the patient agreed to the publication of his personal and clinical details, along with any identifying images. Written informed consent was signed by himself.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lin J, Zhang Y, Bao C, et al. The clinical features and management of empyema caused by streptococcus constellatus. Infect Drug Resist. 2022;15:6267–6277. doi:10.2147/IDR.S382484
2. Lee YJ, Lee J, Kwon BS, et al. An empyema caused by Streptococcus constellatus in an older immunocompetent patient: case report. Medicine. 2021;100(45):e27893. doi:10.1097/MD.0000000000027893
3. Pilarczyk-Zurek M, Sitkiewicz I, Koziel J. The clinical view on streptococcus anginosus group - opportunistic pathogens coming out of hiding. Front Microbiol. 2022;13:956677. doi:10.3389/fmicb.2022.956677
4. Zhang Z, Xiao B, Liang Z. Successful treatment of pyopneumothorax secondary to Streptococcus constellatus infection with linezolid: a case report and review of the literature. J Med Case Rep. 2020;14(1):180. doi:10.1186/s13256-020-02475-w
5. Cai DH, Fang XL. Pyopneumothorax caused by trichomonas tenax and porphyromonas endodontalis coinfection in a patient with previous cerebral infarction: a case report and literature review. Infect Drug Resist. 2022;15:6101–6108. doi:10.2147/IDR.S381859
6. Wu Y, Ye Y, Yang Y, et al. Pyopneumothorax from coinfection by trichomonas tenax and geotrichum capitatum in a child from China: a case report. BMC Infect Dis. 2021;21(1):842. doi:10.1186/s12879-021-06539-0
7. Chrastek D, Hickman S, Sitaranjan D, et al. Streptococcus constellatus causing empyema and sepsis, necessitating early surgical decortication. Case Rep Infect Dis. 2020;2020:4630809. doi:10.1155/2020/4630809
8. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Pol Arch Intern Med. 2022;132:3.
9. Ikematsu Y, Izumi M, Ueno T, et al. Pleural empyema with gas formation caused by mixed infection of edwardsiella tarda with streptococcus constellatus. Respirol Case Rep. 2022;10(3):e0913. doi:10.1002/rcr2.913
10. Sorino C, Mondoni M, Lococo F, et al. Optimizing the management of complicated pleural effusion: from intrapleural agents to surgery. Respir Med. 2022;191:106706. doi:10.1016/j.rmed.2021.106706
11. Hassan M, Patel S, Sadaka AS, et al. Recent insights into the management of pleural infection. Int J Gen Med. 2021;14:3415–3429. doi:10.2147/IJGM.S292705
12. Che Rahim MJ, Mohammad N, Wan Ghazali WS. Pyopneumothorax secondary to Streptococcus milleri infection. BMJ Case Rep. 2016;2016. doi:10.1136/bcr-2016-217537
13. Vulisha AK, Sam R, Nur H, et al. Aggressive Presentation of Streptococcus constellatus. Cureus. 2021;13(4):e14534. doi:10.7759/cureus.14534
14. Pinilla-Monsalve GD, Torres-Cutiva DF, Fernandez-Cubillos JP. Atypical streptococcal meningitis with fatal cerebrovascular complications: a case report. Infect Dis Rep. 2020;12(3):87–96. doi:10.3390/idr12030018
15. Addala DN, Bedawi EO, Rahman NM. Parapneumonic effusion and empyema. Clin Chest Med. 2021;42(4):637–647. doi:10.1016/j.ccm.2021.08.001
16. Porcel JM. Minimally invasive treatment of complicated parapneumonic effusions and empyemas in adults. Clin Respir J. 2018;12(4):1361–1366. doi:10.1111/crj.12730
17. Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev. 2017;3(3):CD010651. doi:10.1002/14651858.CD010651.pub2
18. Klein JR. Dynamic interactions between the immune system and the neuroendocrine system in health and disease. Front Endocrinol. 2021;12:655982. doi:10.3389/fendo.2021.655982
19. van der Spek AH, Fliers E, Boelen A. Thyroid hormone metabolism in innate immune cells. J Endocrinol. 2017;232(2):R67–R81. doi:10.1530/JOE-16-0462
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
