Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Subthreshold Depression: A Systematic Review and Network Meta-Analysis of Non-Pharmacological Interventions
Authors Hao X , Jia Y, Chen J, Zou C, Jiang C
Received 6 July 2023
Accepted for publication 22 September 2023
Published 16 October 2023 Volume 2023:19 Pages 2149—2169
DOI https://doi.org/10.2147/NDT.S425509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xiaofei Hao,1,* Yuying Jia,2,* Jie Chen,2 Chuan Zou,1 Cuinan Jiang3
1Department of General Medicine, Chengdu Fifth People’s Hospital, Chengdu, Sichuan, 611130, People’s Republic of China; 2Department of Outpatient, The General Hospital of Western Theater Command, Chengdu, Sichuan, 610083, People’s Republic of China; 3Department of General Surgery, The Third People’s Hospital of Chengdu & The Affiliated Hospital of Southwest Jiaotong University, Chengdu, Sichuan, 610031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cuinan Jiang, Department of General Surgery, The Third People’s Hospital of Chengdu & The Affiliated Hospital of Southwest Jiaotong University, Chengdu, Sichuan, 610031, People’s Republic of China, Email [email protected]
Background: Subthreshold depression (StD) is considered to be the “precursor” stage of major depressive disorder (MDD), which could cause higher risk of suicide, disease burden and functional impairment. There have been various non-pharmacological interventions for StD. However, the comparison of their effectiveness still lacks sufficient evidence. We performed a systematic review and network meta-analysis to evaluate and rank the efficacy of multiple non-pharmacological interventions targeting StD.
Methods: We conducted a thorough search across various databases including PubMed, Medline, Embase, Web of Science and PsycINFO from inception to December 2022. All included studies were randomized controlled trials (RCTs) of non-pharmacological interventions for patients with StD compared with control group (CG). Several universal scales for measuring depression severity were used as efficacy outcomes. The surface under the cumulative ranking curve (SUCRA) was used to separately rank each intervention using the “Stata 17.0” software.
Results: A total of thirty-six trials were included, involving twenty-eight interventions and 7417 participants. The research found that most non-pharmacological interventions were superior to controls for StD. In each outcome evaluation by different scales for measuring depression, psychotherapy always ranked first in terms of treatment effectiveness, especially Problem-solving Therapy (PST), Behavioral Activation Therapy (BAT), Cognitive Behavioral Therapy (CBT)/Internet-based CBT (I-CBT)/Telephone-based CBT (T-CBT). Since different groups could not be directly compared, the total optimal intervention could not be determined.
Conclusion: Here, we show that psychotherapy may be the better choice for the treatment of StD. This study provides some evidence on StD management selection for clinical workers. However, to establish its intervention effect more conclusively, the content, format and operators of psychotherapy still require extensive exploration to conduct more effective, convenient and cost-effective implementation in primary healthcare. Notably, further research is also urgently needed to find the biological and neural mechanisms of StD by examining whether psychotherapy alters neuroplasticity in patients with StD.
Keywords: subthreshold depression, non-pharmacological intervention, efficacy, comparison, network meta-analysis, systematic review
Introduction
Depression is highly prevalent and ranked third among the global burden of disease by WHO, expected to rise to being the number one ranked disease by 2030.1 This trend poses a substantial challenge for health systems in both developed and developing countries. Whether in the DSM-IV or ICD-10 diagnostic classification system, a diagnosis of major depressive disorder (MDD) requires the time course, number of symptoms and severity to meet certain threshold requirements.
Subthreshold depression (StD) (also called subsyndromal, subclinical or minor depression) was coined by Judd et al2 in 1994 and has been widely accepted by scholars. StD was regarded at a “preclinical” stage of MDD,3 referring to a depressive state with at least two or more depressive symptoms, including mood depression or loss of interest and pleasure, with a duration of at least two weeks, and accompanied by impaired social functioning. This state does not meet criteria for major depressive episodes. In addition, having a higher score than a certain cut-off in self-rated depression scales was also recognized as an alternative definition by researchers.4,5 StD has been a highly prevalent condition, with approximately 2.9%−9.9% incidence rate among adults in primary care, 1.4%−17.2% among adults in community settings,6 and 38.7% among older adults receiving home care.7 And during COVID-19, the proportion of the population meeting the criteria for StD was even as high as 47.8%, suggesting that the epidemic perhaps act as a stressor affecting all population.8 In addition, the state of StD is associated with gender, family status, economic and employment status, disability and impairment associated, health service use and comorbidity,5 the prevalence of StD among patients with diabetes reached 11.6% evaluated by the Patient Health Questionnaire 9-item (PHQ-9).9 Prevention and treatment of cardiovascular diseases also play an important role in preventing depression and other mental disorders.10
The pathophysiological features of depressive disorder are complex. The monoamine hypothesis has prevailed for the pathogenesis of depression, holding that depression is caused by the depletion of 5-HT, norepinephrine, or dopamine in the central nervous system (CNS). The other pathogenesis includes stress, neurotrophins and neurogenesis, excitatory and inhibitory neurotransmission, (epi)genetics, inflammation, the opioid system, myelination, and the gut-brain axis, among others. The neural substrate of depression refers to the abnormalities in neuronal activity and neurotransmitters associated with depression in the brain. Battaglia et al11,12 addressed the role of specific neuropharmacological adjuvants that act on neurochemical synaptic transmission and found that depression can be present with neurotransmitter-related abnormalities, including dysregulation of neurotransmitters such as epinephrine, norepinephrine, and dopamine. In addition, the synaptic plasticity in patients with depression may be affected by abnormal release and reuptake of neurotransmitters, changes in synaptic structure, inflammation and stress response, which in turn affects emotional regulation and cognitive function, and deficits in emotion regulation may also lead to mood disorders.13
Mitochondria are multifunctional organelles which produce cellular energy and play a major role in other cellular functions including homeostasis, cellular signaling, and gene expression, among others. In recent years, researchers have found that mitochondrial abnormalities may lead to disturbances in energy metabolism, which can lead to depressive symptoms. Abnormal mitochondrial function can lead to both an increase in oxidative stress, inflammation and apoptosis, as well as affecting the synthesis and regulation of neurotransmitters, which in turn can affect mood and cognitive function.14,15 Furthermore, Hakamata et al16 found that the blunted interleukin-6 diurnal rhythm predicts depressive symptoms, modulated by amygdala emotional hyporeactivity and gene-stressor interactions. These findings may indicate a potential mechanism underlying vulnerability to depressive disorders, suggesting their early detection, prevention, and treatment through the understanding of immune system dysregulation. To delve into the new neurobiological mechanisms behind neuropathogenesis, stimulation effects, brain responses, researchers have utilized a variety of neuroimaging techniques such as structural and functional magnetic resonance imaging (s/fMRI), electroencephalography (EEG), diffusion tensor imaging (DTI) etc., all of which provide structural, functional, and chemical brain detailed visualizations and measurements of the brain.17–20 The integration of these techniques enables a deeper understanding of the pathophysiology of these disorders and facilitates the development of more effective therapies.
Compared with healthy individuals, StD was associated with a higher risk of suicide, disease burden, and functional impairment.21 Cuijpers et al22 conducted a 6-year longitudinal study on older adults and found that 7.8% of those with StD developed MDD, the rate reached 12% after 3-year follow-up in a Dutch study.23 Early appropriate interventions for StD that can reduce the risk of developing depression have attracted widely attention. Previous meta-analysis indicated that antidepressants did not demonstrate therapeutic advantages for StD over placebos,24 and benzodiazepines were also not determined to their potential therapeutic role.25 Furthermore, according to the Guidelines from the National Institute for Health and Care Excellence (NICE), people with StD should not be recommend pharmacological therapy. Consequently, non-pharmacological treatments currently become important means for StD. Scholars globally were gradually dedicated to exploring in the field. There were various types of methods applied to the treatment of StD, including psychological therapy (ie, cognitive behavioral therapy, problem-solving therapy, behavioral activation, cognitive therapy, mindfulness therapy, etc.), physical activity therapy (ie, aerobic exercise, Chinese Tai Chi, etc.), psychosocial therapy (ie, counseling therapy) and others. In terms of treatment forms, there were not only group face-to-face treatments,26,27 but also self-help or professional guided treatments based on the internet, telephone, email, manual, and so on.28,29
A few previous meta-analyses have evaluated the effectiveness of partial measures for StD. He et al30 allocated non-pharmacological interventions among adults into one of two groups: intervention classes (physical activity, psychosocial intervention, psychotherapy and so on) and individual modalities, and showed that psychotherapy demonstrated statistically significant superiority over conventional treatment. A meta-analysis conducted by Spanish researchers assessed the effectiveness of pure or minimal support self-help interventions based on cognitive, behavioural or cognitive-behavioural treatments in older adults, had further been validated to have short-term therapeutic effects, but the long-term preventive effects were not yet clear.31 Moreover, Cuijpers et al3 first presented evidence showing that psychological interventions had a small to moderate but significant effect on reducing depressive symptoms in adolescents with StD. Jiang et al32 suggested that electroacupuncture or bright light therapy appeared to be the better choices in the treatment of StD.
However, perhaps there were still some differences between trials those included in patient characteristics, study interventions, outcome assessments or study designs, which make comprehensive comparability remain unclear. Therefore, we decided to conduct a new, comprehensive network meta-analysis (NMA) of non-pharmacological interventions to identify and rank the efficacy of multiple therapies for people aged over 12 years old with StD.
Methods
Protocol and Registration
The protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42022355683). It was reported according to Preferred Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (PRISMA).33
Eligibility Criteria
Studies fulfilling the following the “PICOS” inclusion criteria were included in our meta-analysis:
- Population (P): study participants were patients aged over 12 years old with clinically considered StD, described in the Diagnostic and Statistical Manual of Mental Disorders (DSM), International Classification of Diseases (ICD), or Research Diagnostic Criteria (RDC);
- Intervention (I): study participants underwent non-pharmacological interventions, including psychotherapy, physical activity therapy, psychosocial therapy, and others explored;
- Comparison (C): study participants assigned to the control group did not receive active treatment, but only no treatment, treatment as usual (TAU), enhanced TAU, or on the waiting list;
- Outcome (O): the outcome of efficacy will refer to mean overall change on continuous depression severity scales and included study was required to report at least one of the following indicators: Scores of Depression Scale (CES-D), Beck Depression Inventory scale (BDI/BDI-II), the Patient Health Questionnaire-9 (PHQ-9), the Kessler Screening Scale for Psychological Distress (K-6), Hamilton Depression Scale (HAMD/HRSD), Quick Inventory of Depressive symptomatology (QIDS), Geriatric Depression Scale (GDS), Self-rating Depression Scale (SDS);
- Study Design (S): published randomized controlled trials (RCTs) in English.
- Other requirements: articles whose full text could be retrieved and with complete or obtainable data.
Databases and Search Strategy
We systematically searched online the following databases from inception to December 2022 for eligible English-language journal RCTs: PubMed, Medline, Embase, Web of Science, and PsycINFO. Combined with the references or studies of previous studies and systematic reviews, some additional records were manually supplemented. The following key terms were used: (“subthreshold depression” OR “subsyndromal depressive symptoms” OR “subsyndromic depression” OR “subclinical depression” OR “Subsyndromal symptomatic depression” OR “minor depression” OR “StD” OR “SD” OR “SDS”) AND (“randomized clinical trial” OR “randomized controlled clinical trial” OR “RCT”) etc. The search strategy was applied to titles and abstracts. We exported the search results into the reference management software Endnote X9.
Data Extraction
Two researchers independently searched and screened the titles and abstracts of records. Full-text papers were retrieved to determine all potentially eligible studies that met the inclusion criteria. For studies published more than once, we included only the trial with the most complete and informative data. Any ambiguous disagreements were settled by consensus or by a discussion with a third researcher.
The following data were extracted and collected in Microsoft Excel spreadsheets using a standard data extraction form: article title, publication year, first author’s name, setting, sample size, sex distribution, mean age or age range, details of the interventions in each group, mode of intervention (i.e., in-person or digital, group or individual), duration of intervention, outcome data etc.
Assessment of the Risk of Bias in Included Studies
The risk of bias of all included RCTs was assessed by two reviewers independently using the Cochrane Risk Bias tool (By Review Manager 5.4 Software). The indicators of the tool consists of 7 items including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. The judgment results were expressed as low risk of bias, high risk of bias and bias risk uncertain. Disagreements about quality assessment were resolved by discussion until a consensus was reached.
Statistical Analysis
The network meta-analysis was conducted using Stata 17.0 software program to combine direct and indirect evidence of StD. There were several measures of StD, we divided the articles into different groups based on the type of scale and then analyzed each group separately. The effect size (ES) of continuous variables was expressed as a standardized mean difference (SMD) and 95% confidence interval (95% CI). To note that, although 7 articles used the Hamilton scale, their versions were inconsistent including HAMD, HAMD-14, HAMD-17 and HRSD. To avoid bias in research choices and considering similarity of scale types, we included them in a group after discussion and used weighted mean difference (WMD) to represent the ES. We calculated whether there was a significant difference in the inconsistency model. If P>0.05, we chose a consistency model; if P<0.05, an inconsistency model was selected.34 When there was at least one closed loop in the evidence network (refers to a comparison of multiple interventions included in the study and forming a closed loop), local inconsistency test (node-splitting method) and loop inconsistency were required. We obtained the rank of each intervention through the surface under the cumulative ranking (SUCRA), with 0≤SUCRA≤100% (or represented as 0≤SUCRA≤1), the higher the SUCRA value means the better the rank.35 Performing sensitivity analyses to evaluate the stability of the results, and assessing publication bias in interventions using a funnel plot.
Results
Identification of Studies
A total of 26,876 records were initially searched, with 19,640 remained after eliminating duplicates. Their titles or abstracts were screened for this systematic review and 4515 articles remained for full-text review. We finally included 36 eligible articles in this systematic review based on the inclusion criteria. The PRISMA flowchart of detailed study selection process is shown in Figure 1.
 |
Figure 1 Flow diagram for search and selection of the included studies. |
Study Characteristics
The studies were published from 2001 to 2022, and a total of 36 trials were included involving 7417 participants, with 52.22% (n=3873) receiving non-pharmacological interventions, in which 47.78% (n=3544) receiving control treatment. The characteristics of all included studies are summarized in Table 1.
 |
Table 1 Characteristics of Included Trials (n=36) Ordered Chronologically |
Population: The age of study participants ranged approximately from 12 to 90 years old, 65.86% of them were female (4 articles with unclear or not obtainable gender information). The included RCTs were conducted in China (n=7), 36–39,44,45,47 the United States (n=5), 27,52,58,62,65 Japan (n=5), 40,46,48,57,59 the Netherlands (n=5), 50,61,63,64,67 Germany (n=4), 29,51,55,56 Australia (n=3), 28,43,49 Columbia (n=2), 60,66 Thailand (n=1), 41 Croatia (n=1), 54 Spain (n=1), 42 Canada (n=1)26 and Britain (n=1).53 Most studies were two-arm trials (n=32), and remaining were three-arm trials (n=4). The diagnostic criteria for StD are not unified among those studies. According to the statistics, there were 25 studies combining severity rating scales with interviews (to exclude MDD or MDE) to get the diagnose of StD,2 articles combining symptoms with interviews, 7 articles only using scales, and 2 articles single relying on symptoms.
Interventions: In total, there were 28 different non-pharmacological interventions, summarized into 7 categories, including psychotherapy (n=30), physical activity therapy (n=2), psychosocial therapy (n=3), Feldenkrais (n=1), collaboration therapy (n=2), stepped therapy (n=1) video-viewing therapy (n=1). The network plot of all interventions is shown in Figure 2.
Control group: A total of 36 RCTs utilized non-active control conditions, including waitlist (WL; n=3), treatment as usual (TAU; n=20), enhanced TAU (ETAU=10; ie, education, discussion, etc.) and TAU before WL (n=2).
Types of outcome assessment: We found that 15 studies used CES-D (n=2), 12 studies used BDI/BDI-II, 9 studies used PHQ-9, 7 studies used Hamilton Depression Scale HAMD/HRSD, 3 studies used K-6 2 studies used QIDS, 2 studies used GDS and 2 studies used SDS.
Risk of Bias Assessment
Most studies presented a low risk in the randomization process and allocation concealment. There were 11 (30.6%) studies rated as having a high risk of bias for ‘blinding of participants and personnel; this is, though, typically unavoidable in studies of psychological interventions. There were 2 studies reported a high risk of bias for ‘blinding of outcome assessment (the residents in the treating group occasionally revealed their participation in group to the raters, thus introducing potential bias among assessors), and this was rated as unclear in 13 studies (36.1%). The risk of attrition bias was low: only two studies did not address incomplete data. Three studies were rated as having a high risk of selective reporting bias. None of the studies reported a high risk of bias for other biases. All studies presented a low risk in the attrition bias and reporting bias. In terms of other bias, most included studies are assessed as low risk. The results of the risk bias assessment are shown in Figure 3.
 |
Figure 3 (A) Risk of bias summary: each risk of bias item for each included study. (B) Risk of bias graph: each risk of bias item presented as percentage. |
Network Meta-Analysis
CES-D
A total of 15 studies reported the results of CES-D, 2 three-arm trials and 13 two-arm trials, with 14 interventions included. The evidence network plot presented two closed loops (see Figure 4). We conducted a global inconsistency test (P=0.84), a local inconsistency test using node-splitting method (P>0.05) and a loop inconsistency (95% CrI included 0), all showing no significant difference between direct and indirect comparisons. Therefore, a consistency model was selected for analysis. We found that most of the treatments show better efficacy on CES-D compared with CG alone. Interventions with significant statistical differences include: PST (MD−12.30, 95% CrI −20.89, −3.71) (P=0.005), I-CBT (MD −7.94, 95% CrI −13.59, −2.29) (P=0.006), CBT (MD−6.51, 95% CrI −10.72, −2.30) (P=0.002). The SUCRA of CES-D is in order from large to small: PST (90%)>I-CBT (74%)>IPT (68%)>CBT (66%)>G-CT (61%)>Feldenkrais (59%)>G-CBT (44%)>VISA-PWS (42%)>BAT (36%)>PE (26%)>BB-CBT (24)>CG (20%) (see Figure 4).
BDI-II and BDI
There were 10 studies involving 11 interventions using BDI-II for the outcome assessment, 2 three-arm trials and 8 two-arm trials. The node cleavage method showed significant difference between direct and indirect comparisons (P=0.04). Thus, we chose an inconsistency model. According to statistics, PST+BAT (MD −9.04, 95% CrI −15.84, −2.24), ICBT (MD −7.04, 95% CrI −12.21, −1.87), BAT (MD −6.24 95% CrI −11.38, −1.10), CBT (MD −5.74, 95% CrI −10.91, −0.57), T-CBT (MD −5.36, 95% CrI −10.41, −0.31) had positive effects relative to the control group (P<0.05). In addition, the SUCRA of BDI-II is ordered sequentially: PST+BAT (92%)>I-CBT (83%)>BAT (74%)>CBT (67%)>T-CBT (63%)>BAM (51%)>G-CBT (44%)>DI (29%)>MBSR (28%)>CG (12%)>NI (%)> (8%). The network plot and ranking results are presented in Figure 5A.
Figure 5 Continued.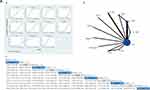
For BDI, 2 studies and 3 interventions were included to evaluate the outcome. The consistency model was used due to inconsistency test result showing no significant difference (P>0.05). From the direct evidence, when compared to CG, CBT (MD −6.10, 95% CrI −9.45, −2.75) (P<0.05) had positive effects, whereas PST (MD −2.30, 95% CrI −6.74, 2.14) (P>0.05) did not. The forest plot and ranking results are presented in Figure 5B.
PHQ-9
To evaluate the PHQ-9 outcome, 9 studies and 10 interventions were included. From the network plot of Figure 6, we found that I-CBT (MD −3.90, 95% CrI −4.51, −3.29), AE (MD −3.07, 95% CrI −3.98, −2.16), MTCC (MD −2.50, 95% CrI −3.60, −1.40), CBT (MD −2.20, 95% CrI −2.79, −1.61), IT-CBT (MD−1.62, 95% CrI −2.46, −0.78), CASPER (MD−1.50, 95% CrI −2.13, −0.87), CBM (MD−1.14, 95% CrI −2.11, −0.17), EBT (MD −0.80, 95% CrI −1.26, −0.34) had better effects (P<0.05) compared to the CG, however STEPEED (MD −0.43, 95% CrI −1.45, 0.59) (P>0.05) did not. The SUCRA of PHQ-9 is as followed: I-CBT (99%)>AE (87%)>MTCC (74%)>CBT (68%)>IT-CBT (50%) >CASPER (47%) >CBM (35%)>EBT (23%)>STEPEED (14%)>CG (2%) (see Figure 6).
HAMD/HRSD
A total of 7 RCTs assessed depression severity for StD in this group, including 3 using HAMD-17, 2 usingHAMD-14, 1 using HAMD and 2 using HRSD. There was no loop structure (see Figure 7) and consistency model was selected (P>0.05). Among 7 interventions, BAT (SMD −0.95, 95% CrI −1.46, −0.43) and IT-CBT (SMD −0.35, 95% CrI −0.58, −0.12) showed better effectiveness compared to CG (P<0.05). No statistical difference was observed in groups of PST+BAT, T-IPC, G-CT and PST+BT. As ranking results and forest plot showed in Figure 7, BAT was the intervention with the highest ranking, with SUCRA 91%.
K6, GDS, SDS, QIDS
There were 3 trials for outcome evaluation with K-6 and using inconsistency model (P<0.05), 2 trials each with GDS, SDS and QIDS, all using consistency model (P>0.05). In the K6 group, T-CBT (MD−3.1, 95% CrI −4.85, −1.35) showed great effectiveness compared to CG and has the highest ranking (SUCRA, 95%). In the GDS group, BAT (MD−3.06, 95% CrI −4.25, −1.87) and G-CBT (MD −3.01, 95% CrI −4.18, −1.84) showed better effectiveness and BAT was the intervention with the highest ranking (SUCRA, 77%), G-CBT the next-highest ranking (SUCRA, 73%). We found no significant difference both in SDS and QIDS group. The ranking results are summarized in Figure 8A and forest map in Figure 8B.
Figure 8 Continued.
Publication Bias and Sensitivity Analysis
When the effect size of each indicator was taken as the abscissa and the standard error as the ordinate-corrected funnel plot (see Figure 9). Each dot represented a direct comparison of different interventions, and the number of points of the same color represented the number of pairwise comparisons in the study. The results showed that all studies were basically distributed on both sides of the midline in the middle, the left and right distribution was roughly symmetrical, suggesting publication bias risk low. There was a few studies deviated far from the regression line, suggesting that there may be small sample studies. Sensitivity analysis was performed by sequentially omitting one study and our studies showed stable.
Discussion
In this network meta-analysis, we compared the effectiveness of 28 non-pharmacological interventions in changing the severity of depression applied 9 kinds of scale. Our study showed evidence that most non-pharmacological treatments for patients with StD had advantages over the control treatment. Furthermore, we found that PST was the best therapy to improve outcome on the CES-D, similarly PST+BAT on the BDI-II, CBT on the BDI, I-CBT on the PHQ-9, BAT on the HAMD/HRSD and GDS, and T-CBT on the K-6.
It’s not difficult to notice that the most effective interventions in each group are all classified as psychotherapy. Based on the above evidence findings, we speculated that psychotherapy especially may have the best efficacy for StD, which is consistent with a previous network meta-analysis showing that psychotherapy especially CBT, may be the most effective intervention for StD.30 They compared all included interventions together, the effect size was expressed as SMD because of the consistency of the scales, so there were differences in statistical methods between us. The effectiveness of psychotherapy in depression has been extensively validated in previous studies among adults, the elderly.68,69 In addition, Cuijpers et al3 found that interventions for StD may have positive acute effects in adolescents. Their team also reported that the types of therapy that have been examined best in primary care settings are CBT, BAT, IPT and PST.70 This has also been mostly validated in our research showing that PST, BAT, CBT, I-CBT, and T-CBT performing better effectiveness for StD.
Kasckow et al58 conducted a pilot study suggesting that a six-to-eight session version of PST may produce improvements in mental health functioning in primary care veterans with StD, which the sessions of PST focused on seven steps: (1) defining the problem; (2) setting a realistic goal; (3) “brainstorming” on potential solutions; (4) considering the advantages and disadvantages of each solution; (5) choosing the best solution; (6) developing an action plan; and (7) reviewing progress. Similar courses have also been implemented in other studies, PST seems well suited for medically ill the elderly given its collaborative and problem-centered approach to daily living with stressful chronic medical conditions.62 However, the intervention effects were preserved until 1-year but not until 8-year follow-up, may indicating its difficult to achieve long-term prevention and necessary to conduct systematic booster sessions.42
BAT is derived from the behavioral model, which was practiced to play an important role in reducing depressive symptoms and increasing behavioral activation for stroke individuals with StD.38 For patients with cognitive and communication impairments, BAT may be an option to consider, which mainly facilitates activities much simpler or even more cost-effective.41
CBT, as an widely used individual intervention, had been demonstrated the superiority in treating mental problems. It was described that CBT could modify dysfunctional thoughts, core beliefs and information processing biases, which are preconditions and risk factors for depression.71 According to our study, I-CBT was the second highest intervention on both the CES-D and BDI-II, others also reported its greater reductions on all the outcomes compared to the WL group at post-intervention.72 I-CBT is an online intervention with many advantages, including overcoming some limitations of face-to-face cognitive-behavioral therapy, 73 being conducted anytime and anywhere, having relatively lower costs, providing privacy protection, 72 helping patients avoid the stigma incurred by seeing a therapist.74 Compared to I-CBT, T-CBT is also a remote CBT but needs to be administered by trained psychotherapists rather than self-help. Digital-based interventions are probably more easily disseminated on a large scale, implying that they save a lot of medical resources and costs.
Over the recent years, neuroimaging studies have identified structural and functional brain changes in patients with depression, including volume reductions in cortical and subcortical structures,75,76 reduced gray matter volume throughout the brain, enlarged lateral ventricles, and white matter microstructural differences.75,77,78 Synaptic activity plays an important role in the network pathways that emerge from cognitive, emotional, and behavioral functions. Fries et al summarize relatively comprehensively the six major hypotheses for the pathogenesis of depression, elucidating how signaling pathways and molecular systems interact in depression and how each pathway or system relates to synaptic transmission.79
There has been a gradual increase in the emphasis on neuroplasticity. It refers to the ability of the nervous system to change its own loops, such as changes in the morphologic structure and functional activity of the central nervous system and peripheral nervous system, i.e., which can readily change the process of information processing. Impaired structural and functional plasticity of neurons in brain regions related to the control of emotions, could in turn affects the clinical manifestations of depression. Based on this, some researchers have proposed the precise modulation of neuroplasticity pathways as a new strategy for antidepressant treatment. Well-designed computerized cognitive training a resulting in activation of the medial prefrontal cortex (mPFC) and improve neural dysfunction in a serious behavioral deficit in schizophrenia.80 The process of synaptic plasticity as LTP-like hippocampal-dependent memory is maintained by fine-tuning mechanisms that control the balance of excitatory and inhibitory neurotransmission.81 Chung et al82 investigated whether cognitive control training (CCT) persistently alters hippocampal neural circuit function. They show that 1) CCT facilitated learning of novel tasks in a new environment for several weeks relative to unconditioned control mice and control mice that avoided the same location when interference was reduced. 2) CCT rapidly altered the function of the entorhinal olfactory cortex-dentate gyrus synaptic circuits resulting in excitatory-inhibitory subcircuitry alterations that persisted for months. 3) CCT increased inhibition by attenuating the dentate response to inputs from the medial entorhinal cortex and, by de-inhibition, by augmenting the response to strong inputs, suggesting an enhanced overall signal-to-noise ratio. These neurobiological perspectives support the neuroplasticity hypothesis that CCT consistently optimizes information processing in neural circuits in addition to storing item-event associations.
However, the effect of physical activity therapy, psychosocial therapy, stepped therapy and others explored did not show the best therapeutic effects. We are mainly considering that the proportion of articles included is small and the statistical power may be insufficient due to the limited sample size. Whether non-psychotherapy has a unique benefit for treatment of StD requires further research.
Limitations
Admittedly, there were some limitations in this network meta-analysis. (1) Many RCTs use inconsistent scales, which limited the comprehensive evaluation of the efficacy of all non-pharmacological interventions included. (2) The studies applying non-psychotherapy interventions are relatively insufficient, more multi-center and high-quality RCTs are needed in the future. (3) Most articles we included were of short-term duration and long-term effects that needed further study. (4) The study languages were limited to English, which may have led to language bias and miss other treatments with national characteristics.
Conclusion
The network meta-analysis showed that psychotherapy may be the most effective intervention for StD compared to other interventions, especially PST, BAT, CBT, Digital-based CBT. This study provides some evidence on StD management selection for clinical workers. However, to establish its intervention effect more conclusively, the content, format and operators of psychotherapy still require extensive exploration to conduct more effective, convenient, and cost-effective implementation in primary healthcare. Considering the limitations of this study, notably, further research is also urgently needed to find the biological and neural mechanisms of StD by examining whether psychotherapy alters neuroplasticity in patients with StD.
Data Sharing Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Acknowledgments
We recognize effort and great work from all team members and appreciate project coordinators, investigators, and data operators.
Author Contributions
All authors made a substantial contribution to the report conception, study design, acquisition of data, execution, analysis and interpretation of data; took part in drafting and revising the article critically; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the Research Project of Sichuan Provincial Health Commission (20PJ215).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psychiatr Res. 2020; 126: 134–140. doi: 10.1016/j.jpsychires.2019.08.002
2. Judd DLL. Subsyndromal symptomatic depression. CNS Drugs. 1994; 1: 399–404. doi: 10.2165/00023210-199401060-00001
3. Cuijpers P, Pineda BS, Ng MY, et al. A meta-analytic review: psychological treatment of subthreshold depression in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2021; 60 (9): 1072–1084. doi: 10.1016/j.jaac.2020.11.024
4. Cuijpers P, Koole SL, van Dijke A, Roca M, Li J, Reynolds CF. Psychotherapy for subclinical depression: meta-analysis. Br J Psychiatry. 2014; 205 (4): 268–274. doi: 10.1192/bjp.bp.113.138784
5. Rodríguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012; 12: 181. doi: 10.1186/1471-244X-12-181
6. Lee YY, Stockings EA, Harris MG, et al. The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychol Med. 2019; 49 (1): 92–102. doi: 10.1017/S0033291718000557
7. Xiang X, Leggett A, Himle JA, Kales HC. Major depression and subthreshold depression among older adults receiving home care. Am J Geriatr Psychiatry. 2018; 26 (9): 939–949. doi: 10.1016/j.jagp.2018.05.001
8. Liao YH, Fan BF, Zhang HM, et al. The impact of COVID-19 on subthreshold depressive symptoms: a longitudinal study. Epidemiol Psychiatr Sci. 2021; 30: e20. doi: 10.1017/S2045796021000044
9. Wang D, Shi L, Li L, et al. Subthreshold depression among diabetes patients in Beijing: cross-sectional associations among sociodemographic, clinical, and behavior factors. J Affect Disord. 2018; 237: 80–86. doi: 10.1016/j.jad.2018.05.016
10. Sobolewska-Nowak J, Wachowska K, Nowak A, et al. Exploring the heart-mind connection: unraveling the shared pathways between depression and cardiovascular diseases. Biomedicines. 2023; 11 (7): 1903. doi: 10.3390/biomedicines11071903
11. Battaglia S, Di Fazio C, Vicario CM, Avenanti A. Neuropharmacological Modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: synaptic transmission and plasticity. Int J Mol Sci. 2023; 24 (6): 5926. doi: 10.3390/ijms24065926
12. Battaglia S, Nazzi C, Thayer JF. Fear-induced bradycardia in mental disorders: foundations, current advances, future perspectives. Neurosci Biobehav Rev. 2023; 149: 105163. doi: 10.1016/j.neubiorev.2023.105163
13. Battaglia S, Cardellicchio P, Di Fazio C, Nazzi C, Fracasso A, Borgomaneri S. Stopping in (e)motion: reactive action inhibition when facing valence-independent emotional stimuli. Front Behav Neurosci. 2022; 16: 998714. doi: 10.3389/fnbeh.2022.998714
14. Tanaka M, Á S, Spekker E, Polyák H, Tóth F, Vécsei L. Mitochondrial impairment: a common motif in neuropsychiatric presentation? The link to the tryptophan-kynurenine metabolic system. Cells. 2022; 11 (16): 2607. doi: 10.3390/cells11162607
15. Tanaka M, Szabó Á, Vécsei L. Preclinical modeling in depression and anxiety: current challenges and future research directions. Adv Clin Exp Med. 2023; 32 (5): 505–509. doi: 10.17219/acem/165944
16. Hakamata Y, Hori H, Mizukami S, et al. Blunted diurnal interleukin-6 rhythm is associated with amygdala emotional hyporeactivity and depression: a modulating role of gene-stressor interactions. Front Psychiatry. 2023; 14: 1196235. doi: 10.3389/fpsyt.2023.1196235
17. Tanaka M, Diano M, Battaglia S. Editorial: insights into structural and functional organization of the brain: evidence from neuroimaging and non-invasive brain stimulation techniques. Front Psychiatry. 2023; 14: 1225755. doi: 10.3389/fpsyt.2023.1225755
18. Bellini H, Cretaz E, Carneiro AM, et al. Magnetic Waves vs. Electric Shocks: a Non-Inferiority Study of Magnetic Seizure Therapy and Electroconvulsive Therapy in Treatment-Resistant Depression. Biomedicines. 2023; 11 (8): 2150. doi: 10.3390/biomedicines11082150
19. Battaglia S, Schmidt A, Hassel S, Tanaka M. Editorial: case reports in neuroimaging and stimulation. Front Psychiatry. 2023; 14: 1264669. doi: 10.3389/fpsyt.2023.1264669
20. Liu M, Xie X, Xie J, et al. Early-onset Alzheimer’s disease with depression as the first symptom: a case report with literature review. Front Psychiatry. 2023; 14: 1192562. doi: 10.3389/fpsyt.2023.1192562
21. Balázs J, Miklósi M, Keresztény A, et al. Adolescent subthreshold-depression and anxiety: psychopathology, functional impairment and increased suicide risk. J Child Psychol Psychiatry. 2013; 54 (6): 670–677. doi: 10.1111/jcpp.12016
22. Cuijpers P, Beekman A, Smit F, Deeg D. Predicting the onset of major depressive disorder and dysthymia in older adults with subthreshold depression: a community based study. Int J Geriatr Psychiatry. 2006; 21 (9): 811–818. doi: 10.1002/gps.1565
23. Tuithof M, Ten Have M, van Dorsselaer S, Kleinjan M, Beekman A, de Graaf R. Course of subthreshold depression into a depressive disorder and its risk factors. J Affect Disord. 2018; 241: 206–215. doi: 10.1016/j.jad.2018.08.010
24. Cameron IM, Reid IC, MacGillivray SA. Efficacy and tolerability of antidepressants for sub-threshold depression and for mild major depressive disorder. J Affect Disord. 2014; 166: 48–58. doi: 10.1016/j.jad.2014.04.078
25. Barbui C, Cipriani A, Patel V, Ayuso-Mateos JL, van Ommeren M. Efficacy of antidepressants and benzodiazepines in minor depression: systematic review and meta-analysis. Br J Psychiatry. 2011; 198 (1): 11. doi: 10.1192/bjp.bp.109.076448
26. Konnert C, Dobson K, Stelmach L. The prevention of depression in nursing home residents: a randomized clinical trial of cognitive-behavioral therapy. Aging Ment Health. 2009; 13 (2): 288–299. doi: 10.1080/13607860802380672
27. Clarke GN, Hornbrook M, Lynch F, et al. A randomized trial of a group cognitive intervention for preventing depression in adolescent offspring of depressed parents. Arch Gen Psychiatry. 2001; 58 (12): 1127–1134. doi: 10.1001/archpsyc.58.12.1127
28. Morgan AJ, Jorm AF, Mackinnon AJ. Email-based promotion of self-help for subthreshold depression: mood Memos randomised controlled trial. Br J Psychiatry. 2012; 200 (5): 412–418. doi: 10.1192/bjp.bp.111.101394
29. Sander LB, Paganini S, Terhorst Y, et al. Effectiveness of a guided web-based self-help intervention to prevent depression in patients with persistent back pain: the PROD-BP randomized clinical trial. JAMA Psychiatry. 2020; 77 (10): 1001–1011. doi: 10.1001/jamapsychiatry.2020.1021
30. He R, Wei J, Huang K, et al. Nonpharmacological interventions for subthreshold depression in adults: a systematic review and network meta-analysis. Psychiatry Res. 2022; 317: 114897. doi: 10.1016/j.psychres.2022.114897
31. Corpas J, Gilbody S, McMillan D. Cognitive, behavioural or cognitive-behavioural self-help interventions for subclinical depression in older adults: a systematic review and meta-analysis. J Affect Disord. 2022; 308: 384–390. doi: 10.1016/j.jad.2022.04.085
32. Jiang X, Luo Y, Chen Y, et al. Comparative efficacy of multiple therapies for the treatment of patients with subthreshold depression: a systematic review and network meta-analysis. Front Behav Neurosci. 2021; 15: 755547. doi: 10.3389/fnbeh.2021.755547
33. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015; 162 (11): 777–784. doi: 10.7326/M14-2385
34. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010; 29 (7–8): 932–944. doi: 10.1002/sim.3767
35. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011; 64 (2): 163–171. doi: 10.1016/j.jclinepi.2010.03.016
36. Ying Y, Ji Y, Kong F, et al. Efficacy of an internet-based cognitive behavioral therapy for subthreshold depression among Chinese adults: a randomized controlled trial. Psychol Med. 2022: 1–11. doi: 10.1017/S0033291722000599
37. Wang R, Cai Y, Lu W, et al. Exercise effect on the gut microbiota in young adolescents with subthreshold depression: a randomized psychoeducation-controlled Trial. Psychiatry Res. 2023; 319: 115005. doi: 10.1016/j.psychres.2022.115005
38. Sun Q, Xu H, Zhang W, Zhou Y, Lv Y. Behavioral activation therapy for subthreshold depression in stroke patients: an exploratory randomized controlled trial. Neuropsychiatr Dis Treat. 2022; 18: 2795–2805. doi: 10.2147/NDT.S392403
39. Au A, Nan H, Sum R, Ng F, Kwong A, Wong S. Cognitive behavioural therapy for adherence and sub-clinical depression in type 2 diabetes: a randomised controlled trial (abridged secondary publication). Hong Kong Med J. 2022; 28 (3): 21–23.
40. Kageyama K, Kato Y, Mesaki T, et al. Effects of video viewing smartphone application intervention involving positive word stimulation in people with subthreshold depression: a pilot randomized controlled trial. J Affect Disord. 2021; 282: 74–81. doi: 10.1016/j.jad.2020.12.104
41. Saisanan Na Ayudhaya W, Pityaratstian N, Jiamjarasrangsi W. Effectiveness of behavioral activation in treating Thai older adults with subthreshold depression residing in the community. Clin Interv Aging. 2020; 15: 2363–2374. doi: 10.2147/CIA.S274262
42. López L, Smit F, Cuijpers P, et al. Problem-solving intervention to prevent depression in non-professional caregivers: a randomized controlled trial with 8 years of follow-up. Psychol Med. 2020; 50 (6): 1002–1009. doi: 10.1017/S0033291719000916
43. Basanovic J, Grafton B, Ford A, et al. Cognitive bias modification to prevent depression (COPE): results of a randomised controlled trial. Psychol Med. 2020; 50 (15): 2514–2525. doi: 10.1017/S0033291719002599
44. Zhang JY, Ji XZ, Meng LN, Cai YJ. effects of modified mindfulness-based stress reduction (MBSR) on the psychological health of adolescents with subthreshold depression: a randomized controlled trial. Neuropsychiatr Dis Treat. 2019; 15: 2695–2704. doi: 10.2147/NDT.S216401
45. Zhang J, Qin S, Zhou Y, Meng L, Su H, Zhao S. A randomized controlled trial of mindfulness-based Tai Chi Chuan for subthreshold depression adolescents. Neuropsychiatr Dis Treat. 2018; 14: 2313–2321. doi: 10.2147/NDT.S173255
46. Yamamoto A, Tsujimoto E, Taketani R, Tsujii N, Shirakawa O, Ono H. The effect of interpersonal counseling for subthreshold depression in undergraduates: an exploratory randomized controlled trial. Depress Res Treat. 2018; 2018: 4201897. doi: 10.1155/2018/4201897
47. Wong SYS, Sun YY, Chan ATY, et al. Treating subthreshold depression in primary care: a randomized controlled trial of behavioral activation with mindfulness. Ann Fam Med. 2018; 16 (2): 111–119. doi: 10.1370/afm.2206
48. Takagaki K, Okamoto Y, Jinnin R, et al. Enduring effects of a 5-week behavioral activation program for subthreshold depression among late adolescents: an exploratory randomized controlled trial. Neuropsychiatr Dis Treat. 2018; 14: 2633–2641. doi: 10.2147/NDT.S172385
49. Singhal M, Munivenkatappa M, Kommu JVS, Philip M. Efficacy of an indicated intervention program for Indian adolescents with subclinical depression. Asian J Psychiatr. 2018; 33: 99–104. doi: 10.1016/j.ajp.2018.03.007
50. Pols AD, Adriaanse MC, van Tulder MW, et al. Two-year effectiveness of a stepped-care depression prevention intervention and predictors of incident depression in primary care patients with diabetes type 2 and/or coronary heart disease and subthreshold depression: data from the Step-Dep cluster randomised controlled trial. BMJ Open. 2018; 8 (10): e020412. doi: 10.1136/bmjopen-2017-020412
51. Ebert DD, Buntrock C, Lehr D, et al. Effectiveness of web- and mobile-based treatment of subthreshold depression with adherence-focused guidance: a single-blind randomized controlled trial. Behav Ther. 2018; 49 (1): 71–83. doi: 10.1016/j.beth.2017.05.004
52. Pan D, Huey SJ, Heflin LH. Ethnic differences in response to directive vs. non-directive brief intervention for subsyndromal depression. Psychother Res. 2019; 29 (2): 186–197. doi: 10.1080/10503307.2017.1325023
53. Lewis H, Adamson J, Atherton K, et al. Coll Aborative care and active surveillance for Screen-Positive EldeRs with subthreshold depression (CASPER): a multicentred randomised controlled trial of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2017; 21 (8): 1–196. doi: 10.3310/hta21080
54. Pibernik-Okanović M, Hermanns N, Ajduković D, et al. Does treatment of subsyndromal depression improve depression-related and diabetes-related outcomes? A randomised controlled comparison of psychoeducation, physical exercise and enhanced treatment as usual. Trials. 2015; 16: 305. doi: 10.1186/s13063-015-0833-8
55. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015; 38 (4): 551–560. doi: 10.2337/dc14-1416
56. Buntrock C, Ebert D, Lehr D, et al. Effectiveness of a web-based cognitive behavioural intervention for subthreshold depression: pragmatic randomised controlled trial. Psychother Psychosom. 2015; 84 (6): 348–358. doi: 10.1159/000438673
57. Imamura K, Kawakami N, Furukawa TA, et al. Effects of an Internet-based cognitive behavioral therapy (iCBT) program in Manga format on improving subthreshold depressive symptoms among healthy workers: a randomized controlled trial. PLoS One. 2014; 9 (5): e97167. doi: 10.1371/journal.pone.0097167
58. Kasckow J, Klaus J, Morse J, et al. Using problem solving therapy to treat veterans with subsyndromal depression: a pilot study. Int J Geriatr Psychiatry. 2014; 29 (12): 1255–1261. doi: 10.1002/gps.4105
59. Furukawa TA, Horikoshi M, Kawakami N, et al. Telephone cognitive-behavioral therapy for subthreshold depression and presenteeism in workplace: a randomized controlled trial. PLoS One. 2012; 7 (4): e35330. doi: 10.1371/journal.pone.0035330
60. Ullmann G, Williams HG. Can Feldenkrais exercises ameliorate subclinical depressive symptoms in older adults? A pilot study. J S C Med Assoc. 2011; 107: 7–10.
61. Joling KJ, van Hout HP, Van’t Veer-Tazelaar PJ, et al. How effective is bibliotherapy for very old adults with subthreshold depression? A randomized controlled trial. Am J Geriatr Psychiatry. 2011; 19 (3): 256–265. doi: 10.1097/JGP.0b013e3181ec8859
62. Gellis ZD, Bruce ML. Problem solving therapy for subthreshold depression in home healthcare patients with cardiovascular disease. Am J Geriatr Psychiatry. 2010; 18 (6): 464–474. doi: 10.1097/jgp.0b013e3181b21442
63. Spek V, Nyklícek I, Smits N, et al. Internet-based cognitive behavioural therapy for subthreshold depression in people over 50 years old: a randomized controlled clinical trial. Psychol Med. 2007; 37 (12): 1797–1806. doi: 10.1017/S0033291707000542
64. Allart-van Dam E, Hosman CM, Hoogduin CA, Schaap CP. Prevention of depression in subclinically depressed adults: follow-up effects on the ‘Coping with Depression’ course. J Affect Disord. 2007; 97 (1–3): 219–228. doi: 10.1016/j.jad.2006.06.020
65. Young JF, Mufson L, Davies M. Efficacy of interpersonal psychotherapy-adolescent skills training: an indicated preventive intervention for depression. J Child Psychol Psychiatry. 2006; 47 (12): 1254–1262. doi: 10.1111/j.1469-7610.2006.01667.x
66. Neugebauer R, Kline J, Markowitz JC, et al. Pilot randomized controlled trial of interpersonal counseling for subsyndromal depression following miscarriage. J Clin Psychiatry. 2006; 67 (8): 1299–1304. doi: 10.4088/jcp.v67n0819
67. Willemse GR, Smit F, Cuijpers P, Tiemens BG. Minimal-contact psychotherapy for sub-threshold depression in primary care. Randomised trial. Br J Psychiatry. 2004; 185: 416–421. doi: 10.1192/bjp.185.5.416
68. Sukhato K, Lotrakul M, Dellow A, Ittasakul P, Thakkinstian A, Anothaisintawee T. Efficacy of home-based non-pharmacological interventions for treating depression: a systematic review and network meta-analysis of randomised controlled trials. BMJ Open. 2017; 7 (7): e014499. doi: 10.1136/bmjopen-2016-014499
69. Cuijpers P, Karyotaki E, Pot AM, Park M, Reynolds CF. Managing depression in older age: psychological interventions. Maturitas. 2014; 79 (2): 160–169. doi: 10.1016/j.maturitas.2014.05.027
70. Cuijpers P, Quero S, Dowrick C, Arroll B. Psychological Treatment of Depression in Primary Care: recent Developments. Curr Psychiatry Rep. 2019; 21 (12): 129. doi: 10.1007/s11920-019-1117-x
71. Godlewska BR, Harmer CJ. Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment. Psychopharmacology. 2021; 238 (5): 1265–1278. doi: 10.1007/s00213-019-05448-0
72. Zhou T, Li X, Pei Y, Gao J, Kong J. Internet-based cognitive behavioural therapy for subthreshold depression: a systematic review and meta-analysis. BMC Psychiatry. 2016; 16 (1): 356. doi: 10.1186/s12888-016-1061-9
73. Andersson G, Titov N, Dear BF, Rozental A, Carlbring P. Internet-delivered psychological treatments: from innovation to implementation. World Psychiatry. 2019; 18 (1): 20–28. doi: 10.1002/wps.20610
74. Gega L, Marks I, Mataix-Cols D. Computer-aided CBT self-help for anxiety and depressive disorders: experience of a London clinic and future directions. J Clin Psychol. 2004; 60 (2): 147–157. doi: 10.1002/jclp.10241
75. Zhuo C, Li G, Lin X, et al. The rise and fall of MRI studies in major depressive disorder. Transl Psychiatry. 2019; 9 (1): 335. doi: 10.1038/s41398-019-0680-6
76. Nolan M, Roman E, Nasa A, et al. Hippocampal and amygdalar volume changes in major depressive disorder: a targeted review and focus on stress. Chronic Stress. 2020; 4: 2470547020944553. doi: 10.1177/2470547020944553
77. Hellewell SC, Welton T, Maller JJ, et al. Profound and reproducible patterns of reduced regional gray matter characterize major depressive disorder. Transl Psychiatry. 2019; 9 (1): 176. doi: 10.1038/s41398-019-0512-8
78. Schmaal L, Pozzi EC, Ho T, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry. 2020; 10 (1): 172. doi: 10.1038/s41398-020-0842-6
79. Fries GR, Saldana VA, Finnstein J, Rein T. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry. 2023; 28 (1): 284–297. doi: 10.1038/s41380-022-01806-1
80. Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012; 73 (4): 842–853. doi: 10.1016/j.neuron.2011.12.024
81. Sánchez-Rodríguez I, Temprano-Carazo S, Jeremic D, et al. Recognition memory induces natural LTP-like hippocampal synaptic excitation and inhibition. Int J Mol Sci. 2022; 23 (18): 10806. doi: 10.3390/ijms231810806
82. Chung A, Jou C, Grau-Perales A, et al. Cognitive control persistently enhances hippocampal information processing. Nature. 2021; 600 (7889): 484–488. doi:10.1038/s41586-021-04070-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







