Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Study on the Interaction Between C3 Gene Polymorphism and Environment in Patients with Type 2 Diabetes Combined with Coronary Artery Disease
Authors Qiu H, Abudureyimu S, Liu M, Liu F, Gao Y
Received 1 November 2023
Accepted for publication 19 March 2024
Published 27 March 2024 Volume 2024:17 Pages 1467—1479
DOI https://doi.org/10.2147/DMSO.S447789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Haitang Qiu,1,* Shajidan Abudureyimu,1,* Mengjia Liu,1 Fen Liu,2 Ying Gao1
1Department of Comprehensive Internal Medicine, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China; 2State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Clinical Medical Research, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ying Gao, Department of Comprehensive Internal Medicine, The First Affiliated Hospital of Xinjiang Medical University, 137 Liyushan South Road, Urumqi, Xinjiang, 830054, People’s Republic of China, Tel +86-991-4362625, Email [email protected]
Objective: This study was conducted to investigate the combined effect of genetic variation in the C3 gene and environmental factors on the risk of type 2 diabetes mellitus(T2DM) and coronary artery disease(CAD) in a population from Xinjiang, China.
Methods: We conducted a hospital-based case-control study with 896 participants (217 with T2DM+CAD and 679 healthy controls). A polymerase chain reaction-ligase detection reaction was used to identify and genotype TagSNPs in the C3 gene, and the influence of the interaction of two SNP loci (rs1047286 and rs11569562) with the environment on T2DM combined with CAD was evaluated through clinical data, statistical analysis of gene frequencies, and the formation of a gene-environment interaction model.
Results: We find that rs11569562 GG is an independent protective factor for T2DM and CAD (OR=0.353, p=0.012), and the variants at its locus may be closely associated with Activated Partial Thromboplastin Time (APTT), lipoprotein a (Lp(a)), Apolipoprotein A (APOA), Aspartate Aminotransferase (AST), Aspartate Aminotransferase (ALT) and AST/ALT levels (all P < 0.05); its GG genotype has significantly lower Gensini score and number of stenoses than the GA and AA genotypes. Multifactorial dimensionality reduction (MDR) finds a strong correlation between rs11569562 and AST (antagonistic effect) (4.44%); the role of rs11569562’s influence remains strong in terms of the independent effects of each attribute (1.72%).
Conclusions: In this study, we find that variants in the C3 gene loci rs11569562 are associated with the incidence of type 2 diabetes mellitus combined with coronary heart disease in a Chinese population. It is expected to be an independent predictor of type 2 diabetes mellitus combined with coronary heart disease in the Chinese population. Rs11569562 may be associated with lipid levels and coagulation molecules.
Clinical Trial Registration: This trial registered on in 2014 at the China Clinical Trials Registry (ChiCTR-TRC-14005114).
Keywords: coronary heart disease, Type 2 diabetes mellitus, complement C3
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, progressive disease that is characterized by years of insulin resistance and hyperinsulinemia preceding the onset of hyperglycemia.1–3 Nearly 9% of adults globally have diabetes, and the number of people with it is rising in many areas.4 More than 415 million persons globally, including 109.6 million persons in China, have been found to have diabetes.5 On a global scale, more than 20% of those aged over 65 have diabetes, and more than 90% of those patients have type 2 diabetes.6 It is commonly accepted that factors related to lifestyle, the environment, and genetics can affect the development of T2DM.7,8 T2DM’s etiology and progression are regulated by complex interactions between numerous genes and several environmental factors.9,10 T2DM is linked to an increased risk of coronary heart disease11 and is the sixth most common cause of disability.12 Having diabetes plus having established atherosclerotic cardiovascular disease (ASCVD) puts people at a significant risk for recurring major adverse cardiovascular events (MACE).13 In fact, cardiovascular disease is the main cause of mortality among diabetic patients. T2DM’s long-term impacts include the potential to increase morbidity and mortality cardiovascular disease (CVD) is the main cause of death in persons with type 2 diabetes (T2DM), and T2DM is a well-known risk factor for CVD.14 In comparison to those without diabetes, patients with T2DM have a 2- to 4-fold increased risk of dying from cardiovascular disease (CVD).15 Therefore, early prediction, identification of at-risk patients, and improved therapeutic management depend on a better understanding of the etiology of CVD and T2DM. Both T2DM and CVD have been linked to risk factors, including modifiable ones like dyslipidemia, obesity, oxidative stress, smoking, exercise, and alcohol use, as well as non-modifiable ones like age, sex, a positive family history, and a person’s genetic susceptibility.16,17
The innate immune system’s complement system removes pathogens and damaged cells from an organism, induces inflammation, and eliminates foreign invaders. C3(complement component 3) plays a crucial role in it.18,19 Complement C3 may be a biomarker of insulin resistance and cardio-metabolic disorders, according to preclinical and clinical studies.20–22 A C3 gene mutation was linked to dyslipidemia and cardiovascular disease and could lead to an increase in circulating C3 concentrations.23,24 The C3 gene has 41 exons that together code for 1663 amino acids and 13 functional domains, and it is found on chromosome 19p13.3–13.2. Before undergoing conformational changes that reveal binding sites for pathogenic cell surface and other complement components, the C3 protein remains inactive physiologically.25,26 Schizophrenic patients with the rs2230199 GG genotype exhibited greater total cholesterol (TC), lower density lipoprotein cholesterol (LDL-C), and C3 levels compared to those with the CC genotype, according to research by Nsaiba et al in 2015.25 In a Tunisian population, a significant positive connection between C3 polymorphism and myocardial infarction was discovered in 2013.27
Few studies have explored the correlation between genetic polymorphisms and the risk of diabetes mellitus combined with coronary heart disease. No studies are focusing on C3 variants based on diabetes mellitus combined with coronary heart disease. This hospital-based study aimed to explore the association between C3 tagSNPs and the risk of type 2 diabetes mellitus combined with coronary artery disease in a Chinese population. Therefore, we conducted this hospital-based case-control study to explore the interaction of C3 gene polymorphisms with the environment and their role in diabetes mellitus combined with coronary heart disease in a Chinese population.
Materials and Methods
Subjects
The Department of First Affiliated Hospital of Xinjiang Medical University conducted this hospital-based case-control study from January 2017 to December 2020 with a total of 881 unrelated adult patients. Of the 896 participants, 217 (140 males and 77 females, mean age (57.61±10.13) years) were patients with CAD+T2DM and 679 (431 males and 248 females, mean age (55.81±9.17) years) were controls with neither CAD nor T2DM. Each participant was Chinese and had a coronary angiography performed on them. At least two skilled cardiologists assessed the results of coronary angiograms. Using the American Diabetes Association’s standards.28 There were 217 participants with T2DM complicated with CVD who had been diagnosed with diabetes with FPG 126 mg/dL or who were on diabetes medication and confused with CAD. A stenosis diameter of more than 50% in at least one major coronary vessel (left main, left anterior descending, left circumflex, right coronary artery, and big branches) was considered a sign of coronary artery disease (CAD). Renal disease, hepatic disease, endocrine disease, metabolic disorders, and autoimmune diseases were among the exclusion criteria. To rule out CAD and T2DM, controls who were chosen from hospital admissions performed a coronary angiography test as well. All of the major coronary arteries’ luminal stenosis must be under 50%.
All parties involved provided their written, informed consent. The Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University accepted the study protocol (approval number, 20170925–02), which complied with the Declaration of Helsinki’s ethical principles.
Lipid Analysis and Biochemical Markers
The morning following hospitalization, when lipid-lowering medications and medications that can impact metabolic parameters were not being used, fasting blood samples were taken. The First Affiliated Hospital of Xinjiang Medical University Laboratory supplied test indicators to identify serum total cholesterol (TC), HDL-C,TG, LDL-C, lipoprotein a (Lp(a)), apolipoprotein A (ApoA), apolipoprotein B (ApoB), and other biochemical indicators. The enzymatic approach was used to test the levels of TG, TC, LDL-C, and HDL-C. An immunoturbidimetric test was used to assess ApoB, ApoA1 and Lp(a).
Selection of C3 Gene Polymorphisms
Venipuncture was used to obtain fasting blood samples from the subjects before performing cardiac catheterization. Blood samples were kept in an EDTA tube (5 mL) made of ethylene diamine tetraacetic acid (EDTA). The plasma was then separated by centrifuging for 5 minutes at 4000 g. Later, peripheral leukocytes’ genomic DNA was extracted using the industry-standard phenol-chloroform procedure. The DNA samples were kept in a freezer at 80°C. SNPs for C3 (rs1047286, rs11569562) were discovered using linkage disequilibrium patterns using r2≥0.8 and MAF≥0.05 as a cutoff, Haploview 4.2 software, and the Phase I&II database of the International HapMap Project website (http://www.hapmap.org). The SNP genotyping was acquired using Genesky’s (Shanghai, China) enhanced multiplex ligation detection reaction (iMLDR) method. Primers for the polymerase chain reaction (PCR) were listed in Table 1. Genotyping was carried out using a blinded procedure without knowledge of any patient-specific clinical data. A 10% duplicate of genotyped samples was done so that genotyping quality could be checked.
 |
Table 1 Primer Information Sheet |
Statistical Analysis
The data collected was analyzed using IBM SPSS version 20.0 software, and the clinical outcomes were also examined. Quantitative data was expressed using standard deviation (SD) and mean values. The ranges and frequencies of distributions for quantitative variables were estimated. Data that showed a normal distribution were compared between two groups using the Student’s t-test and among multiple groups using the ANOVA test. The significance of proportional differences was determined using the Chi-square test (2) with a p-value of 0.05. This test was used to investigate variations in genotype and allele frequencies across groups, as well as deviations from Hardy-Weinberg equilibrium. The relationship between illnesses and C3 gene polymorphisms was assessed using unadjusted odds ratios (OR) and 95% confidence intervals (CI) through a single-variable logistic regression approach. Potential confounders such as age, gender, weight, smoking, and drinking were all accounted for in a logistic regression analysis to assess the risk factors for disease development. The results were conveyed as adjusted odds ratios. Following the regression analyses, we utilized MDR 3.0.2 software to create a gene-environment interaction model to further investigate the effects of these risk factors when they collaborate to cause disease.
Results
Population Characteristics
For both populations, there were no significant differences in age and sex between the T2DM+CAD group and the control group. The distribution of rs11569562 genotypes differed between control and experimental groups (p=0.008). In addition, compared with controls, patients with WBC(p<0.001), NEUT(p<0.001), MONO(p<0.001), EOS(p<0.001), BASO(p<0.001), RBC(p=0.006), PCV(p<0.001), MCV(p<0.001), RDW(p<0.001), PT(p=0.038), APTT(p=0.006), UA(p=0.004), Glu(p<0.001), TG(p<0.001), TC(p=0.01), HDL-C(p<0.001), APOA(p=0.043), Lp(a)(p=0.047), CB(p<0.001),UCB(p<0.001),GLB(p<0.001), A/G(p<0.001), AST(p<0.001), ALT(p=0.001), AST/ALT(p<0.001) and γ-GT(p=0.002) showed significant differences. The demographic, clinical and biochemical data of the enrolled subjects are summarized in Table 2.
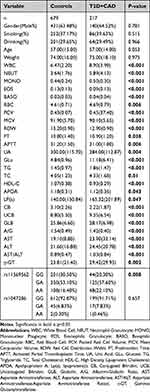 |
Table 2 General Characteristics and Biochemical Variables of the Study Population |
Correlation of rs1047286 and rs 11569526 Genotypes with Clinical Indices
The rs1047286 locus of C3 gene has 3 different genotypes, AA, GA, and GG, of which GG is the wild type and GA and AA are mutations; rs11569562 locus also has 3 different genotypes, AA, GA, and GG, of which AA is the wild type and GA and GG are mutations. rs1047286 polymorphic genotype subgroups between The differences in APTT, Lp(a), AST/ALT levels between rs1047286 polymorphic genotype subgroups were statistically significant (P < 0.05). rs11569562 polymorphic genotype subgroups showed statistically significant differences in sex and age, MCV, APTT, Lp(a), APOA, AST and AST/ALT levels (P < 0.05). None of the differences in smoking and drinking between the rs1047286 and rs11569562 polymorphic genotype subgroups were statistically significant (P > 0.05) (Table 3)
 |
Table 3 Clinical Characteristics and Plasma Fatty Acid Profiles According to rs1047286 and rs 11569526 Genotypes |
Association of C3 Genotypes and Haplotypes Frequencies with T2DM and CAD
The gene polymorphism distribution of rs1047286 and rs11569562 was in accordance with Hardy-Weinberg equilibrium: P>0.05. This indicates that the population is genetically balanced and the data are from the same Mundell population, which is representative of the population. In the conventional, dominant of inheritance (GG vs GA+AA), the experimental group rs11569562 GG genotype was lower than the control group (P =0.008), the G allele was significantly lower than the control group, and the A allele was significantly higher than the control group (P =0.004). This suggests that patients with the GG genotype of rs11569562 may have a lower risk of T2DM+CAD. In the rs1047286 genotype, there was no statistically significant difference between the experimental and control groups (P>0.05). (Table 4)
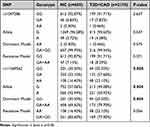 |
Table 4 Allele Frequency and Genotype Frequency Distribution of C3 Polymorphism in Experimental and Control Groups |
In rs11569562, Gensini score (p=0.002) and number of stenoses (p=0.001) were higher in the AA genotype than in the GA and GG genotypes. Gensini score and vascular stenosis number distribution differences among genotypes in rs1047286 were not statistically significant (P>0.05). (Table 5)
 |
Table 5 Single Factor Analysis of Gensini Score and Vascular Stenosis Index in Different Genotypes |
Logistic Regression Analysis of T2DM with CAD in Chinese Population
The indexes were graded and assigned with reference to the 9th edition of diagnostic criteria. (Table 6.)
 |
Table 6 Assignment Table of Each Variable |
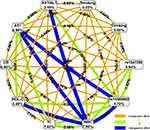
A univariate logistic regression analysis with T2DM with CAD as the dependent variable and rs11569562 and other variables as independent variables revealed that rs11569562, white blood cells (WBC), neutrophils (NEUT), monocytes (MONO), eosinophils (EOS), basophils (BASO), red blood cells (RBC), red blood cell pressure volume (PCV), mean red blood cell volume (MCV), APTT, glucose (GLU), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL-C), lipoprotein a (LP(a)), conjugated Bilirubin(CB), unconjugated bilirubin(UCB), globulin(GLB), globulin, A/G, menthyl aminotransferase (AST), alanine aminotransferase (ALT), menthyl/alanine (AST/ALT), γ-glutamyl transpeptidase (γ-GT), as influencing factors for diabetes and coronary heart disease (OR= 1.43, 7.395, 11.844, 2.575, 0.326, 3.243, 2.318, 2.094, 0.374, 0.525, 33.706, 1.641, 1.947, 2.895, 1.483, 0.349, 1.71, 1.663, 0.573, 6.624, 1.701, 2.357, 1.544, p≤0.05) (this table is omitted). Further logistic regression analysis was performed to find rs11569562, WBC, GLU, TC, HDL-C, CB, AST, AST/ALT as risk factors for type 2 diabetes and coronary heart disease. (Figure 1.)
 |
Figure 1 Adjusted odds ratio (OR) of the logistic regression analysis for T2DM with CVD risk. Abbreviations: OR, odds ratio; CI, confidence interval. |
Association of C3 Gene-Environment Interactions with the Development of Diabetes and Coronary Heart Disease
The best models were determined to be rs1047286, rs11569562, WBC,TC, HDL-C, CB, AST, AST/ALT, Smoking, Drinking models when using MDR to develop multi-order interaction models of gene-gene and gene-environment. Training Balance Accuracy CV = 0.8377, Testing Balance Accuracy CV = 0.7031, CV Consistency = 10/10. Table 7 has all the details.
 |
Table 7 Interaction Model of rs1047286,rs11569562 Gene and Environmental Factors MDR |
A significantly antagonistic effect exists between AST and rs11569562(4.44%), which are all found on the same main fork, according to the interaction dendrogram. The substantial negative interaction is also found between WBC and AST/ALT (5.89%). (Figure 2). As for the primary effects of the various attributes, they were as follows, WBC (7.99%), AST(6.86%), HDL-C (3.59%), AST/ALT(3.19%), rs11569562(1.72%), TC (1.42%), rs1047286(0.94%), CB(0.5%), Smoking(0.03%), Drinking(0%). (Figure 3)
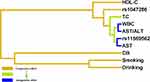 |
Figure 2 The dendrogram of the interaction between rs1047286,rs11569562 gene and gene,and between gene and environment MDR. |
Discussion
To our knowledge, this is the first study to evaluate the relationship between tagSNPs in the C3 gene and environmental factors and T2DM+CAD risk in a Chinese population. Our study provides evidence of a correlation between rs11569562 and an elevated risk of T2DM and CAD statistical analysis.
T2DM, a chronic condition disease, induced by a genetic predisposition together with environmental factors, is a well established risk factor for CAD. T2DM and its related cardiovascular complications propose specific challenges at diverse stages of the life. C3 polymorphisms have been reported to significantly associate with risk for T2DM and CAD, which were considered as influential genetic risk factors. Complement activation is crucial to the host’s immunological defense. However, if the complement component becomes overactive, it can cause several illnesses, such as diabetes and cardiovascular conditions. The complement system’s core element, C3, has been identified and is crucial to the pathophysiology of cardiovascular disorders.22 It has been consistently shown that C3 is frequently linked to various cardiovascular disease (CVD) incidents and prevalent symptoms.29 C3 has been linked to metabolic problems in the past, including excess fat, dyslipidemia, insulin resistance, liver dysfunction, and diabetes. C3 is now becoming more well-acknowledged as a risk factor for cardiometabolism.30 Many genomic studies have identified C3.31,32 Alexander Gudjonsson’s study found a strong correlation between polymorphisms in the C3 gene and CVD.31 In 2005, high levels of C3 have been demonstrated to be independently correlated with the occurrence of type 2 diabetes, at least in men.33,34 Complement C3 might be a biomarker for insulin resistance and cardio-metabolic disorders, according to preclinical and clinical research.20–22 All of these suggested that C3 may contribute to the association between diabetes and heart disease. To our knowledge, no research has examined in-depth the relationship between environmental interactions and C3 (rs1047286,rs11569562) gene polymorphisms and T2DM combined with CAD in the Chinese population. Thus, this small research gap is filled by our current work.
For rs1047286, previous studies have found that the rs1047286 T allele plays a role in the pathophysiology of rheumatoid arthritis, possibly by influencing complement activation by alternative pathways.35 Carriage of the T allele of rs1047286 also has a trend toward the risk of advanced age-related macular degeneration.36 For rs11569562, an SNP (SNP24, rs11569562) located in intron 31 of the C3 gene was associated with adult Bronchial asthma37 and TGG (rs11569562-rs344555-rs2241393) showed a risk or protective effect against schizophrenia38. It is widely recognized that dyslipidemia is a major factor in the development and progression of coronary heart disease. Previous studies have proposed that the rs11569562 locus of C3 was related to lipid levels and inflammatory states.39,40 Our results also find statistically significant differences in genotype distribution of rs11569562 in LP(a), APOA, AST, and AST/ALT levels (p=0.024, 0.033, 0.041, 0.012); the distribution of rs1047286 loci shows statistically significant differences in Lp(a), and AST/ALT levels (p=0.022, 0.018). The distribution of rs11569562 genotypes differs in gender, age, and MCV distribution (p<0.05), which may be due to chance. Previous studies suggest that the number of diseased vessels can indicate the severity of CAD.41 In our study, the rs11569562 variant is determined to reduce the number of stenoses (p=0.001). We also find that the genotype distributions of rs11569562 and rs1047286 differ at the APTT level (p=0.035, 0.004), which, combined with previous findings that ”C3 interacts with fibrinogen to promote clot generation,42,43 we hypothesize that rs11569562 and rs1047286 may be associated with coagulation related.
Prior studies have found that elevated leukocyte levels in diabetic patients are associated with a higher prevalence of CAD.44 In humans, high amounts of HDL-C in plasma were associated with a lower risk of coronary heart disease (CHD).45,46 Elevated concentrations of to TC in serum were associated with an increased risk of coronary heart disease.47 Serum bilirubin was associated with the risk of coronary heart disease.48,49 AST and ALT have previously been used to diagnose coronary heart disease.50 Our regression analysis confirms that WBC (OR=2.555), HDL-C (OR=2.555), GLU (OR=20.166), TC (OR=2.207), CB (OR=0.187), AST (OR=2.666), and AST/ALT (OR=1.863) are the independent risk and protective factors for T2DM+CAD. In addition to this, our study observes that under traditional and dominant inheritance patterns (GG vs GA+AA), the experimental group rs11569562 GG genotype is lower than that of the control group (P = 0.008), the G allele is significantly lower than that of the control group, and the A allele is significantly higher than that of the control group (P = 0.004). This suggests that patients with the GG rs11569562 genotype have a lower risk of developing T2DM+CAD. Regression analysis also showed that the variant of rs11569562 (OR = 0.353) was an independent protective factor for T2DM+CAD. It is well known that abnormal lipid levels and abnormal blood clots exacerbate cardiovascular risk in diabetic patients.51,52 Hess et al also proposed a new aspect of the role of C3 in diabetes in relation to cardiovascular disease risk - The involvement of C3 in low fibrin lysis, which promotes thrombosis.53 Subsequently,
King and Rhodri suggested that inhibition of the interaction between C3 and fibrinogen may help reduce cardiovascular events in diabetic patients.42,43 Combining previous studies and our findings above, we hypothesize that rs11569562 may affect the risk of coronary heart disease in diabetic patients by affecting lipid levels and coagulation-related markers.
To further explore the inter-occurrence of the C3 gene and validated independent factors and the effects of these independent factors when they act together in the disease, we used the MDR approach to construct a gene-environment interaction model. In this model, we add smoking and alcohol consumption to the analysis to explore the effects of C3 gene polymorphisms (rs11569562, rs1047286) and known independent factors validated in this study (WBC, HDL-c, GLU, TC, CB, AST, AST/ALT) on diabetes mellitus with coronary heart disease. The analysis reveals a significant antagonistic effect between rs11569562 and AST (4.44%) and between WBC and AST/ALT (5.89%) when acting together on the disease. Independent main effects are ranked from highest to lowest, WBC (7.99%), AST (6.86%), HDL-C (3.59%), AST/ALT (3.19%),rs11569562 (1.72%), TC (1.42%), rs1047286 (0.94%), CB (0.5%), cigarette smoking (0.03%), alcohol consumption (0%). This suggests that when these independent influences act together in diabetes mellitus with coronary artery disease, the role of rs11569562 (1.72%) on T2DM+CAD remains very significant and may have the potential to diagnose the disease in conjunction with other factors.
The shortcoming of this study is that the cases registered in our study were not newly diagnosed. Given the fact of the retrospective case-control design, results involving other environmental risk factors may be biased due to confounding. Further expanded and replicated studies would help to draw more convincing conclusions and explain the inherent mechanisms.
Conclusions
In conclusion, our study provides the first evidence of an association between the rs11569562 locus of the C3 gene and the risk of developing type 2 diabetes combined with coronary heart disease. We find that the GG phenotype of rs11569562 is an independent protective factor for T2DM and CAD, and variants at its locus may be strongly associated with APTT, lipoprotein(a), APOA, AST, ALT, and AST/ALT levels; and that its GG genotype has a significantly lower Gensini score and number of stenoses than the GA and AA genotypes. Multifactorial dimensionality reduction finds a strong correlation between rs11569562 and AST when applied to T2DM+CAD (antagonistic effect); the effect of rs11569562 on T2DM+CAD remains strong in terms of the independent effects of each attribute. Thus rs11569562 locus is expected to be an independent predictor of type 2 diabetes mellitus combined with coronary heart disease in the Chinese population. Variation at the rs1047286 locus may have an effect on APTT, Lp(a), and AST/ALT levels. In T2DM combined with CAD, the interaction between environmental factors such as smoking and drinking and the two loci of C3 is weak, at least in this study.(Combined Figure 3 and Table 3 results)
Data Sharing Statement
The datasets used and/or analyzed in the study are available from the corresponding author upon reasonable request.
Ethics Approval
The Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University accepted the study protocol (approval number, 20170925-02), which complied with the Declaration of Helsinki’s ethical principles.
Acknowledgments
We thank all the investigators and subjects who participated in this project.
Funding
Science and Technology Innovation Leading Talents Program-High-level Leading Talents in Xinjiang (Grant No.:2022TSYCLJ0023).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ahmad E, Lim S, Lamptey R., et al. Type 2 diabetes. Lancet. 2022;400(10365):1803–1820. doi:10.1016/S0140-6736(22)01655-5
2. Nazarzadeh M, Bidel Z, Canoy D, et al. Blood pressure lowering and risk of new-onset type 2 diabetes, an individual participant data meta-analysis. Lancet. 2021;398(10313):1803–1810. doi:10.1016/S0140-6736(21)01920-6
3. Shlomai G, Neel B, LeRoith D, et al. Type 2 diabetes mellitus and cancer, the role of pharmacotherapy. J Clin Oncol. 2016;34(35):4261–4269. doi:10.1200/JCO.2016.67.4044
4. Zhou B, Lu Y, Hajifathalian K, et al. Worldwide trends in diabetes since 1980, a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi:10.1016/S0140-6736(16)00618-8
5. Shi FH, Shen L, Pan MM, et al. The successful rapid adjustment of blood glucose in a patient with acute coronary syndrome, renal insufficiency, and diabetes, a case report of management coordinated by clinical pharmacists and clinicians. Front Pharmacol. 2020;11:756. doi:10.3389/fphar.2020.00756
6. Huang ES. Management of diabetes mellitus in older people with comorbidities. BMJ. 2016;353:i2200. doi:10.1136/bmj.i2200
7. Toi PL, Anothaisintawee T, Chaikledkaew U, et al. Preventive role of diet interventions and dietary factors in Type 2 diabetes mellitus, an umbrella review. Nutrients. 2020;12(9). doi:10.3390/nu12092722
8. Weihrauch M, Handschin C. Pharmacological targeting of exercise adaptations in skeletal muscle, benefits and pitfalls. Biochem Pharmacol. 2018;147:211–220. doi:10.1016/j.bcp.2017.10.006
9. Park S, Rhee SY, Jeong SJ, et al. Features of long-standing Korean Type 2 diabetes mellitus patients with diabetic retinopathy, a study based on standardized clinical data. Diabetes Metab J. 2017;41(5):393–404. doi:10.4093/dmj.2017.41.5.393
10. Zhang S, Wang S, Puhl MD, et al. Global biochemical profiling identifies beta-hydroxypyruvate as a potential mediator of type 2 diabetes in mice and humans. Diabetes. 2015;64(4):1383–1394. doi:10.2337/db14-1188
11. Emerging Risk Factors C, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease, a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
12. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi:10.1016/S0140-6736(17)30058-2
13. Honigberg MC, Chang LS, McGuire DK, et al. Use of glucagon-like Peptide-1 receptor agonists in patients with Type 2 diabetes and cardiovascular disease, a review. JAMA Cardiol. 2020;5(10):1182–1190. doi:10.1001/jamacardio.2020.1966
14. Wilcox T, De Block C, Schwartzbard AZ, et al. Diabetic agents, from metformin to SGLT2 inhibitors and GLP1 receptor agonists, JACC focus seminar. J Am Coll Cardiol. 2020;75(16):1956–1974. doi:10.1016/j.jacc.2020.02.056
15. Perreault L, Boardman MK, Pak J. The association between Type 2 diabetes and cardiovascular disease, the ”for your sweetheart” survey. Adv Ther. 2019;36(3):746–755. doi:10.1007/s12325-019-0871-9
16. Siddiqui MB, Arshad T, Patel S, et al. Small dense low-density lipoprotein cholesterol predicts cardiovascular events in liver transplant recipients. Hepatology. 2019;70(1):98–107. doi:10.1002/hep.30518
17. Horejsi B, Ceska R. Apolipoproteins and atherosclerosis. Apolipoprotein E and apolipoprotein(a) as candidate genes of premature development of atherosclerosis. Physiol Res. 2000;49(Suppl 1):S63–9.
18. Lidani KCF, Bavia L, Ambrosio AR, et al. The complement system, a prey of trypanosoma cruzi. Front Microbiol. 2017;8:607. doi:10.3389/fmicb.2017.00607
19. Merle NS, Church SE, Fremeaux-Bacchi V, et al. Complement system part i - molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi:10.3389/fimmu.2015.00262
20. Haj Ahmad RM A, Al-Domi HA. Complement 3 serum levels as a pro-inflammatory biomarker for insulin resistance in obesity. Diabetes Metab Syndr. 2017;11(Suppl 1):S229–S32. doi:10.1016/j.dsx.2016.12.036
21. Bratti LOS, Do Carmo IAR, Vilela TF, et al. Complement component 3 (C3) as a biomarker for insulin resistance after bariatric surgery. Clin Biochem. 2017;50(9):529–532. doi:10.1016/j.clinbiochem.2017.02.006
22. Ursini F, Abenavoli L. The emerging role of complement C3 as a biomarker of insulin resistance and cardiometabolic diseases, preclinical and clinical evidence. Rev Recent Clin Trials. 2018;13(1):61–68. doi:10.2174/1574887112666171128134552
23. Jiang H, Guo M, Dong L, et al. Levels of acylation stimulating protein and the complement component 3 precursor are associated with the occurrence and development of coronary heart disease. Exp Ther Med. 2014;8(6):1861–1866. doi:10.3892/etm.2014.2018
24. van Greevenbroek MM, Jacobs M, van der Kallen CJ, et al. Human plasma complement C3 is independently associated with coronary heart disease, but only in heavy smokers (the CODAM study). Int J Cardiol. 2012;154(2):158–162. doi:10.1016/j.ijcard.2010.09.017
25. Nsaiba MJ, Lapointe M, Mabrouk H, et al. C3 polymorphism influences circulating levels of C3, ASP and lipids in schizophrenic patients. Neurochem Res. 2015;40(5):906–914. doi:10.1007/s11064-015-1543-z
26. Wagner EK, Raychaudhuri S, Villalonga MB, et al. Mapping rare, deleterious mutations in Factor H, Association with early onset, drusen burden, and lower antigenic levels in familial AMD. Sci Rep. 2016;6:31531. doi:10.1038/srep31531
27. Leban N, Jraba K, Chalghoum A, et al. Polymorphism of C3 complement in association with myocardial infarction in a sample of central Tunisia. Diagn Pathol. 2013;8:93. doi:10.1186/1746-1596-8-93
28. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi:10.2337/dc14-S081
29. Onat A, Can G, Rezvani R, et al. Complement C3 and cleavage products in cardiometabolic risk. Clin Chim Acta. 2011;412(13–14):1171–1179. doi:10.1016/j.cca.2011.03.005
30. Hertle E, van Greevenbroek MM, Stehouwer CD. Complement C3, an emerging risk factor in cardiometabolic disease. Diabetologia. 2012;55(4):881–884. doi:10.1007/s00125-012-2462-z
31. Gudjonsson A, Gudmundsdottir V, Axelsson GT, et al. A genome-wide association study of serum proteins reveals shared loci with common diseases. Nat Commun. 2022;13(1):480. doi:10.1038/s41467-021-27850-z
32. Khan A, Shang N, Petukhova L, et al. Medical records-based genetic studies of the complement system. J Am Soc Nephrol. 2021;32(8):2031–2047. doi:10.1681/ASN.2020091371
33. Engstrom G, Hedblad B, Eriksson KF, et al. Complement C3 is a risk factor for the development of diabetes, a population-based cohort study. Diabetes. 2005;54(2):570–575. doi:10.2337/diabetes.54.2.570
34. Borne Y, Muhammad IF, Lores-Motta L, et al. Complement C3 associates with incidence of diabetes, but no evidence of a causal relationship. J Clin Endocrinol Metab. 2017;102(12):4477–4485. doi:10.1210/jc.2017-00948
35. Sena L, Oliveira-Tore CF, Skare T, et al. C3 gene functional polymorphisms and C3 serum levels in patients with rheumatoid arthritis. Immunol Invest. 2021;50(8):1027–1041. doi:10.1080/08820139.2020.1800726
36. Lu F, Liu S, Hao Q, et al. Association between complement factor C2/C3/CFB/CFH polymorphisms and age-related macular degeneration, a meta-analysis. Genet Test Mol Biomarkers. 2018;22(9):526–540. doi:10.1089/gtmb.2018.0110
37. Inoue H, Mashimo Y, Funamizu M, et al. Association study of the C3 gene with adult and childhood asthma. J Hum Genet. 2008;53(8):728–738. doi:10.1007/s10038-008-0304-0
38. Zhang S, Zhou N, Liu R, et al. Association between polymorphisms of the complement 3 Gene and schizophrenia in a han chinese population. Cell Physiol Biochem. 2018;46(6):2480–2486. doi:10.1159/000489654
39. Cai G, Li L, Chen Y, et al. Complement C3 gene polymorphisms are associated with lipid levels, but not the risk of coronary artery disease, a case-control study. Lipids Health Dis. 2019;18(1):217. doi:10.1186/s12944-019-1163-8
40. Phillips CM, Goumidi L, Bertrais S, et al. Complement component 3 polymorphisms interact with polyunsaturated fatty acids to modulate risk of metabolic syndrome. Am J Clin Nutr. 2009;90(6):1665–1673. doi:10.3945/ajcn.2009.28101
41. Hu W, Li K, Han H, et al. Circulating levels of CILP2 are elevated in coronary heart disease and associated with atherosclerosis. Oxid Med Cell Longev. 2020;2020:1871984. doi:10.1155/2020/1871984
42. King RJ, Schuett K, Tiede C, et al. Fibrinogen interaction with complement C3, a potential therapeutic target to reduce thrombosis risk. Haematologica. 2021;106(6):1616–1623. doi:10.3324/haematol.2019.239558
43. King R, Tiede C, Simmons K, et al. Inhibition of complement C3 and fibrinogen interaction, a potential novel therapeutic target to reduce cardiovascular disease in diabetes. Lancet. 2015;385(Suppl 1):S57. doi:10.1016/S0140-6736(15)60372-5
44. Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17(5):695–708. doi:10.1016/j.cmet.2013.04.001
45. Lee JS, Chang PY, Zhang Y, et al. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status, the strong heart study. Diabetes Care. 2017;40(4):529–537. doi:10.2337/dc16-1958
46. Husing A, Kabar I, Schmidt HH. Lipids in liver transplant recipients. World J Gastroenterol. 2016;22(12):3315–3324. doi:10.3748/wjg.v22.i12.3315
47. Zhang WJ, Su WW, Li PB, et al. Naoxintong capsule inhibits the development of cardiovascular pathological changes in bama minipig through improving gut microbiota. Front Pharmacol. 2019;10:1128. doi:10.3389/fphar.2019.01128
48. Chen YH, Chau LY, Chen JW, et al. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care. 2008;31(8):1615–1620. doi:10.2337/dc07-2126
49. Hinds TD, Stec DE. Bilirubin, a cardiometabolic signaling molecule. Hypertension. 2018;72(4):788–795. doi:10.1161/HYPERTENSIONAHA.118.11130
50. Elizondo-Montemayor L, Ugalde-Casas PA, Lam-Franco L, et al. Association of ALT and the metabolic syndrome among Mexican children. Obes Res Clin Pract. 2014;8(1):e79–87. doi:10.1016/j.orcp.2012.08.191
51. Ren L, Cui Q, Liu W, et al. Novel GLP-1 analog supaglutide stimulates insulin secretion in mouse and human islet beta-cells and improves glucose homeostasis in diabetic mice. Front Physiol. 2019;10:930. doi:10.3389/fphys.2019.00930
52. Patti G, Cavallari I, Andreotti F, et al. Prevention of atherothrombotic events in patients with diabetes mellitus, from antithrombotic therapies to new-generation glucose-lowering drugs. Nat Rev Cardiol. 2019;16(2):113–130. doi:10.1038/s41569-018-0080-2
53. Hess K, Alzahrani SH, Mathai M, et al. A novel mechanism for hypofibrinolysis in diabetes, the role of complement C3. Diabetologia. 2012;55(4):1103–1113. doi:10.1007/s00125-011-2301-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.