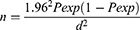Back to Journals » Veterinary Medicine: Research and Reports » Volume 12
Study on Prevalence of Bovine Mastitis and Associated Risk Factors in Dairy Farms of Modjo Town and Suburbs, Central Oromia, Ethiopia
Authors Fesseha H , Mathewos M , Aliye S, Wolde A
Received 4 June 2021
Accepted for publication 24 September 2021
Published 8 October 2021 Volume 2021:12 Pages 271—283
DOI https://doi.org/10.2147/VMRR.S323460
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Haben Fesseha,1 Mesfin Mathewos,1 Saliman Aliye,1 Amanuel Wolde2
1School of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 2College of Agriculture, Department of Animal Health, Jinka University, Jinka, Ethiopia
Correspondence: Haben Fesseha Email [email protected]
Background: In the global dairy industry, mastitis is the main economic significant disease of cattle. Milk and other dairy outputs are scarce in developed countries, including Ethiopia.
Methods: In this cross-sectional investigation in the Modjo district, milk samples were collected aseptically from 384 randomly selected lactating cattle to investigate the prevalence of clinical and sub-clinical mastitis and determine the possible risk factors and isolate bacterial pathogens causing mastitis. Besides, clinical mastitis cases have been reported by veterinarians based on milk, udder, or systemic cow anomalies, whereas the presence of subclinical mastitis was determined using California Mastitis Test (CMT).
Results: The research revealed that subclinical mastitis (71.02%) is more prevalent in dairy farms of the study area than in the clinical type (28.9%). The quarter-level frequency was 36.9%; from which, 34.9% and 3.4% were from subclinical form and blind teat, respectively. There was a significant correlation between the frequency of mastitis in lactating cows (p < 0.05) and factors, such as breed, age, body condition score, herd size, milking mastitic cow at the end, and previous mastitis history. The dominant mastitis-causing agents isolated in this study were Staphylococcus aureus (40.3%), Streptococcus species (24.3%), Coagulase-negative Staphylococcus (12.5%), E. coli (8.3%), Staphylococcus hyicus (3.5%), and Staphylococcus intermedius (1.4%). The high occurrence of mastitis, particularly sub-clinical mastitis, revealed significant economic potential losses in dairy farms in the research district.
Conclusion: Therefore, appropriate measures aimed at increasing the understanding and hygiene milking methods of dairy farmers, routine monitoring for subclinical mastitis, dry cow therapy, and culling of chronically contaminated cows to reduce bovine mastitis and its impact on milk production and food security.
Keywords: California mastitis test, dairy cow, mastitis, risk factor, Modjo
Introduction
Ethiopia has a large livestock resource in Africa with a total of 57.8 million cattle population of which 7.2 million are mainly kept for the processing of milk.1 Milk is one of the most crucial human food. Owing to its important ingredients, it is widely accepted as a full diet.2,3 In Ethiopia, however, milk consumed annually is poor in comparison with other developing countries’ total dairy intake. Local milk production fails to meet the milk requirements of the country because of low inputs and common cattle health conditions.4–7 The growth of the Ethiopian dairy sector will dramatically reduce poverty and nutrition in the region. Nevertheless, in many African countries, this consumer-orientated milk processing, a fast-growing system, is subject to intensification diseases like mastitis and reproductive disorders.5,8,9
Mastitis is a mammalian gland inflammation caused by pathogenic microorganisms that invade the mammary gland. Besides, injury of the teat canal and results in physical, chemical, pathological, and bacteriologic changes in glandular tissues and milk composition. In the dairy business, mastitis is among the most severe and economically important infections throughout the globe. Mastitis causes higher economic losses in milk production as a result of the infected quarters’ inflammation. Bovine mastitis leads to a decrease in milk production, increases culling rate, incurs medical costs, and often leads to severe deaths from infections.3,6,10
Mastitis is of extreme zoonotic importance since the milk is unsafe for human consumption. This is due to the risk of bacterial contamination of the milk from infected cows.3,11,12 Clinical and subclinical mastitis (SCM) are the two types of mastitis. Variations in physical characteristics of milk, udder enlargement, redness, and increase in udder temperature were commonly observed as a clinical manifestation of clinical mastitis, whilst SCM infected dairy cattle display no significant milk or udder changes and can only be detected by somatic cell count (SCC) or bacterial culture.4,10,13,14
Mastitis in cattle is a complex and multifactorial disease, depending on the animal, environmental and pathogenic variables.3,15–17 Contagious pathogens are the most important reservoirs for diseased cows. The prevalent and infectious bacteria identified in different trials were Staphylococcus aureus, Streptococcus agalactiae, Corynebacterium bovis, and Mycoplasma species.6,9,12,18–22 They spread mainly during milking between cows and appear to develop chronic subclinical infections with outbreaks of clinical incidents. Environmental mastitis can be generally identified as an infection of the mammary glands (E. coli, Klebsiella species, Strep. dysgalactiae, and Strep. uberis) and the ecosystem is the principal reservoir in which the cow.5,9,19,20,23,24 Mastitis is most likely attributed to poor milk sanitation, low barn sanitation, lack of teat dipping and the use of lubricant during contact, and lack of treatment in milking cows of various age groups.11,15
Over the past few years, numerous scientists in Ethiopia have researched the frequency of mastitis in dairy herds. Previous studies found that the occurrence of mastitis in the country fell between 23.2% and 81.1%.22,25–28 Although both clinical and subclinical mastitis are serious constraints in most semi-intensive and intensive dairy farms in Ethiopia, including the study area, only a few investigations were conducted, mostly confined to the central part of the country, without representing the prevalence of mastitis in different ecological and management conditions. This study tries to identify both the prevalence of clinical and subclinical mastitis as well as the pathogens that were responsible for the infection. In addition, most previous studies only focus on prevalence studies and there was limited research that investigated the isolation of the pathogens responsible for mastitis. Also, subclinical mastitis received little regard in several of the previous studies, though it is still a major constraint in the dairy industry in most of the country as well as in the current study area. Hence, this research aims at investigating the occurrence and precipitating factors of clinical and subclinical mastitis in dairy farms of Modjo district.
Materials and Methods
Study Site and Animals
The research was conducted from November 2018 to June 2019 in Mojo town and its surroundings in central Ethiopia, located in the East Shewa zone of the Oromia Region. The area has a latitude and longitude of 8°39′N 39°5′E with an elevation between 1788 and 1825 meters above sea level. It is the administrative center of Lome woreda. The study subjects were lactating dairy cows located on different nominated farms in Modjo town and its surroundings.
The study animals were lactating indigenous zebu, Holstein Friesian cross with local zebu breed and jersey breeds that were kept under an intensive, semi-intensive, and extensive management system. In addition, the farms were categorized into small, medium, and large-scale dairy farms based on the dairy cows in the farm. The study animals were classified as young (3–6 years), adult (6–9), and old (>9) based on their dentition.29
Study Design
A cross-sectional study design was used from November 2018 to June 2019 to determine the prevalence of clinical and sub-clinical mastitis, detect the potential risk factors, and isolate bacterial pathogens causing mastitis, from lactating dairy cows. In this research, 90 dairy farms were selected using convenience sampling from total dairy farms in the areas on the basis of accessibility and willingness of the farm owners to participate in the study and grouped into small, medium-sized farms depending on the number of cows. The research included all lactating dairy cows on selected farms.
Sampling Method and Sample Size Determination
In this research, a total of 90 dairy farms were first selected using convenience sampling techniques from total dairy farms in the study area on the basis of accessibility and willingness of the farm owners to participate in the study. Then, the lactating dairy cows were selected from 90 dairy farms using a simple random sampling technique. The total number of lactating dairy cows was determined based on the number of the cattle population in each farm. The sample size was determined on the basis of the Thrusfield,30 formula that uses a 95% confidence level and 5% precision. Since there was no similar study undertaken in the district and thus, a 50% expected prevalence was assumed.
Where, Pexp = expected prevalence, d = absolute precision, n = sample size. Hence, 384 lactating cows were selected for the current study.
Study Methodology
Data Collection Methods
A structured questionnaire survey was conducted to achieve the goals of the study. For this purpose, data regarding farm location and cow-related factors, such as age, parity, body condition score, lactation stage, breed, the existence of a lesion on udder’s skin and milking method, milking, and floor type, were gathered by questioning 110 farm workers and owners. In the present study, three different breed types, namely local zebu, crossbreed (both crosses of Holstein Friesian and Jersey with local zebu breed), and Jersey were included. The study dairy cows were classified as young (three to six years), adults (seven to nine years), and old (greater than nine years old) on the basis of birth records and dentition features; lactation stage was classified into early (1st–3rd months), mid (4–6th months), and late (7th month to the beginning of dry period). The parity was recorded and grouped into few with (one-three calves), moderate (four to six calves), and seven and above calves.
Milk yield was categorized as low (1–5 liters), medium (6–10 liters), and high (greater than 10 liters). The lactation stage was categorized as less than 4 months, 4–8 months, and greater than 8 months. Herd size was also categorized into less than 10, 10–42, and greater than 42; type of floor was also classified as good (if it is made of the concrete floor), bad (if it is not the well-constructed concrete floor) and soil (if it is constructed of mud). The milking method was categorized into hand and machine. The farm hygiene was also categorized as good (clean farm husbandry) and bad (if it has poor farm husbandry). The udder lesion (present vs absent) and hygiene (washing and drying and washing only before and after milking). The farm types were classified as intensive, extensive, and semi-intensive (kept indoor based on the farm management system). Factors such as milking mastitis cow at the end (yes vs no) and previous mastitis history (yes vs no).
Clinical Inspection of Udder and Milk for Detection of Mastitis
Any anomalies of secretions, size abnormalities, consistency, and temperature of all the lactating cows in sampled farms have been carefully investigated. The mammary gland was sensed to harden and the teats to assess the teat canal capacity. Palpatory pain, changes in milk (blood-mixed milk, watery discharges, flakes, pus), and variation in udder consistency were regarded as signs of clinical mastitis and all the physical examination procedures were conducted as per the guidelines of Quinn et al.11
The CMT has been conducted as mentioned by Quinn et al11 to identify the presence of subclinical cases of mastitis. In each cup of the CMT paddle, about two mL of milk and an equivalent amount CMT were added and mixed for 15 seconds. The findings were analyzed based on gel formation and rating as 0 for negativity, 1 for weak positive, 2 for distinct positive, and 3 for the strong positive. Milk samples were graded as proof of subclinical mastitis for CMT 1, 2, and 3 test results.11
Milk Collection
Milk has been collected from both clinical and subclinical mastitis cases using standard milk sampling techniques adopted by the National Mastitis Council.31 First, the dairy cow’s udder and teat orifice has been completely watered, cleaned, and dried before collecting milk samples. In addition, dirt particles and other debris were also cleaned from the udder as well as teat with a dry towel. Then, the teats were further scrubbed with cotton, soaked in 70% alcohol to prevent recontamination. Teats on the far side of the udder were first scrubbed with alcohol and sampled, then those on the near side were sampled later. After a few millimeters of milk were discarded, about 10 mL of milk was collected by keeping the collection container nearly horizontal and all the procedures were conducted as suggested by the National Mastitis Council.31 Finally, all milk samples were labeled, transported within 2 to 3 hrs using an icebox to Veterinary Microbiology Laboratory and examined bacteriologically.
Bacterial Isolation and Identification
We present a bacteriological analysis of milk samples from both clinical and subclinical quarters using the standard bacteriological protocols.11,32 Milk samples obtained from each teat quarter were individually cultured using 7% defibrinated bovine blood on MacConkey agar and blood agar bases and incubated aerobically at 37°C for 24–48 hours. Then, plate growth, morphology, and hemolysis pattern on the blood agar base were subsequently studied. Subcultures were made for the pure identification of isolates.
The growth of bacteria on mannitol salt agar and purple agar was used to identify Staphylococci species. The fermentation of mannitol by S. aureus causes yellow discoloration of the medium. Colonies that show a weak or delayed yellow color on Mannitol Salt Agar (MSA) after 24 hrs of incubation were considered as S. intermedius and colonies that failed to produce any change in the medium were determined as S. hyicus and CNS.11 On the other hand, colonies that were grown on the MSA plate were sub-cultured on nutrient medium broth and incubated at 37°C for 24 hrs. Then, 0.5 mL of rabbit plasma and a drop of the 24 hrs old colonies taken from nutrient broth (NB) were mixed and incubated for 4–24 hrs at 37°C. The clotting of the suspension was evaluated at 30 minutes intervals for the first 4 hrs of the test and then after 24 hrs of incubation. The reaction was considered as coagulase-positive if any degree of clotting was visible.32
The detection of Streptococci species was performed on Edwards’s media according to their growth characteristics. Different biochemical tests, such as Tube coagulase test, catalase test, esculin hydrolysis test, indole production, methyl red test, Voges-Proskauer reaction, urease production, citrate utilization, and sugar fermentation, were used to identify the Staphylococci and Streptococci species.11 Also, pink-colored presumptive E. coli colonies were sub-cultured onto Eosin Methylene Blue (EMB). Colonies with a metallic green sheen on EMB were later characterized microscopically using Gram’s stain. Presumed E. coli colonies were then transferred onto nutrient agar for further identification using biochemical tests. Oxidase reaction, Catalase testing, Triple Sugar Iron (TSI), “IMViC” (indole, methyl red, Voges-Proskauer, and citrate) test, and motility test have been used to identify the E. coli species.11
Data Analysis
All data collected were analyzed using STATA version 13. Descriptive statistics were used to summarize data gathered from the incidence of mastitis, various precipitating factors, CMT, and bacterial isolation. Effects of specific variables (breed, hygienic practice, age, parity, lactation stage, and udder or teat lesion) on the occurrence of mastitis were considered using the chi-square (X2) test. The association between prevalence of mastitis and associated risk factor was assessed using logistic regression analyses. A p-value less than 0.05 was considered statistically significant.
Results
Isolation of Bacterial Pathogens from Clinical and Subclinical Mastitis Cases
Of the total 283 CMT-positive cows tested, 144 cows (50.8%) were sampled for each of their positive quarters from clinical and subclinical cases. Of the total isolates, both contagious pathogens (Staphylococcus and Streptococcus species) and environmental pathogens (E. coli) were involved. Staphylococcus species were the major pathogens out of which Staphylococcus aureus contributed the major share (45.14%). Generally, the predominant bacterial species isolated were Staphylococcus species [Staphylococcus aureus, Staphylococcus intermidus and Staphylococcus hyicus and Coagulase-negative Staphylococcus (CNS)] (52.8%), Streptococcus species (24.3%), and E. coli (8.3%) (Table 1).
 |
Table 1 Isolation Rate of Bacteria Species from Cow with Clinical and Subclinical Mastitis |
Association Mastitis with Bacterial Isolation Rate
According to the investigation, the isolation rate from the subclinical mastitic case 70.8% (102/144) was higher than that of the clinical one 29.2% (42/144). Bacterial species such as E. coli (18.41%), S. intermedius (4.98%), S. hyicus (4.48), CNS (4.98%), Streptococcus species (5.97%), and mixed (growth of different bacterial isolates) were isolated in higher proportion from quarters infected with subclinical mastitis. On the other hand, S. aureus (13.41%) was isolated in a higher proportion from clinically infected mastitic quarters. However, there is no statistically significant association between the isolation rate of different bacterial species and mastitis (clinical and subclinical) cases (Table 2).
 |
Table 2 Isolation Rate of Bacterial Species from Mastitic Quarters in the Study Areas |
Prevalence of Mastitis
The current research revealed the overall occurrence of mastitis was 73.7% (283/384), out of which 28.9% (82/283) of clinical and 71.02% (201/283) of subclinical mastitis cases were identified during the study period (Figure 1).
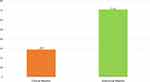 |
Figure 1 Prevalence of clinical and subclinical mastitis in the study area. |
According to the present investigation, 71.02% (201/283) of the lactating dairy cows were found to be infected with subclinical-type mastitis after the California mastitis test (CMT). Based on CMT results and clinical examination, 74.4% of the herds and 73.7% of the cows were positive for mastitis. All quarters of cows (1536) were checked for the presence of gross abnormalities, and it was found that 52 (9.15%) teats were blind, while 15 (0.7%) of them had various types of lesions. Subsequently, CMT was conducted on milk samples from 1536 quarters; out of which 568 (36.9%) were positive for mastitis. Sub-clinical mastitis was the predominant type of mastitis observed at herd 62.2% (56/90), cow 52.34% (201/384), and quarter 34.9% (536/1536) levels. There was a higher prevalence of subclinical mastitis than clinical mastitis at both cow and quarter levels (Table 3).
 |
Table 3 Prevalence of Mastitis at the Cow, Herd, and Quarter Levels |
Out of 1536 teat quarters inspected, 52 (3.4%) teat quarters were blind teat and non-functional. Out of 1484 screened functional teats, 568 (36.9%) teat quarter were infected with a subclinical type of mastitis. The left rare (58.3%) and right front (55.2%) were the most infected teat quarters during the study (Table 4).
 |
Table 4 Prevalence of Subclinical Mastitis and Blind Teat Cases Across the Four Quarters |
Associations of Risk Factors with the Prevalence of Mastitis
The present research showed that factors such as herd size, milking mastitis cow at the end, and the previous history of mastitis have a strong correlation (p < 0.05) with the prevalence of mastitis. Age is a determinant factor in the occurrence of several infections since sometimes it may predispose to stress. Hence, the prevalence of mastitis on the basis of the cows’ age groups revealed that the highest frequency was recorded in ages of 6–9 years (61.5%) when compared to 3–6 years (49.5%), and ≥9 years of age (47.2%). Also, the odd of cows infected with mastitis was 1.76 (95% CI; 1.02–3.03) higher in 7–9-year-old cows than >9-year-old cows 1.23 (95% CI; 1.02–3.03) while holding cows with 3–6 years old (Table 5).
 |
Table 5 Multivariate Logistic Regression Analysis of Risk Factors with Prevalence of Mastitis in the Study Area |
Breed differences also play a key role in mastitis occurrence. At the present research site, three breeds of cows were considered and examined at dairy farm levels. The highest prevalence of mastitis was recorded in Jersey (78.6%) followed by the crossbred of Holstein Friesian × indigenous zebu cows (51.9%), while the lowest was recorded in indigenous zebu (16.7%) breeds. The odds of mastitis infection are 4.27 and 0.11 times greater in the Jersey breed (95% CI; 1.07–17.09) and Holstein Friesian (95% CI; 0.01–1.32), respectively, while indigenous zebu breeds held constant. The highest prevalence (54.1%) was noted in cows that have a parity of 1–3 calves when compared to cows with ≥7 parity cows (50%) and cows that gave 4–6 calves (45.1%). The odds of mastitis infection were 1.08 (95% CI; 0.13–8.95) and 0.87 (95% CI; 0.49–1.55) times greater in cows with parity of seven and above, and cows with parity between four and six, respectively, while cows that gave 1–3 calves held constant.
In the present investigation, milking activities like cleaning and drying of the udder and milking mastitic cows at the end have a major role in the highest prevalence of mastitis and lead to low milk production. In comparison to frequently cleaned cows and low-milk-yielding cows, there was a higher risk of occurrence in cows with poor udder hygienic (53.4%) and medium milk yields (58.04%). The odds of mastitis prevalence is 1.08 times (95% CI; 0.63–1.84) higher in the owner that uses washing as the only method of keeping udder hygiene. On the other hand, the odds of mastitis is 0.94 times greater (95% CI; 0.59–1.49) in cows kept under poor hygienic condition as compared to those in good farm hygiene.
In cows with poor body conditions (BCS), mastitis was higher in poor (60.4%) than medium (52.5%) and good BCS (30%). The prevalence of mastitis is commonly observed in cows with prior history of mastitis (40.3%). Udder lesion and herd sizes have also played a crucial role in mastitis at the current study site. The odds of mastitis is 1.02 times greater in cows having udder lesion in reference to cows free from udder lesion. Also, the odds of mastitis is 0.32 times greater in farms with 10–42 herds size (95% CI; 0.63–1.84) than those farms having greater than 42 (95% CI; 0.63–1.84), while farms with less than 10 herd size kept constant. The prevalence of mastitis was higher with previous no history of mastitis (54.9%), but there is also a 40.3% prevalence in cows with a previous history of mastitis. The odds of mastitis is 2.7 times greater in cows with a previous history of mastistis (95% CI; 1.20–3.91) (Table 5).
Discussion
The outcome of the study revealed that the total occurrence of mastitis was 73.7%. The finding was comparable with Sori et al33 who reported a prevalence of 75.22% from 218 lactating cows in dairy farms of Jimma town, Mekibib et al13 71% from 107 crossbred milking cows in Holeta town, Zeryehun et al28 74.3% from 499 dairy cows in Addis Ababa district, and Tuke et al12 68.11% from 138 dairy cows in Alage ATVET College Dairy Farm.
The current finding was greater than the previous results of Zeryehun and Abera9 64.3% (247/384) in selected districts of Eastern Harrarghe Zone, Bedane et al34 59.1% from 460 lactating Boran breed cows in Borana, Bedacha and Menghistu,35 56.5% from 278 lactating dairy cows in Batu district, and Belina et al20 50.03% from 471 cross and pure Borana breed dairy in the North Shewa and pastoral area of Borana zones, Kitila et al17 39.67% from 532 lactating cows in west Wollega of western Oromia, Ethiopia and Dabele et al7 30.5% from 404 lactating zebu cows in selected (Toke Kutaye, Cheliya, and Dendi) districts of West Shewa Zone, western Ethiopia.
The current result is, nevertheless, less than that of Argaw and Tolosa,36 from 153 lactating cows and Dabash et al,18 from 144 lactating crossbred dairy cows around North Shewa, who reported 89.5% and 88.9%, respectively. According to the reports, mastitis prevalence might vary depending on the husbandry practiced on the farms, breed of cows, and agroecology of the study sites. Furthermore, the majority of dairy cows housed on farms were managed under intensive and semi-intensive husbandry systems, which increased the likelihood of cow-to-cow contact and, as a result, greatly contributed to a greater frequency of mastitis in the area’s dairy cows.37
The quarter-level prevalence revealed that 568 (36.9%) were CMT positive and most of the cases of mastitis at the quarter level were observed in both right fronts and left rare quarters. This was comparable with earlier reports of Zeryehun and Abera,9 who reported 41.4% in selected districts of eastern Harrarghe Zone, Ethiopia. The current quarter-level finding was higher than the report of Bitew et al,38 who reported 12.3% prevalence in and around Bahir Dar town, Girma et al,26 who reported a 10.12% prevalence in Doba District, East Hararghe zone, Dabele et al7 who reported 8.3% in selected (Toke Kutaye, Cheliya, and Dendi) districts of West Shewa Zone, Belachew,39 who reported a prevalence of 6% in Bishoftu and its surrounding, and Zeryehun et al,28 who reported a prevalence 5.2% in Addis Ababa and suburbs, Ethiopia.
This result is lower than the finding of Argaw and Tolosa,36 from 612 teat quarters and Birhanu,40 from 230 examined teat, who stated 63.1% and 52.4%, correspondingly at the teat level. This disparity in frequency might be attributed to regional differences in veterinary services, animal and farm sanitary practices. In Ethiopia, most farmers treat their diseased animals themselves, which contributes to drug abuse, degrades drug quality, and contributes to a greater prevalence of mastitis infection in the area.
From the 1536 examined teat quarters, 52 (3.4%) teat quarters were blind, and most of the cases of mastitis at the quarter level were observed in both right front and left rare quarters. The frequency of blind teats (3.4%) is comparable with the outcome of Kebebew and Jorga,6 who reported 5.5% (33/604) in Ambo town of West Shewa Zone, Oromia, Ethiopia and lower than the report of Kitila et al17 in extensively managed dairy cows in west Wollega, western Oromia, Ethiopia. The mammary gland may become blind if subclinical mastitis is not checked earlier and also if the clinical cases are not treated earlier. Another explanation for the difference in infection across the quarters is that milkers prefer to milk the left front quarter since most of them are right-handed, which raises the likelihood of infection in the left teat quarters.41 This finding is supported by the previous work of Shittu et al41 from Nigeria; however, the study of Abebe et al,5 from Hawassa, south Ethiopia, showed that the hindquarters were more infected by mastitis.
The cost of mastitis is higher because of loss in milk production and non-selling milk losses, veterinary costs, drug costs, labor, and the separation of cows infected with chronic conditions the economic loss of clinical mastitis. The current research showed that the occurrence of 28.9% for clinical mastitis, which was higher than the finding of Zeryehun et al28 19.6% (98/373) in Addis Ababa, Workineh et al42 21.5% (40/186) in the two major farms of Ethiopia, and Mekibib et al13 22.4% (24/107) in Holeta district, Bedane et al34 21.1% (97/460) in Yabello district of Borana zone, Southern Ethiopia, Sori et al43 16.11% (29/180) in Sebeta district, Tuke et al12 16.67% (23/138) in Alage College dairy farm, Zeryehun and Abera,9 12.5% (48/384) in the selected district of Eastern Harrarghe Zone, Ethiopia and Belina et al20 9.5% (45/471) in selected zones of Oromia regional states, Ethiopia.
The present study results, however, were much higher than the result of Dabele et al7 3.2% in selected districts of West Shewa Zone, Oromia, Ethiopia, Bedacha and Menghistu,35 5.3% (15/278) in Batu districts, Bitew et al38 3% (9/302), Moges et al44 in the Gondar district by 0.93% (3/322) and Dabash et al18 8.3% (12/144). This variance in mastitis prevalence between studies might indicate that the illness is complicated, interacting with a number of factors, including management methods, husbandry systems, the environment, causal factors, veterinary service coverage, and a lack of intermammary infusion.
The present study revealed that a statistically significant (p < 0.05) association was observed among the risk factors, such as herd size, previous history of mastitis, and milking mastitic cows at the end. In addition to the shortage of information from the community about dairy practice, this shows that a huge herd size may disturb the status of animal health. The presence of clinical mastitis, which is more elevated in old age animals (>9), has also been observed to be associated with aging. The other significant factor was that of previous mastitis history.
In cows with a history of prior mastitis, the frequency of clinical mastitis was greater than cows without a history of mastitis. This is attributable to the probability of shipment of pathogens to potential parities. Milk yields were also observed to be lower in higher-yielding livestock in this study in relation to clinical mastitis. This will possibly be correlated with a high degree of emphasis in terms of management of higher-than medium- and low-milk animals. The occurrence of clinical mastitis was greater in dairy cattle in the existence of udder lesions compared to cows with the lack of udder lesions.
The current research also reported that subclinical mastitis frequency was 71.02%. This finding was higher than the outcome of Zeryehun and Abera,9 51.8% (199/247) in the Eastern Harrarghe zone, Tuke et al12 51.44% (71/138) in Alage dairy farm, Zeryehun et al28 55.1% (275/373) in Addis Ababa, Bedane et al,34 38.8% (175/460) in Asella, and Mekibib et al,13 44.6% (52/107) in Holeta district, Workineh et al42 38.2% (71/186) from two major Ethiopian dairies. The results of this research were far higher than the outcomes of Bedacha and Menghistu,35 40.6% (113/278) in Batu district, Belina et al,20 40.7% (192/471) in North Shewa, and Borana pastoral area, Sori et al43 36.67% (66/180) in Sebeta district, Moges et al,44 31.67% (102/322) in Gondar district, Dabele et al7 27.2% in three districts of West Shewa Zone, Bitew et al38 25.2% (76/302) in Bahir Dar districts, and Kitila et al17 22.7% in west Wollega, western Oromia, Ethiopia and lower than the findings of Dabash et al,18 who found 80.6% (116/144) in North Shewa zone of Ethiopia.
The incidence of subclinical mastitis in milk cows can rely on environmental factors that play a vital part in the onset of the infection.15 The result indicated that clinical mastitis was lower than subclinical mastitis. Similarly, Belina et al reported that subclinical mastitis was the most prevalent in the selected areas of Oromia regional states.20 This may be due to the indistinguishable and quiet essence of subclinical mastitis, which gives the farmers little focus. Veterinarians, in contrast to the clinical mastitis during therapy, have given greater emphasis to treatment and control efforts.
In general, the invisible loss of subclinical mastitis is not well known to Ethiopian farmers, especially to small farmers because milking among these farmers is a sideline business.9,20 Compared to clinical cases that can be seen clinically, subclinical mastitis cases are not easily detected without the help of screening tests such as CMT, and the cases are mostly neglected since the majority of the dairy owners have less awareness about the disease. The office for animal development, hygiene, and marketing programs should cooperate to raise awareness of subclinical mastitis for dairy farmers in order to increase their milk production in the region. In order to avoid cross-contamination with healthier cattle, mastitis-positive cows should also be milked at the end.
The research also measured the number of parties as a predisposition factor for the occurrence of mastitis. According to Sharf et al,24 dairy cattle were found to be more resilient to mastitis during their first lactation period. Different justifications can be provided to explain the diverse causes of the decrease in teat potency,15 in the current research, the occurrence of subclinical mastitis was higher in the first three months of lactation and then decline at subsequent lactation period. Udder hygiene with the occurrence of subclinical mastitis has also been shown to be statistically important. In this study, in cows with the washed udder, the incidence of subclinical mastitis is higher than in cows with dirty udder. Dirty udder contributes to the spread of infectious and environmental pathogens in and around the teat orifice and may bring a mild subclinical infection.
In cows with udder lesions, the occurrence of subclinical mastitis is lower than in those without udder lesions. This is most likely attributed to therapies for removing the gross lesion. The incidence of subclinical mastitis was also linked with the age of dairy cows, being higher in animals of old age. This may be related to an animal’s ability to protect itself against infectious agents in the immune system. Another important reason for subclinical mastitis was the state of the body. The poor body condition of cows is another key risk factor for an increased incidence of mastitis. This may be related to the decreased cows’ immune status, which predisposes the udder to infection by various opportunistic pathogens.45 Several causes, including malnutrition or parasite infection, and reproductive stress, can lead to poor body conditions.
This research revealed that the highest frequency was observed in high-producing dairy cattle compared to those that produce lower milk yields per lactation period. Radositis et al15 also stated that high-producing dairy cattle are more susceptible to udder inflammation (mastitis) than low-producing cows. This might be as a result of the chance of injuries that are more likely to occur in bigger size udders and this predisposes the udder for infection since it creates the entrance of pathogens and stress due to a high milk yield may also disrupt the cow’s defense system.
According to the current investigation, the frequency of subclinical mastitis was higher in cows found in small herd sizes compared to farms with medium and large herd sizes. This could probably be because of the low attention given to cow management when the farm size is small. In subclinical mastitis, the breed also plays an important role. A significantly higher udder infection was detected in Jersey and crossbred cows than indigenous breeds. This was in agreement with the previous report by Lakew et al,21 who reported a strong correlation between udder infection and breed (crosses and local Arsi breed), and Biffa et al,46 who reported a significant correlation between mastitis and local zebu, Holstein Friesian, and Jersey breeds. Exotic breed cows, such as Holstein Friesian and Jersey breeds, have been found to be more susceptible to mastitis due to the size of the udder, the location of the teat, and the anatomy of the teat canal. Variations in the hereditary makeup of teat canal muscles, teat canal keratin, or teat end form where the pointed end is vulnerable to infection.15
The frequently isolated bacterial genera and species (Staphylococcus aureus, Staphylococcus intermidus, and Staphylococcus hyicus, and Escherichia coli) in the current research agree with the results of Kalla et al,47 and Abera et al25 who identified similar bacterial pathogens. Radositis et al15 described how Staphylococcus aureus commonly contaminates the udder and typically results in a minor long-lasting, and subclinical disease that is transferred through milk to healthy animals, especially during milking procedures. Staphylococcus aureus (40.3%) and Streptococcus 24.3% were predominant isolates. The remaining isolates are Coagulase-negative Staphylococcus CNS (12.5%), E. coli (8.3%), Staphylococcus hyicus (3.5%), and Staphylococcus intermidus (1.4%).
Staphylococcus species [Staphylococcus aureus and Coagulase-negative Staphylococcus (CNS)] are the most common pathogens isolated during the current study period and accounted for 52.8% of all bacterial isolates. This is comparable to the outcomes of Mekonnin et al4 and Workineh et al,42 who described 57.14% and 57% of the overall isolates, respectively. The identification of a greater number of species of Staphylococcus is due to the massive diffusion of bacteria in the teat and udder tissue.10,11,15
The current study revealed that the prevalence of Staphylococcus aureus in clinical mastitis (13.4%) is higher than in subclinical mastitis (4.48%) S. intermedius were isolated in higher proportion from clinical mastitis (6.1%) than subclinical mastitis (4.98%); however, S. hyicus were isolated in higher proportion from subclinical cases mastitis (4.48%) than clinical cases (3.66%). Similarly, Zeryehun and Abera9 reported a higher isolation of Staphylococcus aureus from clinical cases of mastitis as compared to subclinical cases. In contrast to the current study, Dabele et al7 from Toke Kutaye, Cheliya, and Dendi Districts, West Shewa Zone, Oromia, Ethiopia, and Kitila et al17 in west Wollega, western Oromia, Ethiopia. Reported higher cases of subclinical mastitis than clinical mastitis. Furthermore, Staphylococcus species live to persist in the udder, where they generally form a long-term chronic subclinical case that is masked through milk, acting as reservoirs of infection for other healthy cows and transferred during the milking process. When dry cow treatment and post-milking teat dipping are not used, the invariable hold milking technique is used, and the culling rate of chronically infected cows is low, transmission among cows rises.37
In this research, Streptococcus species (24.3%) were also responsible for the existence of mastitis. This finding coincides with that of Kassa et al,16 who reported 23.5%, but much lower than the report of Kingwill et al48 which was 80.95%. This lower incidence of Streptococcus species might be due to the widespread utilization of Penstrip in the field for the treatment of mastitis.
The other important bacterial isolate was Escherichia coli with an 8.3% share of the total isolates. The result was higher than the findings of Mekibib et al,13 4.6% in Holeta district and lesser than the outcome of Mekonnin et al,4 14.29% in and around Wolaita Sodo town. However, the current outcome was far higher than the previous findings of Sori et al,43 in the Sebeta district (0.75%). The main precipitating factor for the higher occurrence of environmental mastitis is teat canal infection by different pathogens that are associated with poor husbandry management in dairy farms.18
In the present study, 12.5% of CNS bacteria species were isolated from mastitic cow milk. This was comparable with the earlier report of Dabele et al7 who reported a 7.9% prevalence of CNS from mastitic zebu cow milk. However, the isolation rate of CNS was much lower than the finding of Zeryehun and Abera,9 who reported a 34.2% from cross-breeds of Holstein-Zebu and local Zebu cows. Few studies have proven CNS isolation from mastitis milk in Zebu cows in Ethiopia. This variation in the frequency of CNS observed from mastitic milk might be attributable to differences in dairy cow breeds, management practices, and laboratory analytical methodologies used in different nations.
Conclusion
This research revealed a significant prevalence of mastitis (73.7%) at the cow and quarter level both in clinical and subclinical mastitis forms in studied farms of Modjo district. Staphylococcus species (45.14%) such as Staphylococcus aureus, Staphylococcus intermidus, Staphylococcus hyicus, Streptococcus species (24.3%), and Escherichia coli (8.3%) were isolated from the raw milk samples and the existence of such a high prevalence of bacterial pathogens was associated with age, breed, body condition score, herd size, milking mastitic cow at the end, and previous mastitis history. Thus, awareness creation for farm owners about regular testing for subclinical mastitis, pre- and post-milking udder washing, and proper sanitation of bedding should be applied to dairy farms to overcome the problem in the study area. In addition, there should be a regular pattern of diagnosis of mastitis in order to minimize the disease prevalence and cull chronically infected animals. In addition, effective prevention strategies should be established, and identifying the most pathogenic species would ensure future research.
Abbreviation
SCM, Subclinical Mastitis; CNS, Coagulase-negative Staphylococcus; CM, clinical mastitis.
Data Sharing Statement
The analyzed data during this study will be provided on request from the corresponding author.
Ethics Clearance
The best practice guidelines for veterinary care were followed and those cattle owners were informed as to the purpose of the study, and that the Wolaita Sodo University of Research Ethics and Review Committee approved the protocol of the study with the reference number WSU 41/22/2241 and the verbally informed consent process in the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was not funded by any organization or institution.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Central Statistical Agency. Report on livestock and livestock characteristics. The Federal Democratic Republic of Ethiopia, Private peasant holdings, Statistical bulletin 570. Addis Ababa, Ethiopia: CSA; 2017.
2. Javaid S, Gadahi J, Khaskeli M, et al. Physical and chemical quality of market milk sold at Tandojam, Pakistan. Pak Vet J. 2009;29:1.
3. Ababu A, Endashaw D, Fesseha H. Isolation and antimicrobial susceptibility profile of Escherichia coli O157: H7 from raw milk of dairy cattle in Holeta District, Central Ethiopia. Int J Microbiol. 2020;2020:8. doi:10.1155/2020/6626488
4. Mekonnin E, Eshetu E, Awekew A, et al. A study on the prevalence of bovine mastitis and associated risk factors in and the surrounding areas of sodo Town, Wolaita Zone, Ethiopia. GJSFR. 2016;16(2):13–15.
5. Abebe R, Hatiya H, Abera M, et al. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12(1):1–11. doi:10.1186/s12917-016-0905-3
6. Kebebew G, Jorga E. Prevalence and risk factors of bovine mastitis in Ambo town of West Shewa Zone, Oromia, Ethiopia. Ethiop Vet J. 2016;20(1):123–134. doi:10.4314/evj.v20i1.10
7. Dabele DT, Borena Snr BM, Admasu P, et al. Prevalence and risk factors of mastitis and isolation, identification and antibiogram of Staphylococcus species from Mastitis positive Zebu cows in Toke Kutaye, Cheliya, and Dendi Districts, West Shewa Zone, Oromia, Ethiopia. Infect Drug Resist. 2021;14:987–998. doi:10.2147/IDR.S295257
8. Lema M, Kassa T, Tegegne A. Clinically manifested major health problems of crossbred dairy herds in urban and periurban production systems in the central highlands of Ethiopia. Trop Anim Health Prod. 2001;33(2):85–93. doi:10.1023/A:1005203628744
9. Zeryehun T, Abera G. Prevalence and bacterial isolates of mastitis in dairy farms in selected districts of eastern Harrarghe Zone, Eastern Ethiopia. J Vet Med. 2017;2017:7. doi:10.1155/2017/6498618
10. Megersa R, Mathewos M, Fesseha H. Isolation and Identification of Escherichia coli from dairy cow raw milk in Bishoftu Town, Central Ethiopia. Arch Vet Anim Sci. 2019;1:1.
11. Quinn PJ, Carter M, Markey B, et al. Clinical Veterinary Microbiology. Virginia, USA: Harcourt Publishers; 2002.
12. Tuke M, Kassaye D, Muktar Y, et al. Bovine Mastitis: prevalence and associated risk factors in alage ATVET College Dairy Farm, Southern Ethiopia. J Vet Sci Tech. 2017;8(04):462. doi:10.4172/2157-7579.1000462
13. Mekibib B, Furgasa M, Abunna F, et al. Bovine mastitis: prevalence, risk factors and major pathogens in dairy farms of Holeta Town, Central Ethiopia. Vet World. 2010;3(9):397–403. doi:10.5455/vetworld.2010.397-403
14. Disassa N, Sibhat B, Mengistu S, et al. Prevalence and antimicrobial susceptibility pattern of E. coli O157: H7 isolated from traditionally marketed raw cow milk in and around Asosa town, western Ethiopia. Vet Med Int. 2017;7:1–7. doi:10.1155/2017/7581531
15. Radositis M, Hinchcliff KW, Done SH, et al. Mastitis in Veterinary Medicine. London, UK: Elsevier Health Sciences; 2016.
16. Kassa F, Ayano AA, Abera M, et al. Longitudinal study of bovine mastitis in Hawassa and Wendo Genet small holder dairy farms. GJSFR. 2014;14:34–41.
17. Kitila G, Kebede B, Wakgari M. Prevalence, aetiology and risk factors of mastitis of dairy cows kept under extensive management system in west Wollega, western Oromia, Ethiopia. Vet Med Sci. 2021;7(3):1–7.
18. Dabash H, Petros A, Fekadu A. Prevalence and identification of bacterial pathogens causing bovine mastitis from crossbred of dairy cows in North Showa Zone of Ethiopia. Glob Vet. 2014;13(2):189–195.
19. Abunna F, Worku H, Gizaw F, et al. Assessment of post-harvest handling practices, quality and safety of milk and antimicrobial susceptibility profiles of Escherichia coli O157: h7Isolated from milk in and around Asella Town, Oromia, Ethiopia. Ann Public Health Res. 2018;5(1):1072.
20. Belina D, Yimer Muktar AH, Tamerat N, et al. Prevalence, isolation of bacteria and risk factors of mastitis of dairy cattle in selected zones of Oromia regional states, Ethiopia. Glob J Med Res. 2016;16(1):39–42.
21. Lakew M, Tolosa T, Tigre W. Prevalence and major bacterial causes of bovine mastitis in Asella, South Eastern Ethiopia. Trop Anim Health Prod. 2009;41(7):1525. doi:10.1007/s11250-009-9343-6
22. Lidet G, Benti D, Feyissa B, et al. Study on prevalence of bovine mastitis in lactating cows and associated risk factors in and around Areka town, Southern of Ethiopia. Afr J Microbiol Res. 2013;7(43):5051–5056. doi:10.5897/AJMR2013.6202
23. Ranjbar R, Dehkordi FS, Shahreza MHS, et al. Prevalence, identification of virulence factors, O-serogroups and antibiotic resistance properties of Shiga-toxin producing Escherichia coli strains isolated from raw milk and traditional dairy products. Antimicrob Resist Infect Control. 2018;7(53):1–11. doi:10.1186/s13756-018-0345-x
24. Sharf A, Umer M, Muhammad G. Mastitis controlling in dairy production Livestock and dairy development department. Punjab, Lahore Pakistan. Agri Soc Sci. 2009;5(3):1–31.
25. Abera M, Demie B, Aragaw K, et al. Isolation and identification of Staphylococcus aureus from bovine mastitic milk and their drug resistance patterns in Adama town, Ethiopia. J Vet Med Anim Health. 2010;2(3):29–34.
26. Girma S, Mammo A, Bogele K, et al. Study on prevalence of bovine mastitis and its major causative agents in West Harerghe zone, Doba district, Ethiopia. J Vet Med Anim Health. 2012;4(8):116–123.
27. Belayneh R, Belihu K, Wubete A. Dairy cows mastitis survey in Adama town, Ethiopia. J Vet Med Anim Health. 2013;5(10):281–287.
28. Zeryehun T, Aya T, Bayecha R. Study on prevalence, bacterial pathogens and associated risk factors of bovine mastitis in small holder dairy farms in and around Addis Ababa, Ethiopia. J Anim Plant Sci. 2013;23(1):50–55.
29. Wakeman D, Pace J. Determining the age of cattle by their teeth. J Am Vet Med Assoc. 1983;121:483.
30. Thrusfield M. Veterinary Epidemiology. John Wiley and Sons; 2018.
31. National Mastitis Council. Microbiological procedures for the diagnosis of udder infection. Arlington, VA: National Mastitis Council Inc; 2004.
32. Markey F, Leonard M, Archambault A, et al. Clinical Veterinary Microbiology.
33. Sori T, Hussien J, Bitew M. Prevalence and susceptibility assay of Staphylococcus aureus isolated from bovine mastitis in dairy farms of Jimma town, South West Ethiopia. J Anim Vet Adv. 2011;10(6):745–749. doi:10.3923/javaa.2011.745.749
34. Bedane A, Kasim G, Yohannis T, et al. Study on prevalence and risk factors of bovine mastitis in Borana pastoral and agro-pastoral settings of Yabello District, Borana zone, Southern Ethiopia. Am Eurasian J Agric Environ Sci. 2012;12(10):1274–1281.
35. Bedacha BD, Menghistu HT. Study on prevalence of mastitis and its associated risk factors in lactating dairy cows in batu and its environs, Ethiopia. Glob Vet. 2011;7(6):632–637.
36. Argaw K, Tolosa T. Prevalence of sub clinical mastitis in small holder dairy farms in Selale, North Shewa Zone, Central Ethiopia. Internet J Vet Med. 2008;5:1.
37. Radositis OM, Gay CC, Hinchcliff KW, et al. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Elsevier Ltd; 2007.
38. Bitew M, Tafere A, Tolosa T. Study on bovine mastitis in dairy farms of Bahir Dar and its environs. J Anim Vet Adv. 2010;9(23):2912–2917. doi:10.3923/javaa.2010.2912.2917
39. Belachew T. Bovine mastitis: prevalence, isolation of bacterial species involved and its antimicrobial susceptibility test around Debrezeit, Ethiopia. J Vet Sci Technol. 2016;7(6):2. doi:10.4172/2157-7579.1000396
40. Birhanu A. Risk factor, isolation and identification of major bacteria and antimicrobial susceptibility test in and around Asella. Haramaya, Ethiopia: Haramaya University; 2008.
41. Shittu M, Abdullahi J, Jibril A, et al. Sub-clinical mastitis and associated risk factors on lactating cows in the Savannah Region of Nigeria. BMC Vet Res. 2012;8(1):134. doi:10.1186/1746-6148-8-134
42. Workineh S, Bayleyegn M, Mekonnen H, et al. Prevalence and aetiology of mastitis in cows from two major Ethiopian dairies. Trop Anim Health Prod. 2002;34(1):19–25. doi:10.1023/A:1013729626377
43. Sori H, Zerihun A, Abdicho S. Dairy cattle mastitis in and around Sebeta, Ethiopia. Intern J Appl Res Vet Med. 2005;3(4):332–338.
44. Moges N, Asfaw Y, Belihu K. A cross sectional study on the prevalence of sub clinical mastitis and associated risk factors in and around Gondar, Northern Ethiopia. Int J Anim Vet Adv. 2011;3(6):455–459.
45. Mungube E, Tenhagen B-A, Kassa T, et al. Risk factors for dairy cow mastitis in the central highlands of Ethiopia. Trop Anim Health Prod. 2004;36(5):463–472. doi:10.1023/B:TROP.0000034999.08368.f3
46. Biffa D, Debela E, Beyene F. Prevalence and risk factors of mastitis in lactating dairy cows in Southern Ethiopia. Int J Appl Res Vet Med. 2005;3(3):189–198.
47. Kalla D, Butswat I, Mbap S, et al. Microbiological Examination of camel (Camelus dromedarius) milk and sensitivity of milk microflora to commonly available antibiotics in Kano, Nigeria Sav. J Agric. 2008;3:1–8.
48. Kingwill R, Neave F, Dodd F, et al. The effect of a mastitis control system on levels of subclinical and clinical mastitis in two years. Vet Rec. 1970;87(4):94–100. doi:10.1136/vr.87.4.94
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.