Back to Journals » Infection and Drug Resistance » Volume 16
Study on Antibacterial Activity and Mechanism of Improved Dian Dao San Against Cutibacterium acnes (C. acnes)
Authors An L, Gong N, Hu T, Wang L, Zhang M, Huang M, Chen G, Tang T , Liu X
Received 18 May 2023
Accepted for publication 26 July 2023
Published 1 August 2023 Volume 2023:16 Pages 4965—4975
DOI https://doi.org/10.2147/IDR.S419161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Lili An,1,2 Nan Gong,3 Taoting Hu,1 Lan Wang,1,2 Mei Zhang,1,2 Minjia Huang,1,2 Gongzhen Chen,1,2 Ting Tang,2 Xin Liu4
1Guizhou University of Traditional Chinese Medicine, Guiyang City, People’s Republic of China; 2Dermatology Department, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang City, People’s Republic of China; 3Beijing Jishuitan Hospital Guizhou Hospital, Guiyang City, People’s Republic of China; 4College of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang City, People’s Republic of China
Correspondence: Ting Tang, Dermatology Department, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang City, People’s Republic of China, Tel +8613511951295, Email [email protected] Xin Liu, College of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang City, People’s Republic of China, Tel +8618886056643, Email [email protected]
Purpose: The hyperproliferation of C. acnes has long been regarded as a primary etiological factor in the development of acne vulgaris (AV). Antibiotics targeting C. acnes have been the mainstay in AV treatment. Meanwhile, C. acnes has developed resistance to numerous antibiotics. IDDS, as traditional Chinese medicine, exhibits potent antibacterial activity against C. acnes. However, the mechanism of IDDS against C. acnes remains unclear.
Methods: In this study, we conducted a systematic investigation in vitro to determine the minimal bactericidal concentration (MBC) and time-kill curves. The MBC and time-kill curves were assessed by quantifying Colony Forming Units countsIn order to establish an in vivo rat ear model of acne, a single intradermal injection of 100μL C. acnes suspension was administered, and oleic acid was applied to the right ear pinna for a duration of 14 days. The intervention involved the utilization of IDDS medications. Additionally, the levels of inflammatory mediators tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10) were assessed using respective ELISA kits, while Hematoxylin and eosin (HE) staining was employed to visualize the rat ear model. The antimicrobial mechanism was investigated through the analysis of mRNA levels using real-time, quantitative PCR. ELISA analysis was performed according to the protocols outlined for energy metabolism and antioxidant system.
Results: Our research has demonstrated that IDDS possesses antibacterial activity against C. acnes both in vitro and in vivo. The mechanisms underlying these effects involve energy metabolism and antioxidant systems.
Conclusion: The data has provided further insights into the mechanism of IDDS against C. acnes, which establishes a robust foundation for the clinical application of IDDS.
Keywords: acne vulgaris, cutibacterium acnes, IDDS, antibacterial mechanisms
Introduction
Acne vulgaris (AV) is an extraordinarily worldwide chronic inflammatory disease with a predictive risk factors.1 It not only seriously damages the appearance of patients, but also continues to affect their physical, mental health and social life.2 AV impacts more than 85% of adolescents.3 More importantly, it has became one of the eight largest epidemic in the world. The pathogenesis of AV involves a multitude of factors, including inflammation, androgens, microorganisms, environmental influences, psychological stressors and other contributing elements.4 Among these, AV caused by microorganisms has attracted adequate interest in recent years.5 The human skin microbiome host a wide variety of microorganisms, including bacteria, viruses, and fungi.6 C. acnes accounts for approximately 90% of the human skin microbiome of healthy adults. Among these, the hyperproliferation of C. acnes has long been considered as one of the primary etiological factor of AV.7
There are various of topical measures available for treating AV including antibiotics, antiandrogens, isotretinoin, glucocorticoids and so on.8–10 Currently, antimicrobial drugs are the primary treatment for AV caused by microorganism.11 However, prolonged use of antibiotics exerts selective pressure and contributes to the development of antibiotic-resistant strains. The literature indicates that the consumption of antibiotics is linked to an increase in bacterial resistance.12 In a decade, the prevalence of multi-resistant C. acnes rose from 34.5% to 93.6%.13 The emergence of multi-resistant C. acnes has presented a significant challenge, necessitate ongoing research for the development of more effective and safe therapeutic agents. Many studies have demonstrated that traditional Chinese medicine (TCM) contains a diverse range of active components including antibacterial activity with the advantage of low drug resistance.14,15 Our previous study proved that Patrinia scabiosaefolia possessed strong antibacterial against methicillin resistant Staphylococcus epidermidis.16 Similarly, Houttuynia cordata showed good antibacterial activity to Staphylococcus aureus.14 Dian Dao San (DDS) is a classic formula to treatment AV in traditional Chinese medicine (TCM), which consists of rhubarb and sulfur, as recorded in Wu Qian’s The Golden Mirror of Medicine during the Qing Dynasty. The therapeutic effect is remarkable; however, it is accompanied by significant adverse reactions. Thus the clinical application has been greatly limited. So, many scholars have studied and improved this method to decrease its adverse reactions. Simultaneously, based on clinical application, this research group discovered that by adjusting the ratio of rhubarb to sulfur to 2:1 and employing the reverse formula (rhubarb:sulfur:1:1), adverse reactions were decrease and without compromising clinical effectiveness.
In our previous research, IDDS exhibited superior antibacterial effect against C. acnes.17 However, the antibacterial activity of IDDS and its potential mechanisms have yet to be thoroughly investigated.
Antibacterial of TCM occur mainly through inhibiting of biofilm formation, efflux pump system, enzyme activity, and destroying bacterial cell membranes, walls and so on.14 In recent years, the antibacterial mechanisms of the Oxidative stress and energy metabolism have gained widespread attention. Oxidative stress is a result of imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense systems, leading to cellular damage.18 The organism possesses a sophisticated network of antioxidant defense mechanisms that function to mitigate oxidative stress-induced damage. Most living organisms possess enzymatic defenses such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GR), as well as non-enzymatic antioxidant defenses including glutathione, thioredoxin, Vitamin C, and Vitamin E. Additionally, they have repair systems in place to protect against oxidative stress. Antioxidant stress is a critical factor in the growth of bacteria.19
In addition, energy metabolism refers to the biochemical processes through which living organisms convert nutrients into usable energy for cellular activities.20 The tricarboxylic acid (TCA) cycle serves as the primary source of cellular energy and participates in numerous metabolic pathways within cells.21 Succinate Dehydrogenase (SDH) and NADP-Malate Dehydrogenase (NAD-MDH) are pivotal enzymes in the TCA cycle. Many studies have shown that microorganisms have been obtained energy from SDH and NAD-MDH.22 Bacterial growth is dependent on the energy metabolism it generates.20
In this study, we conducted a systematic investigation of the in vitro and in vivo antibacterial activity of IDDS against C. acnes, building upon previous research findings. Furthermore, the antibacterial mechanisms were explored.
In short, our aim is to establish a comprehensive theoretical framework that can support the practical implementation of IDDS in clinical settings.
Materials and Methods
Materials
The IDDS prescription (rhubarb and sulfur and Fusidic Acid Cream were obtained from the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine) (rhubarb:20210910, sulfur:20211009, Fusidic Acid Cream:C59765).
The activity detection kit (SOD, CAT, SDH and NADP-MDH) were purchased from Beijing Solarbio Science & Technology Co., Ltd. and Elisa-kit (IL-6, IL-10 and TNF-α) were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd.
C. acnes (ATCC11827) samples were purchased from Shanghai Zhichenhui Biotechnology Company Limited (Shanghai, China).
The study utilized Wistar rats, weighing between 180 and 200 g, procured from SPF (Beijing) Biotechnology Co., Ltd in Beijing, China.
Culture Conditions
Brain heart infusion (BHI) broth (Difco, Hangzhou, China) was incubated under anaerobic conditions at 37°C for 48 hours. The culture concentration of the bacterial suspension was adjusted to approximately 1 × 106 colony-forming units CFU/mL.
Preparation of Extracts
Initially, a mixture was prepared by dissolving 30g of sulfur powder in 30g of glycerin, with the intention of creating an IDDS (rhubarb:sulfur=2:1). To this mixture, 60g of rhubarb powder were added and soaked in a paper. A total of 360 mL of purified water was then added to the mixture. The solution was soaked for 30 minutes, followed by a 30-minute decoction and subsequent filtration. The filtrate was collected, while the residue was boiled with an appropriate amount of water (approximately 180 mL). The resulting solution was simmered over low heat for 15 minutes, and the resulting filtrate was collected. Finally, the process was concluded.
Determination of Minimal Bactericidal Concentration of C. acnes with IDDS
MIC and MBC tests were performed following the Clinical and Laboratory Standards Institute 2017 (CLSI 2017) guidelines using the broth microdilution method. The MIC of IDDS against C. acnes was 62.5mg/mL in our previous study. Minimal bactericidal concentration (MBC) was evaluated as reported previously. Briefly, the MIC of IDDS was determined. MBC refers to the minimum concentration at which no visible bacterial growth is observed after incubating 100 µL subcultures from each well on BHI agar plates at 37°C for 48 h. The assay was performed in triplicate.
Determination of the Time-Kill Curves of C. acnes with IDDS
The time-kill curves test was performed based on the method provided by Qu,23 with minor modifications. Briefly, C. acnes was grown to 1×106CFU/mL in BHI. Add the IDDS to bacterial cultures to achieve final concentrations of 1/4 MIC, 1/2 MIC, MIC, 2 MIC. Control bacterial were cultivated without the additions of IDDS. The tubes containing different drug concentration were subsequently incubated in an anaerobic jar at 37°C for cultivation. Samples were collected at 0, 8, 16, 24, 32, 40, 48 hours and suitably diluted. Subsequently, a uniform application of the bacterial solution (20μL) was made onto BHI Agar medium followed by incubation at a temperature of 37°C for a duration of up to 48 hours. The assay was performed in triplicate.
Antibacterial Activity in vivo
Construction of Rat Ear Acne Model and Evaluation of IDDS Efficacy in Acne Models
The rats were allocated into experimental groups through a randomized block design. All experimental procedures and data analyses were conducted in a blinded fashion. After one week of acclimatization, the rats were randomly selected as control group (C, n = 8) or model group. The rat ear acne model was established by undergoing a single intradermal injection of 100μL C. acnes suspension and applying oleic acid in the right ear pinna for 14 days. Once rat ear acne model was established, the acne rats were divided into the acne model group (M, n = 8) and the IDDS treatment group (I, n = 8) and positive drug controlled group (P, n = 8) according to a random number control table.
Subsequently corresponding treatment for 7 days. The rats in the IDDS group received a dose of 1.0 g d−1 for 7 days. The rats in the Positive drug controlled group received Fusidic Acid Cream treatment. The model group and control group received normal saline treatment. Briefly, rats were anesthetized using intraperitoneal injections of 3% sodiumpen to barbital (SigmaAldrich, United States).
Tissue Bacterial Counts
During the construction of the rat ear acne model and the evaluation of IDDS efficacy, the quantification of viable bacteria in the rat ear acne model was conducted. Rat ears were excised and homogenized in 2mL of nutrient broth using a tissue homogenizer. Bacterial enumeration was carried out by plating 0.25 mL of tissue homogenate (prepared as 1:10 and 1:100 serial dilutions in BHI) onto nutrient agar plates in duplicate, followed by incubation for 48 hours at 37°C under anaerobic conditions. The bacterial counts were expressed as colony-forming units per gram (cfu/g). Each sample was analyzed in triplicate.
Elisa
The investigation involved the examination of cytokines, including TNF-α, IL-6, and IL-10, in treated serum samples from four distinct groups. The samples were subjected to Elisa analysis in triplicate.
Pathological Investigations
Additionally, pathological investigations were conducted on skin tissue sections obtained from rats. These sections were fixed in a 4% formalin solution, embedded in paraffin, and sliced into 3–5μm thick sections. Hematoxylin-eosin staining was performed to facilitate histopathological analysis, and the samples were observed under a light microscope (BX53; Olympus). Samples were assayed in triplicate.
All procedures were conducted in accordance with Animal Experimental Ethical Inspection of Guizhou University of Traditional Chinese Medicine and had been approved by Animal Experimental Ethical Inspection of Guizhou University of Traditional Chinese Medicine.
Antibacterial Mechanisms of C. acnes with IDDS
Bioinformatics Analysis
In our previous study we conducted proteomic analysis of IDDS on C. acnes and subsequently performed additional data analysis in this research. Proteins demonstrating up-regulation or down-regulation were identified using a cutoff value of A or ≤1/1.2-fold, with a statistical significance of p < 0.05. To analyze the sequence data of differentially expressed proteins, gene ontology (GO) was employed for analysis. The most significantly enriched cellular components, molecular functions, and biological processes were selected for analysis. Based on these findings, further investigations were conducted utilizing qPCR technology.
The Quantitative RT-PCR (QPCR)
In order to validate the hypothesis mentioned above, the differential proteins that were identified underwent qPCR analysis for mRNA validation. The bacteria were treated with varying concentrations of medication (1/2 MIC, MIC, 2MIC), along with a control group that did not receive any medication. The selection of modified proteins was narrowed down to seven specific proteins. Additionally, 16srRNA was used as an internal reference, and the primers used for the target genes are listed in Table 1. Total RNA was extracted using the Total RNA extraction kit (Omega, Beijing, China), and cDNA synthesis of C. acnes with and without IDDS was conducted following the manufacturer’s instructions (Takara, Dalian, China). qPCR was performed using SYBR Premix Ex Taq on the StepOne Real-Time PCR System, which is known for its high precision and accuracy. The reaction conditions included an initial incubation at 50°C for 15 minutes, followed by 40 cycles of amplification at 85°C for 5 seconds, and a cooling step at 4°C for 10 mins. The assays were repeated three times.
 |
Table 1 Primers for RT-PCR |
Three replicates were performed on all genes, and the relative fold changes were determined using the 2-ΔΔCt method as previously outlined. The statistical significance of differences between mean values was assessed using Student’s t-test.
The Detection of Enzyme Activity Related to Antioxidant System
The C. acnes were cultured in Brain Heart Infusion (BHI) medium until the cell density reached 1 × 106 colony-forming units per milliliter (CFU/mL). Subsequently, the bacterial cultures were subjected to treatment with an Intra-Dermal Drug Delivery System (IDDS), resulting in final viscosities equivalent to half of the minimum inhibitory concentration (1/2MIC), the MIC, and twice the MIC (2MIC). Additionally, a bacterial fluid control group was included. All groups were incubated at a temperature of 37°C for a duration of 48 hours. The cells were harvested by centrifugation at a speed of 8000 revolutions per minute (rpm) for 5 minutes at a temperature of 4°C. Sonication of the bacteria was performed using a power of 200 W, in the presence of a superoxide dismutase (SOD) and catalase (CAT) enzyme extraction solution. The sonication cycle consisted of 3 seconds of sonication followed by 10 seconds of rest, repeated for a total of 30 cycles. After sonication, the bacteria were subjected to centrifugation at a speed of 3000 rpm for a duration of 10 minutes at a temperature of 4°C. The resultant supernatant was then collected. The activity detection kit was employed to measure the levels of SOD and CAT, while considering the antioxidant system of the bacteria. Samples were assayed in triplicate.
The Detection of Enzyme Activity Related to Energy Metabolism
A culture of C. acnes was established at a concentration of 1×106CFU/mL in BHI medium. Subsequently, C. acnes was cultured at concentrations of 0MIC,1/2MIC, MIC, and 2MIC, followed by incubation for 48 hours at 37°C in an anaerobic chamber. The Cells were collected by centrifugation at 8000 rpm for 5 minutes at 4°C. Sonication was performed on the bacteria in the presence of SDH enzyme extraction solution (using a power of 200W, with 3 seconds of sonication followed by 10 seconds of rest, repeated for 5 minutes). The resulting mixture was then subjected to centrifugation at 11,000 rpm for 10 minutes at 4°C, and the resulting supernatant was collected. The bacteria underwent sonication in the presence of an extraction solution containing NADP-MDH enzyme. The sonication process consisted of a cycle of 3 seconds followed by 7 seconds of rest, repeated for a total of 30 cycles at a power level of 20%. Subsequently, the mixture was centrifuged at 8000 rpm for 10 minutes at a temperature of 4°C, resulting in the collection of the supernatant. The levels of SDH and NADP-MDH were measured using an activity detection kit, which relied on the assessment of bacterial energy metabolism. The samples were analyzed in triplicate.
Statistical Analysis
The means ± SDs were used to present the data. Normality of all data was assessed using the Kolmogorov–Smirnov test, and subsequently, the statistical differences among groups were examined through one-way ANOVA. Significance was determined if p < 0.05, and further confirmed by employing Turkey’s Honest Significant Difference test.
Results
Minimal Bactericidal Concentration and the Time-Kill Curves of IDDS
According to the Clinical and Laboratory Standards Institute (CLSI), the minimum bactericidal concentration (MBC) of IDDS against C. acnes was determined to be 125mg/mL. The efficacy of IDDS against C. acnes was assessed using the time-kill curves method, as depicted in Figure 1. In comparison to the control group, the 1/2 MIC group did not exhibit any interference with the growth of C. acnes. However, after 32 hours of IDDS treatment at the MIC level, a notable reduction in the number of viable cells was observed. Concurrently, the complete eradication of C. acnes was observed at a concentration of 2 times the minimum inhibitory concentration (MIC) of the investigational drug delivery system (IDDS). Additionally, the growth of bacterial colonies and the concentration of IDDS exhibited a direct correlation with both dosage and duration of exposure. The antibacterial properties of IDDS were evident at a concentration of 62.5 mg/mL, while bactericidal effects were observed at a concentration of 125 mg/mL, aligning with prior research findings.
 |
Figure 1 Time-kill curves of IDDS against C. acnes. |
Evaluate the Acne Model
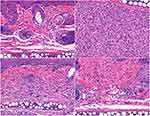
Firstly, we designed to make the rat ear acne model. The color of the auricle epidermis in the normal group was normal and the capillaries were obviously visible and soft. The auricle epidermis of model group rats was rough and dry within the skin. The skin was red and swollen and the tactile sensation of capillaries became hard and thick. And the histological results are presented in Figure 2A and B. Histological analysis of the molding area skin revealed hyperplasia of (Dermal collagen fiber degeneration and necrosis, inflammatory cell infiltration, fibrous hyperplasia). These also showed that the rat acne model was constructed successfully.
Effect of IDDS on the Acne Model
In the blank group, the auricle epidermis of the rats exhibited a normal coloration, with clearly visible and soft capillaries. Conversely, the rats in the model group displayed rough and dry auricle epidermis, accompanied by redness, swelling, and a hardened and thickened tactile sensation of the capillaries. The histological findings are depicted in Figure 2. Notably, in the I and P groups, the skin redness and swelling gradually subsided, while the capillaries regained their normal tactile characteristics. The colony count is depicted in Figure 3. Rats from the M group demonstrated significantly heightened expression of IL-10, IL-6, and TNF-α, as illustrated in Figure 4. Pathological sections are presented in Figure 2C and D. In the M group, there was a notable increase in dermal collagen fiber degeneration and necrosis, inflammatory cell infiltration, and fibrous hyperplasia. Conversely, the I group exhibited skin tissue resembling that of blank and P group rats, with reduced inflammatory cell infiltration and a decrease in the extent of dermal collagen fiber degeneration and necrosis. The blank group did not exhibit any pathological findings.
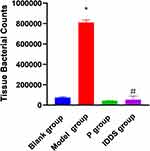 |
Figure 3 Bacterial counts with in rat ear; Drug therapy for acne treatment model. (*p < 0.05 Blank group vs Model group; (#p < 0.05 M vs IDDS group.)). |
 |
Figure 4 Expression levels of the serum inflammatory factors interleukin (IL)-6, IL-10, and tumor necrosis factor alpha (TNF-α) (*p < 0.05 M vs B rats; #p < 0.05 vs IDDS vs M rats). |
Results Obtained from Bioinformatics Analysis
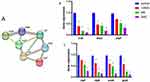
Ultimately, a total of 71 proteins were identified as differently expressed proteins (DEPs), out of which 64 were observed to be up-regulated, while 7 were found to be down-regulated. The down-regulated proteins identified, namely trxB, ahpC, ahpF, sodA, clpp, clpB, and grpE, have all been reported in numerous studies to be involved in antioxidative stress response (as shown in Figure 5A).
The qPCR Results
After subjecting C. acnes to different concentrations of IDDS, RNA was extracted to perform reverse transcription and qPCR amplification. The results demonstrated a significant inhibition of gene expression in C. acnes, specifically for trxB, ahpC, ahpF, sodA, clpp, clpB, and grpE and gap (P < 0.05), as illustrated in Figures 5B and C and 6.
In Antioxidant System and Energy Metabolism in C. acnes Revealed Novel Anti-Bacteria Mechanisms
After administering different concentrations of IDDS to C. acnes, it was observed that the group treated with the drug displayed a statistically significant reduction in the activity of SOD and CAT, SDH, and NADP-MDH compared to the control group (P < 0.05). This outcome is visually represented in Figure 7.
Discussions
C. acnes has long been recognized as one of the main pathogens causing AV,7 and the emergence of multi-drug resistant strains poses a significant challenge.24 Our preliminary investigation revealed that the MIC of IDDS against C. acnes was 62.5mg/mL.17 However, its potential antibacterial effect against C. acnes remains uncertain. So MBC and time-kill curves were explored. The finding indicated that IDDS exhibited antibacterial activity at a concentration of 62.5mg/mL and bactericidal effect at 125mg/mL. which demonstrated that IDDS was bactericide. Similar result has been demonstrated in Liu.16,25 The results demonstrate that IDDS exhibits superior in vitro antibacterial activity. Rat ear acne model was established to explore antibacterial effect in vivo. The results indicated a significant reduction in the number of clones from the IDDS group after treatment, as compared to the model group. To further investigate the relationship between AV and immune regulation, this study examines the impact of serum levels of IL-6, IL-10, and TNF-α on a rat ear acne model treated with IDDS. Serum IL-6, IL-10 and TNF-α are primarily produced by macrophages and serve as natural immune mediators. There were reports of some significance of inflammatory cytokines in the pathogenesis of AV.26 The results of IL-6, IL-10 and TNF-α indicate a significant difference between the IDDS group and the blank group, demonstrating that IDDS exerts anti-inflammatory effects. Meanwhile, histopathological examination revealed multiple inflammatory cell infiltration and fibrous tissue proliferation. Chen’s research demonstrated that tanshinones exhibited superior efficacy in a rat model of acne.27 Therefore, the above studies have demonstrated that IDDS exhibits superior antibacterial and anti-inflammatory effects. Furthermore, we conducted proteomic analysis of IDDS against C. acnes and subsequently investigated the altered proteins. The results indicated that the down-regulated proteins were associated with anti-oxidative stress and energy metabolism. Oxidative stress is defined as the imbalance between the production of reactive oxygen species (ROS) and the endogenous antioxidant defence system.18 However, virtually all of these microorganisms suffer poor growth, even death when they are exposed to oxygen levels that exceed those of their native habitats.28 Defense mechanisms have the ability to counteract the killing effect of ROS.
Antioxidant enzymes CAT and SOD are considered to be the efficient defense barriers to protect organisms from negative impacts of toxicants.29 So the activity of CAT and SOD in antioxidant stress system were investigated. The results displayed (Figure 7) that CAT and SOD treated with IDDS significantly reduced (p < 0.05) when compared with control group. The present study analyzed bacterial CAT and SOD levels to examine the effect of IDDS exercise on oxidant-antioxidant status. Thence, we believed that the IDDS had stronger effect on the two key enzymes in antioxidant enzyme system,30 indicating that IDDS can enhance oxidative stress.
Based on comparative proteomics, the antibacterial mechanism was closely related to energy metabolism. The tricarboxylicacid (TCA) cycle is the main source of cellular energy and participates in many metabolic pathways.21,27 In TCA cycle, MDH participates in the oxidation of malate to oxaloacetate, which is essential for cell growth. Furthermore, in the TCA cycle, SDH catalyzes the oxidation of succinic acid to fumaric acid and transfers electrons from succinic acid to ubiquinol, playing an important role in the energy metabolism of bacteria.22 To further illustrate the impact of the IDDS on C. acnes’ energy metabolism, we assessed the activities of MDH and SDH, which are crucial enzymes associated with the TCA cycle. As illustrated in Figure 7, the findings indicate a reduction in SDH and NADP-MDH activity under IDDS. The above results were consisted with previous research reported by Li et al, that the SDH and NADP-MDH enzyme activity of Botrytis cinerea was significantly suppressed under tea tree oil.30 Chen showed that essential oil treated with methicillin-resistant Staphylococcus aureus suffered a decrease in the activities of TCA-related enzymes and disrupted the TCA pathway.31 Our findings demonstrate that the IDDS exerts an inhibitory effect on the TCA cycle through modulation of key enzyme activities. Despite our study’s strengths and novelty, we acknowledge its limitations. Despite our efforts to clarify the antibacterial mechanism related to anti-oxidative stress and energy metabolism, the precise molecular mechanism remains elusive. Future studies must be conducted to clarify the mechanism.
Conclusion
In summary, our studies demonstrated that IDDS exhibits antibacterial activity against C. acnes both in vitro and in vivo. The IDDS has the potential to attenuate the activities of antioxidant and TCA-related enzymes. Our data has provided further insight into the mechanism of IDDS against C. acnes, which offers a solid foundation for the clinical application of IDDS.
Acknowledgments
We express our gratitude for the financial support provided by the earmarked funding of National Nature Science Foundation of China under Grant number 82260824, Science and Technology Foundation of Guizhou Province under Grant number Qianke He Foundation-ZK [2021]General 08 and QianKeHe Foundation-ZK (2021) General 511, Guiyang Science and Technology Plan Project from the Guiyang Science and Technology Bureau ((2019) 9-2-6), and 2021 National Natural Science Foundation of China Post-Subsidy Fund Scientific Research Innovation Exploration Special Project from the Guizhou University of Traditional ChineseMedicine (no. 2019YFC171250506), and 2022 Graduate Education Innovation Program of Guizhou University of Traditional Chinese Medicine (no: YCXZRS202211), Project of Jia Min Inheritance Studio, a renowned national expert in traditional Chinese medicine: Teaching Letter from the National Administration of Traditional Chinese Medicine [2021] No.270; Provincial Clinical Research Base Construction Project in 2021 (Guizhou Provincial Health Commission), Project No. 1415.
Disclosure
There are no conflicts of interest declared by the authors in this work.
References
1. Eichenfield DZ, Sprague J, Eichenfield LF. Management of acne vulgaris: a review. JAMA. 2021;326(20):2055–2067. doi:10.1001/jama.2021.17633
2. Xiang Y, Lu J, Mao C, et al. Ultrasound-triggered interfacial engineering-based microneedle for bacterial infection acne treatment. Sci Adv. 2023;9(10):eadf0854. doi:10.1126/sciadv.adf0854
3. Habeshian KA, Cohen BA. Current issues in the treatment of Acne vulgaris. Pediatrics. 2020;145(Suppl 2):S225–S230. doi:10.1542/peds.2019-2056L
4. Oulès B, Philippeos C, Segal J, et al. Contribution of GATA6 to homeostasis of the human upper pilosebaceous unit and acne pathogenesis. Nat Commun. 2020;11(1):5067. doi:10.1038/s41467-020-18784-z
5. Do TH, Ma F, Andrade PR, et al. TREM2 macrophages induced by human lipids drive inflammation in acne lesions. Sci Immunol. 2022;7(73):eabo2787. doi:10.1126/sciimmunol.abo2787
6. Réno B, Dagnelie MA, Khammari A, Corvec S. The skin microbiome: a new actor in inflammatory acne. Am J Clin Dermatol. 2020;21(Suppl 1):18–24. doi:10.1007/s40257-020-00531-1
7. Castillo DE, Nanda S, Keri JE. Propionibacterium (Cutibacterium) acnes bacteriophage therapy in acne: current evidence and future perspectives. Dermatol Ther. 2019;9(1):19–31. doi:10.1007/s13555-018-0275-9
8. Wang S, Jiang R, Meng T, et al. Stem cell membrane-coated isotretinoin for acne treatment. J Nanobiotechnology. 2020;18(1):106. doi:10.1186/s12951-020-00664-9
9. Chovatiya R. Acne treatment. JAMA. 2021;326(20):2087. doi:10.1001/jama.2021.16599
10. Bagatin E, Costa CS. The use of isotretinoin for acne - an update on optimal dosing, surveillance, and adverse effects. Expert Rev Clin Pharmacol. 2020;13(8):885–897. doi:10.1080/17512433.2020.1796637
11. Bunick CG, Keri J, Tanaka SK, et al. Antibacterial mechanisms and efficacy of sarecycline in animal models of infection and inflammation. Antibiotics. 2021;10(4):439. doi:10.3390/antibiotics10040439
12. Maeda T, Iwasawa J, Kotani H, et al. High-throughput laboratory evolution reveals evolutionary constraints in Escherichia coli. Nat Commun. 2020;11(1):5970. doi:10.1038/s41467-020-19713-w
13. Schafer F, Fich F, Lam M, Gárate C, Wozniak A, Garcia P. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52(4):418–425. doi:10.1111/j.1365-4632.2011.05371.x
14. Wu Z, Deng X, Hu Q, et al. Houttuynia cordata Thunb: an ethnopharmacological review. Front Pharmacol. 2021;12:714694. doi:10.3389/fphar.2021.714694
15. Li J, Feng S, Liu X, et al. Effects of traditional Chinese medicine and its active ingredients on drug-resistant bacteria. Front Pharmacol. 2022;13:837907. doi:10.3389/fphar.2022.837907
16. Liu X, An L, Zhou Y, Peng W, Huang C. Antibacterial mechanism of Patrinia scabiosaefolia against methicillin resistant Staphylococcus epidermidis. Infect Drug Resist. 2023;16:1345–1355. doi:10.2147/IDR.S398227
17. Ren S, Wang W, Jia M, et al. Proteomic analysis of the antibacterial effect of improved DianDaoSan against Propionibacteriu macnes. Evid Based Complement Alternat Med. 2022;2022. doi:10.1155/2022/3855702
18. Guo Y, Liu Y, Zhao S, et al. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat Commun. 2021;12(1):7094. doi:10.1038/s41467-021-27428-9
19. Piccini C, Cai G, Dias MC, et al. Olive varieties under UV-B stress show distinct responses in terms of antioxidant machinery and isoform/activity of RubisCO. Int J Mol Sci. 2021;22(20):11214. doi:10.3390/ijms222011214
20. Liu L, Zeng X, Zheng J, Zou Y, Qiu S, Dai Y. AHL-mediated quorum sensing to regulate bacterial substance and energy metabolism: a review. Microbiol Res. 2022;262:127102. doi:10.1016/j.micres.2022.127102
21. Arnold PK, Jackson BT, Paras KI, et al. A non-canonical tricarboxylic acid cycle underlies cellular identity. Nature. 2022;603(7901):477–481. doi:10.1038/s41586-022-04475-w
22. Kang W, Suzuki M, Saito T, Miyado K. Emerging role of TCA cycle-related enzymes in human diseases. Int J Mol Sci. 2021;22(23):13057. doi:10.3390/ijms222313057
23. Qu Q, Wang J, Cui W, et al. In vitro activity and in vivo efficacy of isoliquiritigenin against Staphylococcus xylosus ATCC 700404 by IGPD target. PLoS One. 2019;14(12):e0226260. doi:10.1371/journal.pone.0226260
24. Dessinioti C, Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. 2017;35(2):163–167. doi:10.1016/j.clindermatol.2016.10.008
25. Liu X, An L, Ren S, Zhou Y, Peng W. Comparative proteomic analysis reveals antibacterial mechanism of Patrinia scabiosaefolia against methicillin resistant Staphylococcus epidermidis. Infect Drug Resist. 2022;15:883–893. doi:10.2147/IDR.S350715
26. Li S, Sun W, Zhang K, et al. Selenium deficiency induces spleen pathological changes in pigs by decreasing selenoprotein expression, evoking oxidative stress, and activating inflammation and apoptosis. J Anim Sci Biotechnol. 2021;12(1):65. doi:10.1186/s40104-021-00587-x
27. He Y, Yang Q, Zhang T, et al. Pathogenic characteristics of Th17 cells based on the IL-17 signaling pathway in the regulation of sebaceous gland lipoprotein metabolism in an acne rat model. Iran J Immunol. 2021;18(3):203–209. doi:10.22034/iji.2021.88231.1855
28. Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11(7):443–454. doi:10.1038/nrmicro3032
29. Jing M, Han G, Wan J, et al. Catalase and superoxide dismutase response and the underlying molecular mechanism for naphthalene. Sci Total Environ. 2020;736:139567. doi:10.1016/j.scitotenv.2020.139567
30. Li Z, Shao X, Wei Y, et al. Transcriptome analysis of Botrytis cinerea in response to tea tree oil and its two characteristic components. Appl Microbiol Biotechnol. 2020;104(5):2163–2178. doi:10.1007/s00253-020-10382-9
31. Chen J, Tang C, Zhang R, et al. Metabolomics analysis to evaluate the antibacterial activity of the essential oil from the leaves of Cinnamomum camphora (Linn.) Presl. J Ethnopharmacol. 2020;253:112652. doi:10.1016/j.jep.2020.112652
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.