Back to Journals » Journal of Inflammation Research » Volume 15
Studies on HBcAg-rBlo t 5-21 Fusion Protein Vaccine That Alleviates Blomia tropicalis Airway Inflammation
Authors Pei Y , Xiao Z, Wei S, Peng M, Luo C, Wang D
Received 16 July 2022
Accepted for publication 6 November 2022
Published 18 November 2022 Volume 2022:15 Pages 6343—6355
DOI https://doi.org/10.2147/JIR.S380526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yechun Pei,1– 3,* Zhengpan Xiao,1,2,* Shuangshuang Wei,1,2 Meiqi Peng,1,2 Chenghui Luo,1,2 Dayong Wang2,3
1School of Life Sciences, Hainan University, Haikou, People’s Republic of China; 2Laboratory of Biopharmaceuticals and Molecular Pharmacology, School of Pharmaceutical Sciences, Hainan University, Haikou, People’s Republic of China; 3One Health Institute, Hainan University, Haikou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yechun Pei, Department of Biosciences, School of Life Sciences, Hainan University, 58 People’s Road, Haikou, Hainan, 570228, People’s Republic of China, Email [email protected] Dayong Wang, Laboratory of Biopharmaceuticals and Molecular Pharmacology, School of Pharmaceutical Sciences, Hainan University, 58 People’s Road, Haikou, Hainan, 570228, People’s Republic of China, Email [email protected]
Background: In tropical and subtropical areas, allergens from the dust mite species Blomia tropicalis are common causes of allergic rhinitis and asthma. Blomia tropicalis has two main allergens: Blo t 5 and Blo t 21.
Aim: To generate a chimeric virus-like particle containing HBcAg, Blo t 5 and Blo t 21 that can treat allergies caused by Blomia tropicalis.
Methods: To produce allergic asthma in mice, prokaryotic expression and purification of Blomia tropicalis allergens rBlo t 5, rBlo t 21, and recombinant fusion allergen rBlo t 5– 21 were utilized in the study. We created a hepatitis B core antigen (HBcAg) and rBlo t 5– 21 fusion prokaryotic expression plasmid. HBcAg-rBlo t 5– 21 was purified after expression and tested by transmission electron microscopy (TEM). Furthermore, the protein HBcAg-rBlo t 5– 21 was employed as a protein vaccination.
Results: In allergy-induced mouse model experiments, the fusion allergen rBlo t 5– 21 was more effective than the individual allergens rBlo t 5 and rBlo t 21 at inducing allergy. We found that vaccinating allergic mice with the recombinant fusion protein vaccine HBcAg-rBlo t 5– 21 alleviated allergy symptoms elicited by the rBlo t 5– 21 allergen. Vaccination with HBcAg-rBlo t 5– 21 resulted in a decrease in total serum IgE levels, suppression of anaphylaxis, and reduction of inflammatory cell infiltration into lung tissue as compared to the PBS group.
Conclusion: HBcAg-rBlo t 5– 21, a protein vaccine containing both the hepatitis B core antigen and the Blomia tropicalis fusion allergen rBlo t 5– 21, could be a suitable vaccination for preventing allergy disorders caused by Blomia tropicalis.
Keywords: Blomia tropicalis, vaccine, hepatitis B core antigen, asthma
Introduction
The prevalence of allergic diseases and asthma is increasing worldwide, particularly in low- and middle-income countries.1 House dust mite (HDM) is a predominant source of indoor aeroallergen, which induced allergic diseases including allergic rhinoconjunctivitis, allergic asthma, atopic eczema and other allergic skin diseases.2,3 And it is now clear that dust mite allergy is a key contributor to asthma in many parts of the world.4 Blomia tropicalis is a mite that is considered a house dust mite of tropical and sub-tropical areas, sensitization to this mite is very common in South America and Southeast Asia.4 Specific IgE level induced by Blomia tropicalis sensitization was significantly higher in patients with persistent asthma compared to intermittent asthma.5
So far, allergen-specific immunotherapy (AIT) is the only potential disease modification therapy for HDM allergic subjects.5 With the efforts of doctors and researchers in the field of allergy, the efficacy, safety, standardization and strategy of AIT are constantly developing.6–10 At present, the possibility of using recombinant protein as a substitute for crude allergen extract can significantly affect the current AIT regimen.11,12 As a result, various recombinant allergens have been produced for AIT from different allergens (such as pollen, animal dander, mites and various foods).13–17 Blo t 5 and Blo t 21 are known major allergens in Blomia tropicalis, and studies demonstrate that they include key epitopes that can be recognized by IgE antibodies in people allergic to Blomia tropicalis antigens.18 Despite these allergens have very similar three-dimensional (3D) structures, they do not cross-react, although co-sensitization is a common feature.18–21 The major linear IgE epitopes of Der p 5 and Blo t 5 are involved in species-specific recognition.22 Traditionally, OVA, an egg allergen, has been used in animal asthma models (5pc6), but has been almost replaced by respiratory allergens, such as Dermatophagoides pteronyssinus23–25 and Blomia tropicalis.26–28 And a new mouse model of severe asthma caused by Blo t 5 has been developed, which results in extensive lung inflammation, a strong adoptive and host Th2 response, airway remodeling, and the formation of IL4/IL-13-dependent iBALT.29
AIT is only disadvantageous to individuals sensitized by mild allergens because successful treatment is dose-dependent; therefore, successful treatment may be related to allergen concentration.30 Therefore, it is necessary for individuals sensitized by mild allergens to improve the immunogenicity of low-dose allergens. Hepatitis B core antigen (HBcAg) is an efficient, safe and widely used virus-like particles (VLP) vector due to its self-assembly into virus-like particles and strong immunogenicity.31 They cause T-cell-dependent and T-cell-dependent immune responses and can be easily manipulated.21 The fusion of HBcAg immunodominant internal sites reduced the immunogenicity and antigenicity of HBcAg and most significantly enhanced the immunogenicity of inserted foreign epitopes.32 The present study aimed to generate HBcAg-rBlo t 5–21 chimeric virus-like particles, which can potentially treat the allergic reaction caused by Blomia tropicalis.
Materials and Methods
Animals
Adult female BALB/c mice (6–8 weeks old) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China) and housed in a pathogen-free environment with free access to food and water ad lib.
Design of Expression Vectors of Recombinant Allergens of Blomia Tropicalis
The CDS sequences of Blo t 5 and Blo t 21 were obtained from GenBank (GenBank: U59102 and GenBank: DQ788677.1, respectively). After codon optimization, the sequences of rBlo t 5, rBlo t 21, and rBlo t 5–21 were synthesized and inserted into the pQE80L vector between BamHI and SalI restriction sites. A truncated version of HBcAg gene (coding for HBcAg lacking the arginine-rich C-terminal domain, amino acids 1 through 149) was obtained from GenBank (GenBank: GU827636.1). In the truncated HBcAg gene, the corresponding sequences of 79th amino acid residue P and 80th amino acid residue A were replaced by the rBlo t 5–21 gene sequence. Glycine-rich flexible amino acid sequences (G4S)2G4 (Sequence: GGCGGCGGCGGCTCTGGTGGTGGTGGTTCTGGCGGTGGCGGT) are located on either side of rBlo t 5–21. Then, the chimeric HBcAg-rBlo t 5–21 sequence was inserted into the vector pQE80L. The resultant expression vector was named as pQE80L-HBcAg-rBlo t 5–21.
Expression of Recombinant Protein
Expressions of all of the recombinant proteins were induced at 37℃ with 0.1 mM of IPTG. After 4–5 h, cells were harvested, resuspended in native lysis buffer, and disrupted by sonication. The fusion proteins were purified by Ni-NTA agarose (cat. No. 30210; Qiagen, Hilden, Germany). Imidazole was then removed by ultrafiltration. According to the manufacturer’s instruction, bacterial endotoxins were removed using the ToxinEraser™ Endotoxin Removal Kit (Cat. No. L00338, GenScirpt, USA). The endotoxin level was determined using the ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit. Endotoxin-free protein contains less than 0.15 EU/mL of endotoxin. The Endotoxin-free protein was concentrated to 1 mg/mL in PBS.
Transmission Electron Microscopy
The purified rBlo t 5–21 and HBcAg-rBlo t 5–21 protein (2 mg/mL in PBS) were loaded onto a grid and stained with saturated uranyl acetate for 2 min at room temperature. The samples were dried and visualized under a transmission electron microscope (Hitachi, Tokyo, Japan) at a nominal magnification of 100,000–250,000.
Immunization, Sensitization and Vaccination
On Days 0 and 7, native BALB/c mice (6–8 weeks old) were injected intraperitoneally with 200 μg rBlo t 5–21 mixed in 100 μL Alum (50 mg/mL Al(OH)3; Sigma-Aldrich, USA) for rBlo t 5–21 protein vaccination. Intratracheal challenges were then carried out. In brief, mice were anesthetized with isoflurane, and then 100 μg of rBlo t 5–21 was delivered to the back of each animal’s tongue on Days 14–20. Sensitized mice were vaccinated subcutaneously with either PBS or 100 μg of HBcAg-rBlo t 5–21 in 100 μL PBS on day 35 and boosted on day 42.
Acute Systemic Anaphylaxis
Sensitized mice were challenged intravenously with 50 μg of rBlo t 5–21 per 100 µL PBS to induce anaphylaxis. Body temperatures were taken using a rectal probe digital thermometer shortly after intravenous antigen challenge and monitored for up to 90 minutes. The area above the curve was determined using Prism (GraphPad 6.02 Software, Inc., San Diego, CA, USA).
Ear Prick Tests
Mice were injected intravenously with 200 µL of Evans blue solution (0.5%; Sangon Biotech, Shanghai, China) and anesthetized 30 min later with isoflurane. Afterward, a drop of rBlo t 5–21 solution (10 μg/20 μL PBS) was placed onto the outer ear skin. Pricks were made into the ear skin with 23-gauge needles (SteriLance, Suzhou, China). For intradermal ear prick tests, an rBlo t 5–21 solution (10 μg/10 μL PBS) was injected intradermally into the ears. Extravasations of dye began almost immediately after the antigen challenge. The mice were euthanized an hour later, and their ears were processed for densitometry analysis of dye leakage. For this purpose, the area of dye leakage was defined within the densitometer by gating around the leaked dye. The magnitude of dye leakage per ear was determined by a combination of the size and color intensity of the pricked area, which was reported as net intensity by the densitometer.
Histology and Inflammation Score
Lung samples from mice were collected from each group, fixed in 4% paraformaldehyde and embedded in paraffin blocks. Sections were then cut and fixed. Antigen retrieval was accomplished by boiling the slides in 0.01 M citrate buffer (pH 6.0) followed by hematoxylin and eosin (H&E) staining with for evaluation of cell invasion and analyzed under a light microscope for determining histology changes.
Lung inflammation was blindly scored. A value of 0 to 3 per criterion was adjudged to evaluate the degree of peribronchial inflammation. A value of 0 was for no inflammation, a value of 1 was for occasional cuffing with inflammatory cells, a value of 2 for most bronchi surrounded by thin layer (one to five cells) of inflammatory cells and a value of 3 was for most bronchi surrounded by a thick layer (more than five cells thick) of inflammatory cells. Each group used 5 mice for H&E staining and scoring analysis.
Determination of Antigen-Specific IgG, IgG1, IgG2a, and IgE
For determination of rBlo t 5–21-specific IgG/IgG1/IgG2a, 96-well ELISA plates (Sangon Biotech, Shanghai, China) were coated with 10 µg of rBlo t 5–21 in carbonate buffer at 4℃ overnight. The plates were rinsed five times with PBS containing 0.05% Tween (PBST), then blocked with 2% nonfat milk solution for 2 hours before being washed five times more with PBST. Serial dilutions of sera were added to the plates and incubated for 2 h at 37℃. The plates were then washed five more times with PBST. Thereafter, HRP-conjugated rabbit anti-mouse IgG (Sangon Biotech, Shanghai, China), HRP-conjugated goat anti-mouse IgG1 (cat. No. PA1-74421, Thermo Fisher Scientific, Waltham, MA, USA), and HRP-conjugated goat anti-mouse IgG2a (cat. No. A10685, Thermo Fisher Scientific, Waltham, MA, USA) antibodies were incubated at 37℃ for 2 h. Plates were washed five times with PBST, followed by the addition of the substrate 3,3’,5,5’-tetramethylbenzidine (Cat. No. C520026, Sangon Biotech, Shanghai, China) and incubated in the dark for 30 min. Then, the stop solution is added to the 96-well plates (Buffer D of EL-TMB Chromogenic Reagent kit, Cat. No. C520026, Sangon Biotech, Shanghai, China). Optical densities were measured at 450 nm.
For the determination of rBlo t 5–21-specific IgE, 96-well plates were coated with 10 µg of rBlo t 5–21 in carbonate buffer at 4℃ overnight. After washing with PBST and blocking with 2% nonfat milk solution for 2 h at 37℃, the sera of individual mice were diluted 1:5 and incubated for 2 h at 37℃. Goat anti-mouse IgE (cat. No. ab19967; Abcam, Cambridge, UK) antibody was added and incubated at 37℃ for 2 h. After washing five times with PBST, plates were incubated with an HRP-conjugated rabbit anti-goat IgG (Sangon Biotech, Shanghai, China) antibodies for 2 h at 37℃. Thereafter, plates were washed five times with PBST, followed by the addition of the substrate 3.3’,5,5’-tetramethylbenzidine (Cat. No. C520026, Sangon Biotech, Shanghai, China) and incubated in the dark for 30 min. Then, added stop solution to stop raction (Buffer D of EL-TMB Chromogenic Reagent kit, Cat. No. C520026, Sangon Biotech, Shanghai, China). Optical densities were measured at 450 nm.
Statistical Analysis
All statistical analysis comparing two different groups were performed with two tailed Student’s t-tests. The resulting p values are either indicated in figure legends or with asterisks in figures (*p < 0.05; **p < 0.01).
Results
Expression and Validation of Recombinant Fusion Proteins
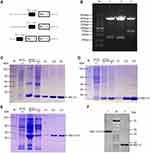
The recombinant plasmids PQE80L-rBlo t 5, PQE80L-rBlo t 21, and PQE80L-rBlo t 5–21 were constructed as shown in Figure 1A. The recombinant plasmid was digested by BamHI and SalI and detected by gel electrophoresis (Figure 1B). All positive bands were detected in the corresponding location (rBlo t 5, Lane 1; rBlo t 21, Lane 2; and rBlo t 5–21, Lane 3). Blomia tropicalis recombinant allergens were expressed and purified as full-length fusion proteins containing N-terminal His-tags (6×His). Once the fusion proteins were purified, their tags were not removed. Each His-tagged protein (rBlo t 5, rBlo t 21, and rBlo t 5–21) produced an expected band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 1C–E). Validation of recombinant fusion proteins (rBlo t 5 and rBlo t 5–21) was detected by Western blotting using biotin4D9 anti-Blo t 5 (Figure 1F).
Establishment of the Allergic Mouse Model
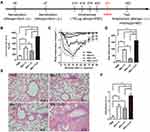
To investigate the efficacy of the virus-like particle vaccine constructed in our study, we established an allergic mouse model. BALB/c mice were sensitized and intratracheal challenged with rBlo t 5, rBlo t 21 and rBlo t 5–21 (Figure 2A). Since the significant increase in IgE level is a typical feature of type I hypersensitivity, we measured the total IgE level in the serum of sensitized mice (Figure 2B). Compared with the groups induced by PBS, rBlo t 5, and rBlo t 21, the total IgE level of the sensitized mice induced by rBlo t 5–21 was significantly increased.
To assess acute systemic anaphylactic reactions, sensitized mice were challenged intravenously with corresponding allergens to induce anaphylactic syndromes as documented by a rapid drop in temperature (eg rBlo t 5-induced allergic mouse model group, mice were injected with rBlo t 5 through tail vein). However, mice in the PBS group were injected with rBlo 5–21 recombinant allergen. As shown in Figure 2C and D, rBlo t 5–21-induced allergic mouse model group experienced anaphylaxis with severe drops in body temperature.
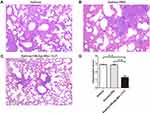
Airway inflammation was evaluated with hematoxylin and eosin staining. The mice were sacrificed, and their lung tissue was removed and fixed by 4% paraformaldehyde. The tissues were then stained with hematoxylin and eosin after paraffin sectioning. According to Figure 2E, the infiltration of inflammatory cells in rBlo t 5–21-, rBlo t 21-, and rBlo t 5-induced mice were significantly higher than that in the PBS group. The Invading cells in the rBlo t 5–21 induce group was significantly higher than those in the rBlo t 5-induced or rBlo t 21-induced group (Figure 2F). The results showed that the best induction effect in mouse allergic model was rBlo t 5–21, followed by rBlo t 5 and rBlo t 21.
Expression and Validation of Recombinant Fusion Protein HBcAg-rBlo t 5-21
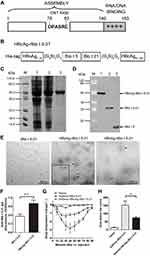
We displayed the major Blomia tropicalis recombinant fusion protein rBlo t 5–21 in a highly ordered fashion on HBcAg particle. The details of the constructs are schematically shown in Figure 3A and B. The HBcAg-rBlo t 5–21 protein was purified (lane 3, Figure 3C) and characterized by were detected by Western blotting using biotin4D9 anti-Blo t 5 (Cat. No, MA-4D9, Indoor Biotechnologies, USA) (Figure 3D). To confirm that fusion protein HBcAg-rBlo t 5–21 can form virus-like particles (VLP) structures, the purified HBcAg-rBlo t 5–21 protein was analyzed by transmission electron microscopy (TEM). TEM micrographs confirmed the presence of spherical, capsid-like particles (Figure 3E, middle and right, black arrow), whereas no such structures were observed in the preparation of rBlo t 5–21 protein (Figure 3E, left).
In order to evaluate whether HBcAg-rBlo t 5–21 has strong immunogenicity, normal mice were randomly divided into two groups. Mice were subcutaneously immunized with rBlo t 5–21 and HBcAg-rBlo t 5–21 on day 0 and day 14, respectively, and serum samples were collected on day 21 to detect IgG levels. As shown in Figure 3F, the HBcAg-rBlo t 5–21 immunized group was able to induce higher levels of IgG than the rBlo t 5–21 immunized group. Furthermore, the rBlo t 5–21-induced allergic mice were randomly divided into two groups, which were immunized with rBlo t 5–21 and HBcAg-rBlo t 5–21, respectively. We found that HBcAg-rBlo t 5–21 could alleviate the decrease of body temperature caused by acute systemic anaphylaxis. However, the body temperature of mice immunized with rBlo t 5–21 protein decreased significantly (Figure 3G and H). These results indicated that HBcAg-rBlo t 5–21 has good immunogenicity and may be used to inhibit anaphylaxis induced by Blomia tropicalis.
Vaccination with HBcAg-rBlo t 5-21 Can Alleviate rBlo t 5-21-Induced Allergic Symptoms
To investigate whether the HBcAg-rBlo t 5–21 vaccine could treat the established allergic inflammation, BALB/c mice were sensitized and intratracheal challenged with rBlo t 5–21 allergen, then randomly divided into two groups and immunized with PBS or HBcAg-rBlo t 5–21. Naïve mice were negative control (Figure 4A). Mice in naive group have very low IgE levels and sensitive mice have very high IgE levels on day 34 (Figure 4B). The allergic mice vaccinated with HBcAg-rBlo t 5–21 led to a significant decrease in IgE titers at Days 41 and 48 (Figure 4B). However, the titers of IgE were still at a high level. To assess whether vaccinated mice were protected from allergic reactions, all groups were challenged intravenously with rBlo t 5–21, and body temperature was monitored for a maximum of 90 min after challenge. A severe drop in body temperature was observed in mice that had been treated with PBS (Figure 4C and D). In contrast, HBcAg-rBlo t 5–21-treated allergic mice were completely protected from anaphylactic reactions.
Figure 4 Continued.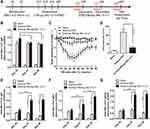
Vaccination with HBcAg-rBlo t 5–21 led to a significant increase in rBlo t 5–21-specific IgG, IgG1, and IgG2a titers at Days 41 and 48 (Figure 4E–G). In addition, the ratio of IgG1 to IgG2a in HBcAg–rBlo t 5–21 immunized group gradually decreased significantly, but there was no significant difference in PBS immunized group on days 34, 41 and 48 (Figure 4H).
To inquire into the effects of vaccination on local allergic reactions, ear skin prick tests with rBlo t 5–21 were performed in a different set of mice. Extravasations of Evans blue began almost immediately after the allergen challenge. We can observe that the dye leakage was very obvious in the asthma model group and the asthma-PBS group, while the dye leakage circle was significantly reduced in the asthma-HBcAg-rBlo t 5–21 group (Figure 4I). Statistical analysis of Evans blue leakage circle also showed that asthma-HBcAg-rBlo t 5–21 group was significantly smaller than the other two groups (Figure 4J).
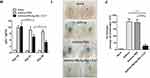
We next sought to assess whether vaccinated animals have improved pulmonary inflammation. As shown in Figure 5, as compared with the asthma model group (Figure 5A) and asthma-PBS group (Figure 5B), the infiltration of cells in lung tissue of the HBcAg-rBlo t 5–21-treated group (Figure 5C) was significantly lower. The cell infiltration of lung tissue in the PBS-treated group was similar to that in the untreated asthma model mice (Figure 5D).
Discussion
In the present study, an allergic mice model was successfully induced using the recombinant allergen rBlo t 5–21 of Blomia tropicalis (Figure 2), which provides a mouse model for further studies on vaccines and drugs against anaphylaxis induced by Blomia tropicalis. We also describe a therapy for a Blomia tropicalis allergy based on immunization with the recombinant Blomia tropicalis allergen rBlo t 5–21 displayed on VLPs derived from the hepatitis B virus (HBcAg-rBlo t 5–21). One advantage of the HBcAg-VLP is that it can be successfully expressed in multiple prokaryotic and eukaryotic expression systems. For example, expressed in an Escherichia coli expression system,33 in a yeast expression system,34 in an adenoviral vector expression system,35 in human HeLa cells,36 and expressed in plants,37 and the like. Furthermore, foreign antigens fused into the most exposed region on the capsid surface,38 encompassing the immunodominant c/e1 B cell epitope of HBcAg,39 are particularly immunogenic for B cells. In this study, we generated fusions of the HBcAg protein with rBlo t 5–21 protein flanked by flexible linkers in the c/e1 epitope (Figure 3B). The chimeric protein, termed HBcAg-rBlo t 5–21, was observed to form VPLs by transmission electron microscopy (Figure 2E). Foreign antigens, fused into the c/e1 epitope of HBcAg, can be displayed outside the core particles and did not prohibit the formation of VLPs, such as the green fluorescent protein (GFP),40 and the outer surface protein C (OspC) of B. Burgdorferi.41
Allergic diseases mainly depend upon the response of Th2 cells, which leads to the production of IL-4 and IgE.42 The imbalance of Th1/Th2 is the main immunological mechanism of allergic diseases. To change the balance of Thl/Th2 by enhancing Th1 or canceling Th2 reaction has always been the main direction of the immune pathway for the prevention and treatment of allergies and asthma. The most direct pathways include injecting cytokines to induce the activation of the Th1 pathway [eg, interferon (IFN) γ, IL-12, and IL-18] or blocking antibodies that inhibit the action of Th-2-related cytokines (eg, anti-IL-4, anti-IL-5, anti-IL-9, and anti-IL-13).43 IFN-γ and IL-2 in the local environment are the key factors to guide the differentiation of Th0 into Th1 cells. Th1 cells can secrete IFN-γ, IL-2, and tumor necrosis factor and mainly promote cellular immune responses.
IFN-γ can promote the transformation of antibodies synthesized by B-cells into IgG2a and IgG3, while IFN-γ can inhibit the production of IgG1 and IgE. Additionally, Th2 can induce B-cells to produce IgG1, and IL-4 can inhibit the production of IgG2a.44 In this study, after the allergic model mice were treated with HBcAg–rBlo t 5–21 protein, it was found that the levels of IgG1 and IgG2a in the model mice were significantly increased (Figure 4F and G), indicating that the Th1 and Th2 reactions in the model mice were enhanced, while the ratio of IgG1/IgG2a was decreased significantly (Figure 4H), indicating that the Th1 type reaction in mice was stronger than that of the Th2 type. Acute systemic stress anaphylaxis and ear prick tests showed that HBcAg-rBlo t 5–21 immunization relieved the symptoms of allergic reaction in mice when reexposed to the same allergen (Figure 4C and I). At the same time, it was found that the invasion of inflammatory cells in the HBcAg–rBlo t 5–21-treated group was significantly lower than that in the untreated group and PBS-treated model mice (Figure 5). These results suggest that the recombinant virus-like granule protein HBcAg–rBlo t 5–21 has a certain effect on allergic symptoms in mice. It also provides an idea for the prevention and treatment of inhaled and ingested allergens.
However, HBcAg-rBlo t 5–21 can alleviate the allergic symptoms induced by rBlo t 5–21. Whether HBcAg-rBlo t 5–21 can inhibit the allergic reactions induced by Blomia tropicalis remains to be further verified. This is one limitation in this paper. The main reason is that it is difficult to raise Blomia tropicalis in Hainan. Another reason is that the whole extract of Blomia tropicalis commercially available is not very effective. We may solve this problem by means of in-house cultivation in our next article. It was also observed that HBcAg-rBlo t 5–21 could alleviate allergic symptoms induced by rBlo t 5–21, including inhibition of body temperature drop (Figure 4C), reduction of lung cell invasion, etc. However, IgE remained at a high level (Figure 4B). This is mainly because IgE takes longer time to fall. This is the defect of protein desensitization by multiple small injections,45–47 not the deficiency of this article itself. This article is intended as a basic exploration to build a more complex tolerant vaccine, and our subsequent work will focus on vaccine research and development around more rapid IgE decline.
Conclusions
In summary, HBcAg-rBlo t 5–21, a protein vaccine containing both the hepatitis B core antigen and the Blomia tropicalis fusion allergen rBlo t 5–21, can alleviate the allergic symptoms induced by rBlo t 5–21. It could be a novel AIT vaccine candidate for preventing allergy disorders caused by Blomia tropicalis.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author Yechun Pei.
Statement of Ethics
This study protocol was reviewed and approved by the Animal Ethics Committee of Hainan University, approval number [HNUAUCC-2022-000113], guided by the national law of Regulations for the Administration of Affairs Concerning Experimental Animals (Revision 2017).
Acknowledgment
We sincerely thank Shuang Geng (Tsinghua University) and Qingyun Li (Washington University, St. Louis) for their valuable suggestions on this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was financially supported by the grants from Science and Technology Project of Hainan Province (ZDYF2022SHFZ059) and National Natural Science Foundation of China (31860726; 32160837) to Yechun Pei, and the grants from Natural Science Foundation of Hainan Province (821RC1053) and National Natural Science Foundation of China (32160214) to Dayong Wang.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7:12. doi:10.1186/1939-4551-7-12
2. Frankland AW. Asthma and mites. Postgrad Med J. 1971;47:178–180. doi:10.1136/pgmj.47.545.178
3. Platts-Mills TA, De Weck AL, Aalberse RC, et al. Dust mite allergens and asthma--a worldwide problem. J Allergy Clin Immunol. 1989;83:416–427. doi:10.1016/0091-6749(89)90128-0
4. Guilleminault L, Viala-Gastan C. Blomia tropicalis: un acarien sous les tropiques [Blomia tropicalis: a house dust mite in the tropics]. Rev Mal Respir. 2017;34:791–801. French. doi:10.1016/j.rmr.2016.10.877
5. Susanto AJ, Rengganis I, Rumende CM, Harimurti K. The differences in serum quantitative specific ige levels induced by dermatophagoides pteronyssinus, dermatophagoides farinae and Blomia tropicalis sensitization in intermittent and persistent allergic asthma. Acta Med Indones. 2017;49:299–306.
6. Colloff MJ. Effects of temperature and relative humidity on development times and mortality of eggs from laboratory and wild populations of the European house-dust mite Dermatophagoides pteronyssinus (Acari: pyroglyphidae). Exp Appl Acarol. 1987;3:279–289. doi:10.1007/BF01193165
7. Trombone AP, Tobias KR, Ferriani VP, et al. Use of a chimeric ELISA to investigate immunoglobulin E antibody responses to Der p 1 and Der p 2 in mite-allergic patients with asthma, wheezing and/or rhinitis. Clin Exp Allergy. 2002;32:1323–1328. doi:10.1046/j.1365-2745.2002.01455.x
8. Pytelková J, Lepšík M, Sanda M, Talacko P, Marešová L, Mareš M. Enzymatic activity and immunoreactivity of Aca s 4, an alpha-amylase allergen from the storage mite Acarus siro. BMC Biochem. 2012;13:3. doi:10.1186/1471-2091-13-3
9. Jacquet A. Innate immune responses in house dust mite allergy. ISRN Allergy. 2013;2013:735031. doi:10.1155/2013/735031
10. Carnés J, Iraola V, Cho SH, Esch RE. Mite allergen extracts and clinical practice. Ann Allergy Asthma Immunol. 2017;118:249–256. doi:10.1016/j.anai.2016.08.018
11. Ferreira F, Wolf M, Wallner M. Molecular approach to allergy diagnosis and therapy. Yonsei Med J. 2014;55:839–852. doi:10.3349/ymj.2014.55.4.839
12. Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351–357. doi:10.1016/j.jaci.2015.12.1299
13. Wallner M, Hauser M, Himly M, et al. Reshaping the Bet v 1 fold modulates T(H) polarization. J Allergy Clin Immunol. 2011;127:1571–1578.e1579. doi:10.1016/j.jaci.2011.01.064
14. Banerjee S, Weber M, Blatt K, et al. Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol. 2014;192:4867–4875. doi:10.4049/jimmunol.1400064
15. Curin M, Weber M, Thalhamer T, et al. Hypoallergenic derivatives of Fel d 1 obtained by rational reassembly for allergy vaccination and tolerance induction. Clin Exp Allergy. 2014;44:882–894. doi:10.1111/cea.12294
16. Comberiati P, Colavita L, Minniti F, et al. Utility of specific IgE to Ara h 2 in Italian allergic and tolerant children sensitized to peanut. Int J Mol Cell Med. 2016;5:160–166.
17. Kinaciyan T, Nagl B, Faustmann S, Kopp S, Wolkersdorfer M, Bohle B. Recombinant Mal d 1 facilitates sublingual challenge tests of birch pollen-allergic patients with apple allergy. Allergy. 2016;71:272–274. doi:10.1111/all.12781
18. Carvalho Kdos A, de Melo-Neto OP, Magalhães FB, et al. Blomia tropicalis Blo t 5 and Blo t 21 recombinant allergens might confer higher specificity to serodiagnostic assays than whole mite extract. BMC Immunol. 2013;14:11. doi:10.1186/1471-2172-14-11
19. Chan SL, Ong TC, Gao YF, et al. Nuclear magnetic resonance structure and IgE epitopes of Blo t 5, a major dust mite allergen. J Immunol. 2008;181:2586–2596. doi:10.4049/jimmunol.181.4.2586
20. Naik MT, Chang CF, Kuo IC, et al. Roles of structure and structural dynamics in the antibody recognition of the allergen proteins: an NMR study on Blomia tropicalis major allergen. Structure. 2008;16:125–136. doi:10.1016/j.str.2007.10.022
21. Tan KW, Ong TC, Gao YF, et al. NMR structure and IgE epitopes of Blo t 21, a major dust mite allergen from Blomia tropicalis. J Biol Chem. 2012;287:34776–34785. doi:10.1074/jbc.M112.348730
22. Lahiani S, Dumez ME, Khemili S, Bitam I, Gilis D, Galleni M. Cross-reactivity between major IgE epitopes of family 5 allergens from dermatophagoides pteronyssinus and Blomia tropicalis. Int Arch Allergy Immunol. 2019;178:10–18. doi:10.1159/000492871
23. Cates EC, Fattouh R, Wattie J, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi:10.4049/jimmunol.173.10.6384
24. Johnson JR, Wiley RE, Fattouh R, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–385. doi:10.1164/rccm.200308-1094OC
25. Fattouh R, Pouladi MA, Alvarez D, et al. House dust mite facilitates ovalbumin-specific allergic sensitization and airway inflammation. Am J Respir Crit Care Med. 2005;172:314–321. doi:10.1164/rccm.200502-198OC
26. Baqueiro T, Russo M, Silva VM, et al. Respiratory allergy to Blomia tropicalis: immune response in four syngeneic mouse strains and assessment of a low allergen-dose, short-term experimental model. Respir Res. 2010;11:51. doi:10.1186/1465-9921-11-51
27. Barboza R, Câmara NO, Gomes E, et al. Endotoxin exposure during sensitization to Blomia tropicalis allergens shifts TH2 immunity towards a TH17-mediated airway neutrophilic inflammation: role of TLR4 and TLR2. PLoS One. 2013;8:e67115. doi:10.1371/journal.pone.0067115
28. Zhou Q, Ho AW, Schlitzer A, et al. GM-CSF-licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis. J Immunol. 2014;193:496–509. doi:10.4049/jimmunol.1303138
29. Chua YL, Liong KH, Huang C-H. Blomia tropicalis –specific TCR transgenic Th2 cells induce inducible BALT and severe asthma in mice by an IL-4/IL-13–dependent mechanism. J Immunol. 2016;197:3771–3781. doi:10.4049/jimmunol.1502676
30. Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–397. doi:10.1111/j.1365-2222.2009.03443.x
31. Wynne SA, Crowther RA, Leslie AG. The crystal structure of the human hepatitis B virus capsid. Mol Cell. 1999;3:771–780. doi:10.1016/S1097-2765(01)80009-5
32. Schödel F, Peterson D, Hughes J, Wirtz R, Milich D. Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: i. presentation of foreign epitopes. J Biotechnol. 1996;44:91–96. doi:10.1016/0168-1656(95)00118-2
33. Edman JCHRA, Valenzuela P, Valenzuela P, et al. Synthesis of hepatitis B surface and core antigens in E. coli. Nature. 1981;291:503–506. doi:10.1038/291503a0
34. Miyanohara AIT, Araki M, Araki M, et al. Expression of hepatitis B virus core antigen gene in saccharomyces cerevisiae. J Virol. 1986;59:176–180. doi:10.1128/jvi.59.1.176-180.1986
35. Jean-Jean OLM, Will H, Hans W, et al. Expression mechanism of the hepatitis B virus (HBV) C gene and biosynthesis of HBe antigen. Virology. 1989;170:99–106. doi:10.1016/0042-6822(89)90356-5
36. Hirschman SZPP, Garfinkel E, Garfinkel E, et al. Expression of cloned hepatitis B virus DNA in human cell cultures. Proc Natl Acad Sci USA. 1980;77:5507–5511. doi:10.1073/pnas.77.9.5507
37. Huang Z, Santi L, LePore K, Kilbourne J, Arntzen CJ, Mason HS. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine. 2006;24:2506–2513. doi:10.1016/j.vaccine.2005.12.024
38. Conway JF, Watts NR, Belnap DM, et al. Characterization of a conformational epitope on hepatitis B virus core antigen and quasi equivalent variations in antibody binding. J Virol. 2003;77:6466–6473. doi:10.1128/JVI.77.11.6466-6473.2003
39. Salfeld J, Pfaff E, Noah M, Schaller H. Antigenic determinants and functional domains in core antigen and e antigen from hepatitis B virus. J Virol. 1989;63:798–808. doi:10.1128/jvi.63.2.798-808.1989
40. Kratz PA, Böttcher B, Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc Natl Acad Sci USA. 1999;96:1915–1920. doi:10.1073/pnas.96.5.1915
41. Skamel C, Ploss M, Böttcher B, et al. Hepatitis B virus capsid-like particles can display the complete, dimeric outer surface protein C and stimulate production of protective antibody responses against Borrelia burgdorferi infection. J Biol Chem. 2006;281:17474–17481. doi:10.1074/jbc.M513571200
42. Georas SN, Guo J, De Fanis U, Casolaro V. T-helper cell type-2 regulation in allergic disease. Eur Respir J. 2005;26:1119–1137. doi:10.1183/09031936.05.00006005
43. Chung RGSAKF, Chung KF. Future treatments of allergic diseases and asthma. Br Med Bull. 2000;56:1037–1053. doi:10.1258/0007142001903526
44. Lefranc GCH, Van Loghem E, Van Loghem E, et al. Simultaneous absence of the human IgG1, IgG2, IgG4 and IgAl subclasses: immunological and immunogenetical considerations. Eur J Immunol. 1983;13:240–244. doi:10.1002/eji.1830130312
45. James LK, Shamji MH, Walker SM, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–516.e501–505. doi:10.1016/j.jaci.2010.12.1080
46. Zhao D, Lai X, Tian M, et al. The functional IgE-blocking factor induced by allergen-specific immunotherapy correlates with IgG4 antibodies and a decrease of symptoms in house dust mite-allergic children. Int Arch Allergy Immunol. 2016;169:113–120. doi:10.1159/000444391
47. Tourdot S, Airouche S, Berjont N, et al. Efficacy of sublingual vectorized recombinant Bet v 1a in a mouse model of birch pollen allergic asthma. Vaccine. 2013;31:2628–2637. doi:10.1016/j.vaccine.2013.03.041
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.