Back to Journals » Infection and Drug Resistance » Volume 13
Studies on 11 Cases of Spinal Epidural Abscess and Literature Review
Authors Dai G, Li S, Yin C, Sun Y, Xu D , Wang Z, Luan L , Hou J, Wang T
Received 8 April 2020
Accepted for publication 4 September 2020
Published 29 September 2020 Volume 2020:13 Pages 3325—3334
DOI https://doi.org/10.2147/IDR.S257398
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Guohua Dai, Shuzhong Li, Chuqiang Yin, Yuanliang Sun, Derong Xu, Zhongying Wang, Liangrui Luan, Jianwen Hou, Ting Wang
Affiliated Hospital of Qingdao University, Qingdao 266003, People’s Republic of China
Correspondence: Ting Wang Tel +86 18661809505
Email [email protected]
Objective: In the present study, we aimed to describe the clinical features, diagnosis, treatment, and prognosis of spinal epidural abscess (SEA).
Methods: The complete clinical data of 11 SEA patients who were treated in our hospital system from January 2015 to June 2018 were retrospectively analyzed. Moreover, the clinical features, diagnosis, treatment, and prognosis of 642 SEA cases collected from the foreign literature from 2010 to 2019 were also investigated.
Results: Among our 11 SEA patients, nine cases had purulent inflammation, two cases had tuberculosis, two cases had infection caused by Staphylococcus aureus, one case had infection caused by Streptococcus constellatus, one case had infection caused by Klebsiella pneumoniae, five cases showed negative bacterial culture, and two cases had Mycobacterium tuberculosis. All 11 cases showed focal spinal pain, eight cases exhibited neurological deficits, and six cases experienced fever. Nine of the 11 cases involved the lumbosacral spine, one case involved the thoracic spine, and one case involved the cervical spine. Eight patients had a longer course of disease (> 2 weeks), all 11 patients had vertebral osteomyelitis, and nine patients had intervertebral discitis. One patient had motor dysfunction of arms and legs, one patient had lower limb motor dysfunction, one patient had limb numbness, one patient experienced relapse after the conservative treatment, and one patient experienced relapse after the surgical treatment. The follow-up time was 15– 24 months.
Conclusion: The classic diagnosis of triads (focal spine pain, neurological deficit, and fever) was less specific for SEA. MRI examination, blood culture, tissue culture, and biopsy could be used for the diagnosis for SEA. Suppuritis was a common cause of SEA. Early detection, early diagnosis and early treatment, as well as the selection of the most suitable treatment regimen based on comprehensive evaluation, played crucial roles in a better prognosis of SEA. There was no statistically significant difference in terms of the general condition, diagnosis, treatment and prognosis between the patients with negative and positive culture results (P> 0.05). For SEA patient with negative culture, antibiotic treatment should be used empirically.
Keywords: epidural abscess, clinical features, diagnosis and treatment, prognosis
Introduction
Spondylitis (spinal infection) includes vertebral osteomyelitis (vertebral body infection), intervertebral discitis (intervertebral disc infection) and spinal epidural abscess (SEA).1 Etiologically, spondylitis can be divided into purulent, granulomatous (tuberculosis, brucellosis, or fungal infection) and parasitic.2 As a rare spinal infection, SEA is often difficult to diagnose in the early stage.3 Purulent dilation within a narrow spinal canal can cause spinal cord injury through mechanical compression, vascular injury, or spinal instability,4 leading to paraplegia, limb paralysis, and even death5 in the later stages. Pathogenic bacteria can enter the epidural space through continuous diffusion or blood-borne diffusion.6 Previous study has shown that several conditions, such as diabetes, intravenous drug abuse, human immunodeficiency virus (HIV) infection, spinal degenerative diseases, recent spinal trauma or surgery, and epidural injection of drugs, are risk factors for SEA.7 The incidence of SEA is estimated to be 1.2–8/10,000. Due to the increase in the aging population of multiple comorbidities and the application of advanced imaging technology,8 the diagnosis rate and incidence of SEA continue to rise.9
Clinical Manifestations
There were five male and six female patients aged 52–78 years in the present study, and their average age was 64 years. Among these 11 patients, there were two cases of Staphylococcus aureus infection, one case had infection caused by Streptococcus constellatus, one case had infection caused by Klebsiella pneumoniae, five cases showed negative bacterial culture, and two cases had infection caused by Mycobacterium tuberculosis. Moreover, nine cases involved lumbar sacral spine, one case involved thoracic spine, and one case involved cervical spine. Abscesses were located on the ventral side of the dural sac in eight cases, on the dorsal side in two cases, and on both ventral and dorsal sides in one case. All 11 cases showed varying degrees of focal spinal pain, eight cases showed neurological deficits, and six cases experienced fever. The course of the disease in eight patients (from symptom onset to presentation) was >14 days, and the course of the disease in three patients was 10 days, with an average duration of 24 days. Four patients developed bacteremia (positive blood culture), nine patients had intervertebral discitis, and 11 patients had vertebral osteomyelitis. Comorbidities included hypertension (4/11), coronary heart disease (1/11), history of depression (1/11), infection with other sites (1/11), postoperative polypectomy (1/11) and tubal ligation (1/11) (Tables 1–4).
 |
Table 1 Clinical Characteristics of 11 Cases of Epidural Abscess in This Study |
 |
Table 2 Imaging Findings and Diagnosis of 11 Patients in This Study |
 |
Table 3 Treatment and Prognosis of 11 Patients in This Study |
 |
Table 4 Classification of 642 Cases in the Literature and 11 Cases in This Study |
Inclusion Criteria
Inclusion criteria were set as follows: (1) physical examination, laboratory examination or histopathological examination showed spinal infection; (2) MRI showed epidural abscess with or without vertebral osteomyelitis and intervertebral discitis.
The Course of Treatment
All patients underwent examinations of neutrophil count (WBC, white blood cell), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), X-ray, CT, MRI, blood culture and tissue culture, as well as histopathological examination. Among the 11 patients, one (1/11) had elevated WBC count, 10 (10/11) had elevated CRP level, and 11 (11/11) had elevated ESR. There were four cases (4/11) with positive blood culture and three cases (2/11) with positive tissue culture. Results of bacterial culture showed that there were two cases of infection caused by S. aureus, one case of infection caused by S. constellatus, one case of infection caused by K. pneumoniae, five cases of negative bacterial culture, and two cases of infection caused by M. tuberculosis. Histopathological examination revealed that there were nine cases of suppurative inflammation and two cases of tuberculosis. CT-guided biopsy was performed in three cases, two of which showed negative results. Intraoperative histopathological examination was carried out in eight cases, three of which showed positive results (3/8) (Tables 1–3).
Treatment Plan
All these 11 patients were given intravenous infusion of vancomycin+levofloxacin upon admission, and the treatment plan was adjusted according to the culture results. For culture-positive patients, sensitive antibiotics were selected based on the drug sensitivity results. Culture-negative patients underwent the broad-spectrum antibiotic treatment, and the duration of antimicrobial treatment was determined based on the periodic examinations of WBC, CRP, and ESR levels as well as spinal MRI results (Tables 2 and 3).
Results
Three cases underwent conservative treatment, two of which showed improved symptoms, and one case experienced relapse. Eight cases underwent surgical treatment, four of which showed improved symptoms, one case had left limb motor dysfunction, one case had left limb motor dysfunction (conservative treatment was given first, and then it was changed to surgery after treatment), one case had left limb numbness, and one case experienced relapse. There was no statistically significant difference in terms of the general condition, diagnosis, treatment and prognosis between the patients with negative and positive culture results. The follow-up time was 15–24 months (Tables 3 and 5).
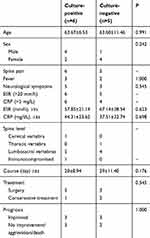 |
Table 5 Comparison of Negative and Positive Cultures |
Typical Case
A 58-year-old woman was admitted to the hospital due to persistent low back pain, low fever and weakness in both lower limbs. The patient had a history of fever 20 days ago, the weakness of both lower extremities occurred 5 days ago, and such symptoms had gradually deteriorated, accompanied by a reduced sensation in the lower limbs. She had a history of hypertension, but no history of intravenous drug abuse, diabetes and HIV infection. On physical examination, it was found that the key muscle strength of the right and left lower extremities was 3 and 2, respectively. Laboratory examination showed that the WBC count was 9.12×109/L (neutrophils 72.7%, lymphocytes 20.4%, monocytes 6.5%), the ESR was 84 mm/h, and the CRP level was 65.17 mg/L. Plain radiographs showed that the lumbar spine was convex to the left, and the L4-S1 intervertebral space was narrowed. Cervical spine CT examination showed reduced effective area of the L3-S1 spinal canal. Lumbar MRI showed a space-occupying lesion located in the epidural space behind L4/L5. The space-occupying lesion exhibited a low signal on the T1-weighted image and a high signal on the T2-weighted image. T1 image-enhanced MRI showed annular enhancement around the lesion.
The diagnosis of this patient was considered as an SEA. Therefore, the patient was treated with posterior lumbar surgery in a timely manner. Pus gushed out when the L4/L5 intervertebral disc was incised during the operation. Abscesses and abnormal inflammatory granulation tissue were completely removed during L3/L4 laminectomy. Pedicle screw was fixed from L3 to L5. Postoperative pathological examination of granulation tissue showed purulent inflammation. According to the drug sensitivity test results of bacterial culture, antibiotic treatment was used.
The lower back pain was gradually reduced postoperatively. At 10 days after surgery, the sensory function of the limbs was almost completely restored, and the key muscle strength of both lower limbs was grade 3. At 18 months of follow-up, the key muscle strength of both lower limbs was grade 4 (Tables 1–3 and Figures 1–4).
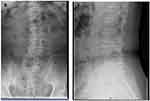 |
Figure 1 X-ray, Anterior and lateral position of lumbar spine before surgery, L3–5 Vertebral bone destruction. |
 |
Figure 2 T1WI, epidural abscess in the spinal canal of L3–5. |
 |
Figure 3 T2WI, epidural abscess in the spinal canal of L3–5. |
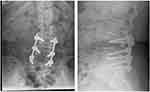 |
Figure 4 Postoperative X-ray, L3–5 Instrumentation, (posterior). |
SEA in the Literature
Of the 642 cases in the literature (Tables 4 and 6), 60% were male patients aged 17–94 years. The most common symptoms are local spinal pain (63%), followed by impaired nerve function (55%) and fever (51%). Methicillin-sensitive S. aureus (43%) is the most common pathogen, followed by methicillin-resistant S. aureus (23%) (Table 4). The most common violations are lumbar spine (46%), followed by thoracic spine (42%), cervical spine (26%) and sacral spine (23%). Moreover, 65% of cases have received surgical treatment. In addition, 43% of patients exhibit improved symptoms, while 12% of patients die during hospitalization or within 90 days.
 |
Table 6 Clinical Characteristics of 11 Cases in This Study and 642 Cases in the Literature |
Discussion
SEA is relatively rare in clinical practice, and it affects the spine. In recent years, the diagnosis rate of SEA has been increased as clinicians have paid more attention to such condition.9 In the present study, the male number advantage was not obvious due to the small number of cases, while male patients account for 60% in the 642 cases reported the literature, which is consistent with previous literature reports. The most common risk factors for SEA include diabetes, intravenous drug abuse, infection (skin, vertebrae, lungs, and urogenital organs) and alcohol abuse.10 The cases with diabetes, intravenous drug abuse, and immune function suppression in the literature account for 26%, 22% and 28%, respectively. Artenstein et al9 have set up a control group, and the results have shown that diabetes is prevalent, and no difference in the prevalence of diabetic patients is found. Among the 11 cases in our study, no diabetic patients were found.
In our cases or cases from the literature, the most common clinical manifestations of SEA are focal spinal pain, neurological deficits, and fever. This is the typical diagnosis of SEA.11 However, these features are not “typical” in our study and literature reports, and the specificity is poor,12 resulting in the prolonged treatment of many SEA patients in our study, and leading to delayed diagnosis and treatment.3 For patients with back pain and fever, it is crucial to suspect SEA before developing neurological deficits,13 and we must actively exclude SEA to avoid the occurrence of serious complications.14 The common site of SEA invasion was the lumbar spine in our study, which is consistent with previous reports.15 SEA is a clinically rare spinal infection. In the diagnosis of spinal infections, CRP and ESR tests are often highly specific,16 and they can be used as an indicator to monitor the treatment effect. The positive rates in our study were 91% and 100% for these above-mentioned two tests, respectively.
X-ray and CT are convenient and quick approaches, and can better describe the degree of bone involvement, while their performances in the diagnosis of SEA are not as good as MRI. Spinal MRI is the first choice for the diagnosis of SEA.6,15 When the patient has back pain, neurological deficits or fever symptoms, and hematological examination shows elevated levels of CRP and ESR, early spinal MRI should be performed as soon as possible. Early identification of SEA can avoid serious complications. SEA often does not exist in isolation. It is often associated with osteomyelitis and spondylodiscitis, which require the spinal MRI identification. Osteomyelitis and spondylodiscitis are usually caused by bacterial seeding of the vertebral endplate. In some cases, infections spread to the epidural space, resulting in SEA. In the present study, there were 10 cases associated with osteomyelitis, and eight cases associated with spondylodiscitis. When an epidural abscess formation is highly suspected in the clinical practice, MRI is a feasible approach to confirm the diagnosis. The typical manifestations are low signal on T1-weighted image, high signal on T2-weighted image, heterogeneous signal and enhanced thick-walled abscess on T1-weighted image with enhanced image strengthen.1 The final determination of the pathogenic bacteria requires blood culture, tissue culture and histopathological examination. The blood culture rate and tissue culture rate in our current study were relatively low, including five cases of negative cultures, compared with the study of Kim et al.17 This discrepancy might be related to the three points as follows: (1) use of antibiotics before specimen collection; (2) low-dose or low-level infection; and (3) false negative biopsy of the infected site. According to previous reports,18,19 CT-guided puncture or open biopsy can improve the positive culture rate. For cases with negative bacterial culture, histopathological examination can help identify pyogenic inflammation, tuberculosis, brucella, fungi and so on.20 In the cases presented in our study, or cases reported in the literature, most of them are purulent inflammation caused by S. aureus, which is consistent with recent literature reports.5
Currently, there are no guidelines for the standardized treatment of SEA, and there are still great differences in the best clinical treatment of SEA.4 Most of the pathogenic bacteria reported in the recent literature21 are Gram-positive cocci and mainly S.aureus. Vancomycin should be used early for empirical antibiotic treatment22 to avoid serious complications due to delayed diagnosis, such as neurological deficit. According to the 2015 American Society of Infectious Diseases guidelines,22 primary vertebral osteomyelitis should be treated with antimicrobial therapy for at least six weeks. The cases in our study and those reported in the literature were treated with antibacterial therapy for more than six weeks. There is no clear treatment guideline for SEA with negative bacterial culture, and such treatment mainly depends on empirical medication.23 With the increase of culture-negative patients in clinical practice,24 it is more and more important to improve the understanding and clinical treatment of negative SEA culture. The vast majority of our cases (in the literature and in our present study) are caused by Gram-positive cocci, mainly S. aureus (67%). Therefore, empirical use of vancomycin is appropriate in SEA patients with negative culture.15,25 In the present study, there was no statistically significant difference in terms of the general conditions, laboratory tests, and prognosis between the culture-negative and culture-positive patients. Therefore, it was appropriate to treat culture-negative SEA patients with empirical treatment. Historically, early surgery in combination with antibacterial treatment has been the mainstream treatment.4 In the past 10 years, the medical management of SEA has been chosen by more and more patients.21 Most of the patients in the literature and in our present study chose the surgery in combination with antibacterial therapy. The difference is the timing of surgery. The surgical indications for SEA patients are mainly based on data obtained from retrospective studies.26 Based on many successful medical management reports,27 conservative treatment can be performed first when there is no neurological deficit or only mild neurological deficit present. When the conservative treatment shows a poor effect or the condition deteriorates, surgical treatment can be performed. Patients with neurological deficits should be treated with surgery immediately because currently there is no effective evidence on how much neurological deficits can be reversed. The biggest treatment problem is still the population with mild neurological deficits. With the failure rate of delayed surgery reaching 40%, recent studies have focused on predictive models for conservative management failure of SEA.14,21
Despite surgical interventions and extended duration of intravenous antibiotic therapy, the prognosis of the nervous system is still worthy of attention. The goals of surgical treatment are proper nerve decompression, control of the source of pathogenic bacteria, and spinal stabilization. Previous studies4,10,28,29 have not clearly supported or opposed the surgical intervention in all cases of SEA. Therefore, we must analyze the specific situation before better evidence is found. We should not only evaluate the neurological status of the patient at the time of admission, the duration of neurological deficit, the development of neurological deficit, speed and location of abscess, increase of inflammatory markers, age, and diabetes, but also consider the patient’s own health status, economic conditions, and family situation in order to achieve a comprehensive evaluation and choose the most suitable treatment regimen for the patient.
Conclusions
The classic diagnosis of triads (focal spine pain, neurological deficits, and fever) is less specific for the diagnosis of SEA. MRI examination, blood culture, tissue culture and biopsy can be used for the diagnosis. Suppuritis is a common cause of SEA. Early detection, early diagnosis and early treatment, as well as the selection of the most suitable treatment regimen based on comprehensive evaluation play crucial roles for a better prognosis of SEA. There is no statistically significant difference in terms of the general condition, diagnosis, treatment and prognosis between the patients with negative and positive culture results. For SEA patients with negative culture, antibiotic treatment should be used empirically.
This study has certain limitations. First, the number of cases is small. Second, the study design is a retrospective study, which is lack of a more careful description of the cases. Third, a more accurate study requires a prospective, multicenter, randomized controlled trial.
Data Sharing Statement
All the case details can publish when approval is obtained from the Institutional Review Board (IRB) Affiliated Hospital of Qingdao University.
Ethics Approval and Consent to Participate
The present study was approved by the Institutional Review Board (IRB) of Affiliated Hospital of Qingdao University.
This study was approved by the Institutional Review Board (IRB) Affiliated Hospital of Qingdao University. All personal details were erased before analysis to cover patient data confidentiality and comply with the Declaration of Helsinki.
Consent for Publication
Written informed consent was obtained from all of the above patients for publication of this research and any accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lazzeri E, Bozzao A, Cataldo MA, et al. Joint EANM/ESNR and ESCMID-endorsed consensus document for the diagnosis of spine infection (spondylodiscitis) in adults. Eur J Nucl Med Mol Imaging. 2019;46(12):2464–2487.
2. Chong BSW, Brereton CJ, Gordon A, et al. Epidemiology, microbiological diagnosis, and clinical outcomes in pyogenic vertebral osteomyelitis: a 10-year retrospective cohort study. Open Forum Infect Dis. 2018;5(3):ofy037. doi:10.1093/ofid/ofy037
3. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975–981. doi:10.1016/j.amjmed.2017.03.009
4. Suppiah S, Meng Y, Fehlings MG, et al. How best to manage the spinal epidural abscess? A current systematic review. World Neurosurg. 2016;93:20–28. doi:10.1016/j.wneu.2016.05.074
5. Vakili M, Crum-Cianflone NF. Spinal epidural abscess: a series of 101 cases. Am J Med. 2017;130(12):1458–1463. doi:10.1016/j.amjmed.2017.07.017
6. Van Baarsel ED, Kesbeh Y, Kahf HA, et al. Spinal epidural abscess secondary to gram-negative bacteria: case report and literature review. J Community Hosp Intern Med Perspect. 2020;10(1):60–64. doi:10.1080/20009666.2019.1705009
7. Bond A, Manian FA. Spinal epidural abscess: a review with special emphasis on earlier diagnosis. Biomed Res Int. 2016;2016:1614328. doi:10.1155/2016/1614328
8. Digiorgio AM, Stein R, Morrow KD, et al. The increasing frequency of intravenous drug abuse-associated spinal epidural abscesses: a case series. Neurosurg Focus. 2019;46(1):E4. doi:10.3171/2018.10.FOCUS18449
9. Artenstein AW, Friderici J, Holers A, et al. Spinal epidural abscess in adults: a 10-year clinical experience at a tertiary care academic medical center. Open Forum Infect Dis. 2016;3(4):ofw191. doi:10.1093/ofid/ofw191
10. Prasad GL. Spinal epidural abscess-conservative or operative approach: a management dilemma. World Neurosurg. 2017;103:945–947. doi:10.1016/j.wneu.2017.03.002
11. King C, Fisher C, Brown PCM, et al. Time-to-completed-imaging, survival and function in patients with spinal epidural abscess: description of a series of 34 patients, 2015–2018. BMC Health Serv Res. 2020;20(1):119. doi:10.1186/s12913-020-4973-5
12. Yang H, Shah AA, Nelson SB, et al. Fungal spinal epidural abscess: a case series of nine patients. Spine J. 2019;19(3):516–522.
13. Alerhand S, Wood S, Long B, et al. The time-sensitive challenge of diagnosing spinal epidural abscess in the emergency department. Intern Emerg Med. 2017;12(8):1179–1183. doi:10.1007/s11739-017-1718-5
14. Babic M, Simpfendorfer CS, Berbari EF. Update on spinal epidural abscess. Curr Opin Infect Dis. 2019;32(3):265–271. doi:10.1097/QCO.0000000000000544
15. Khursheed N, Dar S, Ramzan A, et al. Spinal epidural abscess: report on 27 cases. Surg Neurol Int. 2017;8:240. doi:10.4103/sni.sni_105_17
16. Sato K, Yamada K, Yokosuka K, et al. Pyogenic spondylitis: clinical features, diagnosis and treatment. Kurume Med J. 2019;65(3):83–89. doi:10.2739/kurumemedj.MS653001
17. Kim CJ, Song KH, Park WB, et al. Microbiologically and clinically diagnosed vertebral osteomyelitis: impact of prior antibiotic exposure. Antimicrob Agents Chemother. 2012;56(4):2122–2124. doi:10.1128/AAC.05953-11
18. Foreman SC, Schwaiger BJ, Gempt J, et al. MR and CT imaging to optimize CT-guided biopsies in suspected spondylodiscitis. World Neurosurg. 2017;99(726):e7. doi:10.1016/j.wneu.2016.11.017
19. Ran B, Chen X, Zhong Q, et al. CT-guided minimally invasive treatment for an extensive spinal epidural abscess: a case report and literature review. Eur Spine J. 2018;27(Suppl 3):380–385.
20. Li T, Liu T, Jiang Z, et al. Diagnosing pyogenic, brucella and tuberculous spondylitis using histopathology and MRI: a retrospective study. Exp Ther Med. 2016;12(4):2069–2077. doi:10.3892/etm.2016.3602
21. Shah AA, Ogink PT, Nelson SB, et al. Nonoperative management of spinal epidural abscess: development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546–555. doi:10.2106/JBJS.17.00629
22. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–46. doi:10.1093/cid/civ482
23. Dogan M, Simsek AT, Yilmaz I, et al. Evaluation of empirical antibiotic treatment in culture negative pyogenic vertebral osteomyelitis. Turk Neurosurg. 2019;29(6):816–822.
24. Menon KV, Sorour TM. Epidemiologic and demographic attributes of primary spondylodiscitis in a middle eastern population sample. World Neurosurg. 2016;95:31–39. doi:10.1016/j.wneu.2016.07.088
25. Shweikeh F, Hussain M, Sangtani A, et al. Cervical spine epidural abscess: a single center analytical comparison to the literature. Spinal Cord Ser Cases. 2017;3:17036. doi:10.1038/scsandc.2017.36
26. Alton TB, Patel AR, Bransford RJ, et al. Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J. 2015;15(1):10–17. doi:10.1016/j.spinee.2014.06.010
27. Adogwa O, Karikari IO, Carr KR, et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. 2014;20(3):344–349. doi:10.3171/2013.11.SPINE13527
28. Wang TY, Harward SC, Tsvankin V, et al. Neurological outcomes after surgical or conservative management of spontaneous spinal epidural abscesses: a systematic review and meta-analysis of data from 1980 through 2016. Clin Spine Surg. 2019;32(1):18–29. doi:10.1097/BSD.0000000000000762
29. Ropper AE, Ropper AH, Longo DL. Acute spinal cord compression. N Engl J Med. 2017;376(14):1358–1369. doi:10.1056/NEJMra1516539
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
