Back to Journals » International Journal of General Medicine » Volume 9
Streptococcus pneumoniae sepsis as the initial presentation of systemic lupus erythematosus
Authors Erdem I , Omar S, Kara Ali R, Gunes H, Eren Topkaya A
Received 26 January 2016
Accepted for publication 12 July 2016
Published 8 September 2016 Volume 2016:9 Pages 315—317
DOI https://doi.org/10.2147/IJGM.S105070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ilknur Erdem,1 Senay Elbasan Omar,1 Ridvan Kara Ali,1 Hayati Gunes,2 Aynur Eren Topkaya2
1Department of Infectious Diseases, 2Department of Medical Microbiology, Faculty of Medicine, Namik Kemal University, Tekirdag, Turkey
Objective: Infections are among the most important causes of morbidity and mortality in patients with systemic lupus erythematosus (SLE) but are rare initial presentation of the disease. Therefore, in this study, we describe a case of Streptococcus pneumoniae sepsis in a young woman with previously undiagnosed SLE.
Case report: A 23-year-old female patient was admitted to our outpatient clinic complaining of high fever (40°C), chills, fatigue, generalized myalgia, and cough with brown sputum for 5 days. Blood cultures grew gram-positive coccus defined as S. pneumoniae using standard procedures. Antinuclear antibody was positive at a titer of 1/1,000, and anti-double-stranded DNA was positive at 984 IU/mL. She was diagnosed with SLE. Her respiratory symptoms and pleural effusion were considered to be due to pulmonary manifestation of SLE.
Conclusion: The underlying immunosuppression caused by SLE could have predisposed the patient to invasive pneumococcal disease. It may also occur as a primary presenting feature, although a rare condition.
Keywords: Streptococcus pneumoniae, sepsis, systemic lupus erythematosus
Introduction
Infections are an important cause of mortality and morbidity in patients with systemic lupus erythematosus (SLE). Bacteria are the most common agents, followed by viruses and fungi. The most common types of infections in SLE patients are respiratory, urinary, skin, and soft tissue infections. Case reports and small series indicate that patients with SLE have an increased frequency and severity of Streptococcus pneumoniae infections, accounting for 6%–18% of all bacterial infections in these patients. S. pneumoniae typically causes pneumonia in SLE patients, but meningitis, sepsis, and soft tissue infections have also been reported. S. pneumoniae is an important and well-known cause of bacteremia and sepsis in immunocompetent and immunocompromised patients. There are only few reported cases of S. pneumoniae sepsis as the initial presentation of SLE.1–6 In this study, we report a case of S. pneumoniae sepsis in a young woman with previously undiagnosed SLE.
Case report
A 23-year-old female patient was admitted to our outpatient clinic complaining of high fever (40°C), chills, fatigue, generalized myalgia, and cough with brown sputum for 5 days. On her physical examination, she appeared mildly confused but was awake, alert, and oriented. Her temperature was 39.6°C; respiratory rate, 30 breaths per minute; pulse, 84 beats per minute; and blood pressure, 110/70 mmHg. Physical examination also showed that she had rales and decreased breath sounds over the left base. The rest of the examination was normal.
Laboratory data on admission were as follows: hemoglobin, 7.6 g/dL; white blood cell count, 16,900/mm3 (neutrophils 91%); platelets, 41,000/mm3; C-reactive protein, 300 mg/dL (reference range [RR] 0–5 mg/L); erythrocyte sedimentation rate, 146 mm/h; serum creatinine, 0.74 mg/dL (RR 0.5–1.2 mg/dL); aspartate aminotransferase, 59 IU/mL (RR 0–32 IU/L); alanine aminotransferase, 22 IU/mL (RR 0–35 IU/L); alkaline phosphatase, 303 IU/L (RR 35–114 IU/L); lactate dehydrogenase, 364 IU/L (RR 0–250 IU/L); gamma-glutamyl transpeptidase, 134 IU/L (0–40 IU/L); albumin, 2.2 g/dL (RR 3.5–5.5 g/dL); d-dimer, 0.667 µg/mL (RR 0–0.5 µg/mL); procalcitonin, 64.05 ng/mL (RR 0–0.5 ng/mL); ferritin, 1,116 ng/mL (RR 13–150 ng/mL). Urine: protein was 3+, and there was microscopic hematuria.
Blood, urine, and sputum cultures and serological tests were requested. Bilateral lower lobe pulmonary infiltration and right-sided pleural effusion has been shown on the posteroanterior chest X-ray (Figure 1). Cefotaxime (4×2 g/d) and moxifloxacin (1×400 mg/d) intravenous therapy was started as initial empiric therapy for suspected community-acquired sepsis. In addition, supportive therapy including fresh frozen plasma, red cells, albumin, isotonic fluids, oxygen, 2 L/min was given. Blood cultures grew gram-positive coccus defined as S. pneumoniae using standard procedures. Minimum inhibitory concentration of penicillin of this strain was reported to be 0.09. It was also susceptible to both macrolides and fluoroquinolones. Ultrasound-guided thoracentesis was performed to determine the cause of pleural effusion. It was exudate with no growth in culture. It was learned that the patient was not vaccinated against pneumococcus. The serologic tests for legionella, mycoplasma and parvovirus were negative. Antinuclear antibody was positive at a titer of 1/1,000, and anti-double-stranded DNA was positive at 984 IU/mL. She was diagnosed with SLE. The patient was referred to the rheumatology clinic of a hospital to complete the current treatment, because of elevated titers of autoantibodies against antinuclear antibody and anti-double-stranded DNA. The patient provided written informed consent to publish patient data.
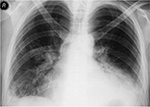  | Figure 1 Chest X-ray of patient showing bilateral lower lobe pulmonary infiltration with right-sided pleural effusion. |
Discussion
SLE is a chronic autoimmune disease that can affect any organ or system of the human body. Its presentation and course are variable, ranging from indolent to fulminant. Infection is one of the leading causes of morbidity and mortality in patients with SLE. These patients demonstrate an increased risk of infection even if they are not treated with immunosuppressants. Possible factors increasing this risk are abnormalities in the complement system, impaired chemotaxis and phagocytosis of macrophages and polymorphonuclear cells, abnormal T-cell mediated cytotoxicity, and functional asplenia. Goldblatt et al2 demonstrated that deposition of C3b/iC3b on S. pneumoniae is significantly reduced in serum from patients with SLE compared with non-SLE rheumatic disease controls and healthy individuals. This may contribute to the increased susceptibility of SLE patients to S. pneumoniae and possibly to other pyogenic bacterial infections.1,2 Hypocomplementemia was considered an important risk factor of infection in our patient.
S. pneumoniae is a Gram-positive diplococcus. There are ~90 known serotypes, of which the top ten account for >60% of infections worldwide. The spectrum of pneumococcal infections can range from asymptomatic pharyngeal colonization to mucosal disease (otitis media, sinusitis, and pneumonia) to invasive infections (meningitis, endocarditis, bacteremia, and sepsis). Underlying diseases or conditions that predispose patients to pneumococcal infections include defective antibody formation (congenital or secondary to conditions such as chronic lymphocytic leukemia, lymphoma, and HIV infection); complement deficiency or dysfunction; neutropenia or neutrophilic dysfunction; splenectomy or splenic dysfunction; prior respiratory infections and inflammatory conditions such as cigarette smoking, asthma, and chronic obstructive pulmonary disease. Other risk factors include diabetes mellitus, renal insufficiency, liver cirrhosis, malnutrition, glucocorticosteroid therapy, alcoholism, cold exposure, stress, fatigue, and excess likelihood of exposure to S. pneumoniae (such as that occurs in day care centers and military training camps).7
Pneumococcal bacteremia and sepsis are life-threatening diseases. They can occur with or without pneumococcal pneumonia. Despite advances in antimicrobial therapy, pneumococcal bacteremia remains a significant cause of morbidity and mortality worldwide. The overall case-fatality rate for bacteremia is ~20% but may be as high as 60% among elderly patients. Patients with asplenia who develop bacteremia may experience a fulminant clinical course. A β-lactam antibiotic is the mainstay of therapy for pneumococcal infection. Intravenous therapy, particularly ceftriaxone and cefotaxime, is recommended for treatment of bacteremia and most often, addition of vancomycin to the initial therapy in case of meningitis.7–9 Cefotaxime treatment was given in our case. Thoracentesis and sputum cultures were negative. Her respiratory symptoms and pleural effusion were considered to be due to pulmonary manifestation of SLE.
Conclusion
The underlying immunosuppression caused by SLE could have predisposed the patient to invasive pneumococcal disease. It may also occur as a primary presenting feature, although a rare condition.
Disclosure
The authors report no conflicts of interest in this work.
References
Danza A, Ruiz- Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–1294. | ||
Goldblatt F, Yuste J, Isenberg DA, Rahman A, Brown J. Impaired C3b/iC3b deposition on Streptococcus pneumoniae in serum from patients with systemic lupus erythematosus. Rheumatology. 2009;48(12):1498–1501. | ||
Naveau C, Houssiau FA. Pneumococcal sepsis in patients with systemic lupus erythematosus. Lupus. 2005;14(11):903–906. | ||
Elliott JA, Copeman A, Davies K. Pneumococcal sepsis as the first presentation of systemic lupus erythematosus (SLE) in a six year old boy. Arch Dis Child. 2011;96:A78. | ||
Shaughnessy MK, David N, Williams DN, Segal B. Severe infection with encapsulated bacteria as the initial presentation of systemic lupus erythematosus: two case reports and a review of the literature. JMM Case Rep. 2014;1(2):1–4. | ||
Webster J, Williams BD, Smith AP, Hall M, Jessop JD. Systemic lupus erythematosus presenting as pneumococcal septicaemia and septic arthritis. Ann Rheum Dis. 1990;49(3):181–183. | ||
Janoff EN, Musher DM. Streptococcus pneumoniae. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell Douglas and Bennett’s Principles and Practice of Infectious Diseases, 8th ed. Philadelphia, PA: Elsevier Saunders; 2015:2310–2327. | ||
Christensen JS, Jensen TG, Kolmos HJ, Pedersen C, Lassen A. Bacteremia with Streptococcus pneumoniae: sepsis and other risk factors for 30-day mortality – a hospital-based cohort study. Eur J Clin Microbiol Infect Dis. 2012;31(10):2719–2725. | ||
Iinuma Y, Hirose Y, Ken Kumagai TT, et al. Rapidly progressive fatal pneumococcal sepsis in adults: a report of two cases. J Infect Chemother. 2007;13(5):346–349. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
