Back to Journals » Cancer Management and Research » Volume 14
Stereotactic Body Radiotherapy for Locally Advanced Pancreatic Cancer Using Optical Surface Management System – AlignRT as an Optical Body Surface Motion Management in Deep Breath Hold Patients: Results from a Single-Arm Retrospective Study
Authors Kaučić H , Kosmina D, Schwarz D, Mack A, Čehobašić A, Leipold V, Avdićević A, Mlinarić M, Lekić M , Schwarz K , Banović M
Received 31 March 2022
Accepted for publication 22 June 2022
Published 12 July 2022 Volume 2022:14 Pages 2161—2172
DOI https://doi.org/10.2147/CMAR.S368662
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Hrvoje Kaučić,1,2 Domagoj Kosmina,3 Dragan Schwarz,4– 6 Andreas Mack,7 Adlan Čehobašić,2,3 Vanda Leipold,2,3 Asmir Avdićević,1 Mihaela Mlinarić,3 Matea Lekić,1 Karla Schwarz,8 Marija Banović9
1Department of Radiosurgery and Radiotherapy, Special Hospital Radiochirurgia Zagreb, Sveta Nedelja, Croatia; 2University Josip Juraj Strossmayer in Osijek – Medical Faculty Osijek, Osijek, Croatia; 3Department of Medical Physics, Special Hospital Radiochirurgia Zagreb, Sveta Nedelja, Croatia; 4Department of Surgery, Special Hospital Radiochirurgia Zagreb, Sveta Nedelja, Croatia; 5Department of Surgery, Medical Faculty of University in Rijeka, Rijeka, Croatia; 6Department of Surgery, University Josip Juraj Strossmayer in Osijek – Faculty of Dental medicine and Health, Osijek, Croatia; 7Swiss NeuroRadiosurgery Center, Swiss Clinical NeuroScience Institute, Zürich, Switzerland; 8University of Zagreb, Medical Faculty, Zagreb, Croatia; 9Department of Endocrinology, Polyclinic Leptir, Zagreb, Croatia
Correspondence: Hrvoje Kaučić, Specijalna Bolnica Radiochirurgia Zagreb, Ulica Dr. Franje Tuđmana 4, Sveta Nedelja, 10431, Croatia, Tel +385 91 5622 191, Email [email protected]
Purpose: To assess the efficacy and safety of stereotactic body radiotherapy for patients with unresectable, locally advanced pancreatic cancer using Optical Surface Management System – AlignRT (OSMS-AlignRT) as an optical body surface motion management in deep breath hold.
Patients and Methods: Forty-five patients diagnosed with locally advanced pancreatic cancer were treated with stereotactic body radiotherapy in 3 or 5 fractions, and received varying BED10 (median 79.5 Gy) from April 2017 to December 2020. All patients were treated in deep breath hold with OSMS-AlignRT used as optical body surface motion management. Thirty-three patients received systemic treatment before and/or after stereotactic body radiotherapy, and twelve patients received no systemic treatment. In this retrospective, observational, single-arm study, primary endpoints were overall survival and freedom from local progression (ie, local control). Secondary endpoints were progression-free survival and toxicity. Actuarial survival analysis and univariate analysis were investigated.
Results: Data from forty-five patients were analyzed. Median follow-up was 15 months. One-year freedom from local progression and survival were 95.5% and 71.1%, respectively. Median progression-free survival was 14 months. Median overall survival from diagnosis for all patients was 17 months, and 19 months for patients alive at the time of analysis. No patient had >G2 toxicity.
Conclusion: Stereotactic body radiotherapy for locally advanced pancreatic cancer using OSMS-AlignRT as optical body surface motion management in deep breath hold patients is an effective and safe local treatment option, with no >G2 toxicity, and could be a promising therapeutic option with acceptable toxicity, either as a single treatment or in a multimodal regimen. OSMS-AlignRT provided accurate and reliable body surface motion management during stereotactic body radiotherapy.
Keywords: AlignRT, locally advanced pancreatic cancer, Optical Surface Management System, pancreatic SBRT, surface guided radiotherapy
Plain Language Summary
- Patients diagnosed with unresectable, locally advanced pancreatic cancer represent a particular problem in oncology, due to the challenges in local treatment.
- As there are still no published data on OSMS-AlignRT as optical body surface motion management during stereotactic body radiotherapy for locally advanced pancreatic cancer, our study aimed to evaluate the efficacy and safety of this treatment approach.
- During stereotactic body radiotherapy, OSMS-AlignRT reliably monitors both steadiness of patients’ deep breath hold and all additional possible movements (eg, drifts).
- The tumor and organs at risk are more clearly visible on planning MSCT and on daily cone beam CT scans in deep breath hold, which makes contouring, treatment planning, and daily image guidance easier and more reliable compared with free breathing.
- During the treatment in deep breath hold, the organs at risk move away from the tumor, which makes them easier to spare from excess of radiation.
- Stereotactic body radiotherapy in deep breath hold requires compliant patient to be adequately conducted.
- Stereotactic body radiotherapy as a local treatment for locally advanced pancreatic cancer using OSMS-AlignRT for body surface motion management presented in our study as an effective, safe, and well tolerated option with favorable clinical outcomes: median overall survival was 17 months, 1-year local control and 1-year survival were 95.5% and 71.1%, respectively, with no > G2 toxicity.
Introduction
Pancreatic cancer is generally one of the deadliest cancers, and will potentially represent the second leading cause of cancer death by 2030.1 Median survival for resected patients following adjuvant therapy ranges from 20–28 months.2–5 In general, 50–80% of patients are affected by locoregional relapse, over 70% by systemic relapse; for radically resected patients, 5-year survival rate is around 10–20%, and 30% experience isolated locoregional disease progression.6,7
The subgroup of patients with surgically unresectable, locally advanced pancreatic cancer (LAPC) represents a particular problem in oncology, due to the challenges in local treatment. In recent years, stereotactic body radiotherapy (SBRT) has emerged as an effective and safe form of local treatment for patients with LAPC.8,9
Motion management plays a crucial role in sparing surrounding organs at risk (OARs) during SBRT. The duodenum, the stomach, and the small bowel are among the most highly radiosensitive OARs in the abdomen and it is critical to spare them from any excess radiation.10–12 Mitigation of respiratory motions is typically achieved with commercially available abdominal compressing devices. Respiratory gating uses correlation of the tumor’s position in selected phase of breathing cycle with external surrogate, and this correlation accounts only for respiratory motion.13 Generation of the 4D-CT internal tumor volume (ITV) is another technique to compensate for tumor movements, but ITV does not accurately represent the daily tumor motions, as significant discrepancies of the abdominal target’s positions are frequently observed between planning multi-sliced computed tomography (MSCT) and daily cone-beam computed tomography (CBCT) for the same patients.14 Deep breath hold (DBH) during SBRT is also a possible motion management technique, but requires adequate monitoring to be sufficiently accurate.15 Intrafractional fiducial-implanted tumor tracking technique is most often used in robotic arm based linacs.16,17 However, not all patients are candidates for fiducial implantation, due topossible unfavorable anatomy or medical conditions that prevent them from safe implantation, and general contraindications for contrast-enhanced CT (MSCT). Magnetic resonance (MR) - linacs use on-board cine-MR imaging devices for intrafractional motion management. The technique is non-invasive and very accurate, but some gating uncertainties still remain due to the time delay of the MRI.
The primary goal of this study was to evaluate the efficacy and safety of SBRT for LAPC using OSMS-AlignRT as optical body surface motion management in deep breath hold patients. Furthermore, we aimed to report our clinical outcomes (overall survival, local and distal disease control, and toxicity) with this treatment approach, to compare them with previously reported data (achieved with different motion management techniques and fractionation regimens), and to present our experiences and workflow with this motion management technique. After thoroughly conducting a search of Pub Med Central database, we found no published data on the use of OSMS-AlignRT during SBRT for LAPC. Surface guidance is most commonly used during radiotherapy for thoracic and abdominal targets – breast,18,19 lung, liver and adrenal tumors.20
Patients and Methods
Patients
Medical data of 45 consecutive patients diagnosed with LAPC were analyzed in this retrospective, single-arm, single-institution, observational study, approved by the Institutional ethics committee. All patients were treated from April 2017 to December 2020, and were available for regular follow-up. Patients’ characteristics are shown in Table 1.
 |
Table 1 Patients’ Characteristics |
Prior to treatment, all patients were considered and approved for SBRT by our institution’s multidisciplinary tumor board. Inclusion criteria were: unresectable, histologically proven pancreatic adenocarcinoma, age ≥18, ECOG 0–2, radiologically negative regional lymph nodes and no signs of distant metastasis, gastric or duodenal obstruction, and no previous abdominal radiotherapy. Unresectable pancreatic cancer was defined according to the arterial and venous criteria for resectability status.2,21 All procedures performed were in accordance with the national medical ethical standards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Standard written informed treatment consent was signed by, and obtained from every patient.
As our Institution does not provide systemic therapy, it was indicated and provided by each patient’s referring medical oncologists’ team, and administered appropriately at least seven days before and after SBRT, due to the possible toxicities.
Optical Surface Management System – AlignRT
OSMS-AlignRT (Vision RT, London, UK) is an optical monitoring system of body surface movements during radiotherapy, consisting of three camera units.22 Each unit uses a projector of a pseudo-random speckle pattern of red light that illuminates the patient’s body surface, and image sensors to three dimensionally reconstruct the patient’s body surface contour in thousands of points (Figure 1A). The system tracks any movements of the body contour in real time with a frequency of 5–10 Hz and submillimeter accuracy, is non-invasive, and does not use ionizing radiation for tracking. It can be used both for patient positioning and motion tracking. Furthermore, combined with CBCT, it allows for more reliable image guidance. According to the manufacturer’s specifications, the lag time of the system is < 100 milliseconds.
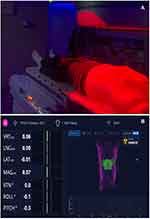 |
Figure 1 OSMS-AlignRT – pseudo-random speckle pattern of red light on patient body surface (A). ROI (green) on a body contour (magenta) (B). |
The default body contour (DBC) is defined as a patient’s body contour on planning MSCT. Region of interest (ROI) is a part of the body surface contour, best suited for tracking, defined by user, typically the region of upper abdomen above the PTV (Figure 1B). During the treatment, OSMS-AlignRT monitors the alignment of a patient’s body contour ROI on the treatment table with an ROI of DBC. Gating windows allow tolerable mismatch of ROIs in any direction within specified values. While ROIs are aligned within the gating windows, OSMS-AlignRT is “in green” and the beam is “on”. When the ROIs’ mismatch exceeds the gating windows, OSMS-AlignRT goes “in red” and automatically shuts off the beam.
Stereotactic Body Radiotherapy
Patients were in supine position either on a wing-board or vacuum pillow, with their arms above the head. No further immobilization was used. Treatments were delivered by a Varian EDGE linear accelerator (Varian Medical Systems, Palo Alto, California, USA). DBH was in inhale phase for all patients.
A contrast-free MSCT scan in DBH with a slice thickness of 1 mm was acquired for all patients for the purpose of treatment planning. Routinely, contrast-free MRIs (T1 and T2) of the abdomen in DBH were also acquired for all patients, and subsequently coregistered (with deformable registration methods) with planning MSCT. The clinical target volume (CTV) was defined as the gross tumor volume (GTV) with no additional margins. CTV was delineated on the T1 or T2 images of the MRI, and further corrected on MSCT scan, as needed. OARs were contoured on planning MSCT scans. If the lesion or the OARs were not clearly visible on contrast-free imaging, additional contrast-enhanced MSCT (with late arterial phase) and/or contrast-enhanced MR of the abdomen were acquired and subsequently coregistered (with deformable registration methods) with planning MSCT.
SBRT plans were optimized and delivered using Volumetric Arc Therapy. Beam energy of 6 and/or 10 MV, with Flattening Filter Free photon beams was used for all patients, to achieve best dose distributions, while having plans with low modulation and high QA passing rates. Biological effective dose was calculated using alpha/beta = 10 Gy for tumor (BED10). Three fractionation regimens were used (corresponding BED10 and number of patients treated are listed respectively):
- 5 x 8 Gy (BED10 = 72 Gy), 22 patients (48.9%)
- 3 x 12 Gy (BED10 = 79.2 Gy), 12 patients (26.7%)
- 5 x 9 Gy (BED10 = 85.5 Gy), 11 patients (24.4%)
Median BED10 was 79.2 Gy.
The optimal fractionation regimen for each patient was chosen to achieve the goal of OARs sparing (Table 2).23,24 The primary OARs were: the stomach, the duodenum, and the small intestine. The dose-volume constraints for them were:
- 3-fraction: V (1 cm3) < 31.4 Gy, V (5 cm3) < 23.2 Gy, V (10 cm3) < 16.7 Gy.
- 5-fraction: V (1 cm3) < 36 Gy, V (5 cm3) < 25.5 Gy and V (10 cm3) < 18.5 Gy.
 |
Table 2 Summary of Planning Dose–Volume Objectives |
We followed Radiation Therapy Oncology Group (RTOG) recommendations for the dose–volume constraints for other OARs.
The dose was applied heterogeneously, and there was no planning constraint on the maximum dose as long as it was located inside the planning target volume (PTV). Average maximum of the prescription dose was 126% (± 5.2%). Average Conformity Index was 1.10 ± 0.05. A required target coverage V (98–99.5%) of the prescribed dose for the PTV was 80% (Figure 2). Target coverage was prioritized over OAR sparing as long as previously mentioned OAR constraints were met. No replans were done.
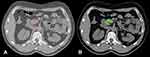 |
Figure 2 Typical GTV (A) and dose distribution (80–130% of prescribed isodose) in colorwash (B). |
The therapeutic position of the tumor was defined as the anatomical position of the tumor on the planning MSCT in DBH. OSMS-AlignRT was used as gating management. Gating windows were set to 3 mm, according to manufacturer’s specifications for abdominal lesions.
During daily treatments, the workflow was as follows:
- Prior to treatment, OSMS-AlignRT was loaded with DBC from planning system for the patient.
- Initial positioning of the patient in free breathing on the treatment table was performed using laser guidance and CBCT.
- The patient was instructed to take and hold deep breath the same way as during the MSCT planning, and alignment of the ROI of body contour on a treatment table with the ROI of the DBC was confirmed with OSMS-AlignRT (“in green”).
- The CBCT in DBH with OSMS-AlignRT “in green” was then repeated to further check the alignment of the GTV on planning MSCT with the GTV on CBCT, and patient’s position was corrected as needed.
- Patient was instructed to breathe freely for a while.
- Patient was then instructed to take and hold deep breath again, the treatment started when OSMS-AlignRT came “in green” and as long as OSMS-AlignRT remained “in green”, the treatment beam was “on”.
- When the patient could not hold the breath anymore, or body contour moved out of gating window for any other reason (OSMS-AlignRT “in red”), the beam was automatically shut off.
Steps 5.– 7 were then repeated to the end of the fraction.
Given the possible additional movements of the tumor in the body, the PTV to CTV margin was 5 mm for all patients which was, in our opinion, sufficient to compensate for residual/intrinsic tumor motions, with no need for further compensation for patient’s positioning uncertainties before or during the fraction, for several reasons:
- CBCT with OSMS-AlignRT “in green” was performed before the start of each fraction, to check the patient’s positioning and alignment of the GTV on planning MSCT with the GTV on CBCT.
- The same routine was repeated regularly after 50% of planned dose delivery to re-check the patient’s position and to correct any eventual mismatch.
- If OSMS-AlignRT detected significant and permanent deviation of ROIs at any time during the fraction, the same routine was repeated.
According to the system’s reports, the “beam on” was on average 10–15% of total treatment time, calculated from the first beam engage to the end of the fraction. Average time per fraction on a treatment couch was 30 minutes for patients treated in 5 fractions, and 42 minutes for patients treated in 3 fractions.
Patients’ Preparations for the Treatment in DBH
Prior to treatment planning, all patients were “tested” on OSMS-AlignRT, to determine if they could adequately (repeatedly and consistently for at least 20 seconds) hold their breath. If needed, further breathing coaching was performed, and the test was repeated. Also, all patients were provided with written recommendations on diet, including instructions to stop taking food and drinks 2–3 hours before the treatment, and to take proton-inhibitors and antiflatulent drugs (eg, simethicone) to reduce flatulence and weight loss during the treatment, all in order to minimize daily anatomical variations. For the same reasons, the time from the planning to the start of the treatment was kept as short as possible (on average 7–10 days).
Response Evaluation and Follow-Up
Follow-up was regularly scheduled every three months after SBRT by the treating radiation oncologist with clinical examination and a contrast-enhanced MSCT scan. Local and distant control were defined according to RECIST criteria.25 Acute and late toxicity was scored according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v4.03.
Statistical Analysis
The conformity index (CI) was defined as the ratio of the volume of the 80% isodose line to the volume of the PTV. Endpoints were: overall survival (OS), freedom from local progression or local control (FFLP/LC), progression-free survival (PFS), and toxicity rate. One-year survival was calculated as the ratio of patients that survived at least one year (12 months) and all patients. FFLP was calculated from the time of diagnosis to the first finding of local progression. Local relapse was defined as radiological progression of the primary lesion within the PTV. PFS was calculated from the time of diagnosis to the first assessment of disease progression. Patients that did not develop disease progression were censored at the date of the last scan. OS was calculated from the time of diagnosis to death. FFLP, PFS, and OS rates were calculated by Kaplan-Meier method; the Log rank test statistic was used for univariate analysis. A significant difference was considered when P ≤ 0.05.
Results
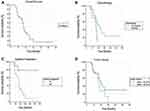
Median follow-up was 15 months (range 5–56 months). Median OS was 17 months (range 6–56 months). Fifteen (33.3%) patients were alive at the time of analysis. Median follow-up in this subgroup was 18 months (range 13–56 months) and median OS was 19 months (range 13–56 months). Median time from diagnosis to SBRT was 3 months (range 1–8 months). Table 3 summarizes the results for FFLP, PFS and OS from diagnosis, and OS from the treatment (OSt). In Figure 3A an actuarial curve for OS is shown.
 |
Table 3 Summary of the Actuarial Analysis for FFLP, PFS, OS and OSt |
Thirty-three patients (73.3%) received systemic treatment. Twelve patients (26.7%) received no systemic treatment (Table 1). Nineteen patients (57.5%) received chemotherapy before (2–4 cycles) and after SBRT, and 14 patients (42.5%) received chemotherapy only after SBRT. On actuarial analysis, patients that received chemotherapy had better OS (P = 0.024) (Figure 3B). No patient was surgically treated or re-irradiated during follow-up.
Five patients (11%) had radiological local disease progression (at 7, 12, 14, 20 and 25 months, all accompanied by distal progression). Nine patients (20%) had radiological local regression. The remaining 31 patients (69%) had radiologically stable local disease. Median PFS was 14 months (range 5–25 months). Thirty-five patients (77.8%) had systemic progression of the disease, and ten (22.2%) had stable state. On actuarial analysis, patients that had no systemic progression had better OS (P = 0.024) (Figure 3C).
Median GTV was 45 cm3 (range 7.2–136.2 cm3) and median PTV was 103.8 cm3 (range 32.8–217.6 cm3). After thorough statistical analysis, we found no impact of tumor volume on survival (P = 0.1577) (Figure 3D).
Twenty-nine (64.4%) patients had grade 1 or 2 acute toxicities: nausea, fatigue, and abdominal spasm or pain, that were successfully treated with symptomatic treatment. Five patients (11.1%) had G2 late toxicity (abdominal spasm or pain, and/or gastroesophageal reflux), developed 6 months or more after SBRT, that were successfully treated with symptomatic treatment. No acute or late toxicity G3 (bleeding from gastrointestinal track or perforations) was reported.
Discussion
In the last years SBRT in the treatment of LAPC has been investigated with a goal of confirming the assumed impact of LC on OS, impact of dose escalation on LC, and the importance of adequate motion management.
Zhu et al in study from 2019 concluded that BED10 ≥ 60 Gy might be required to achieve better LC in pancreatic cancer.26 Comito et al used SBRT for LAPC with an abdominal compression for motion mitigation, with BED10 = 78.8 Gy. One-year FFLP and OS were 87% and 85% respectively, and median OS was 19 months. Significant positive correlation between FFLP as well as OS was shown.27 Seo used respiratory gating for motion management during SBRT for a total of 79 patients with LAPC, with median BED10 = 47.6 Gy. Median OS was 16 months, and the 1-year OS, PFS and FFLP rates were 85%, 57%, and 96%, respectively. Isolated local progression occurred in 7 patients (11%), distant metastasis in 51 patients (80%), and both in 6 patients (9%), respectively.28
In numerous other studies published in the last years, 1-year FFLPs were reported ranging from 40–96%, median OSs were ranging from 10–20 months, and applied BED10s were ranging from 37.5–102.6 Gy.11,27–37 Clinical outcomes presented in our study are rather comparable with those from previously cited studies, with 1-year FFLP of 95.5%, 1-year OS of 71.1%, median OS of 17 months, and BED10 of 79.2 Gy. The number of enrolled patients and median follow-up time in our study are also comparable with those from previously cited studies. Similar clinical outcomes were reported recently, in 2020, by Chuong et al from their experience with 5-fraction adaptive MR-guided SBRT for LAPC,38 and in pooled analysis from Mahadevan et al of tumor control probability models to evaluate the comparative effectiveness of the various SBRT treatment regimens used in the treatment of patients with LAPC (HyTEC review).39
According to our experience, OSMS-AlignRT provided accurate and reliable body surface motion tracking during all treatments. Lens et al in their study, monitored solely steadiness of breath hold, using SpiroDynr’X system, which registered airflow through a mouthpiece with a spirometer, and reported significant residual tumor movements.15 We used OSMS-AlignRT along with CBCT for initial patient positioning, and to monitor both steadiness of breath hold and any possible additional patient’s movements (eg, drifting, coughing), that could contribute to overall tumor’s motions during the treatment. Additional intrinsic/residual tumor motions (eg, caused by peristalsis, heartbeat) were principally compensated for with PTV to CTV margins.
It is our impression that the accurate and reliable body surface motion management of patients’ DBH and additional movements during the treatments, provided by OSMS-AlignRT, allowed us to safely apply ablative doses to the tumor, while keeping the doses to the OARs within the recommended dose-volume constraints. This approach could have contributed to favorable clinical outcomes and acceptable toxicity presented in our study. In our experience, planning and conducting SBRT for LAPC in DBH had the following advantages: 1) clear visibility of the tumor and OARs on planning MSCT and on daily CBCTs, which made contouring, treatment planning and daily image guidance easier and more reliable, 2) moving the OARs away from the GTV, which made them easier to spare from the excess radiation, and 3) no need for abdominal compression or rigid immobilization of the patients. The following disadvantages were observed, as well: 1) need for patient’s strict compliance during all steps of the treatment, 2) favoring the patients with a better performance status, and 3) prolongation of the treatment time on a table.
The majority of treated patients (77.8%) had systemic (regional and/or distal) progression of the disease during follow-up, and had statistically significant inferior OS. Furthermore, statistically significantly better OS was observed for patients that received chemotherapy before and/or after SBRT. Local control in our study was satisfactory, as only 5 patients (11.1%) had local disease progression during follow-up. We performed thorough statistical analysis to determine possible “cut off” value of tumor volume that would yield statistically significant impact on survival. As no such value was found, we presented the actuarial curve of survival for patients with tumor volume larger and smaller than median tumor volume. This finding is contrary to previously published data. Toxicity profile of SBRT was very acceptable, with 64.4% of patients experiencing mild to moderately acute toxicities.
Conclusion
Our results indicate that SBRT for LAPC using OSMS-AlignRT as optical body surface motion management provides favorable clinical outcomes and allows for safe dose delivery with acceptably low toxicities, but requires compliant patients. This treatment could be considered as an effective, safe, and well tolerated local therapeutic option for LAPC, as a single treatment, and probably even more effective as part of multimodal treatment.
Future studies and trials are needed to evaluate the role of OSMS-AlignRT for optical body surface motion tracking during SBRT for primary and secondary malignancies of other localizations.
Abbreviations
BED, biological effective dose; CBCT, cone beam computed tomography; CTV, clinical target volume; Dmax, maximal dose; FFF, flattening filter free; FFLP, freedom from local progression; FOLFIRINOX, fluorouracil/leucovorin/irinotecan/oxaliplatin; GTV, gross tumor volume; Gy, Gray; IMRT, intensity modulated radiotherapy; LAPC, locally advanced pancreatic cancer; LC, local control; MSCT, multi slice computed tomography; OAR, organs at risk; OS, overall survival; PFS, progression-free survival; PTV, planning target volume; RECIST, Response Evaluation Criteria in Solid Tumors; RTOG, Radiation Therapy Oncology Group; SBRT, stereotactic body radiotherapy; V (dose), volume receiving dose (in Grays).
Data Sharing Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Ethics Approval and Informed Consent
This study protocol was approved by the ethics committee of Special Hospital Radiochirurgia Zagreb (code number: 02/2021, date: 8th December 2021). All procedures performed were in accordance with the national medical ethical standards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Standard written informed treatment consent was signed by, and obtained from every patient. All data in the current study had no personal identifiers and were kept confidential.
Consent for Publication
We confirm that the details of any images can be published, and that the persons providing consent have been shown the article contents to be published.
Acknowledgments
The authors would like to express their gratitude to the following radiation therapists: Jelena Trajković, Sanja Brezovec and Marica Keser for their care and efforts while introducing this technology in our institution; and Dr. Iva Andrašek MD for her help in conducting research and investigation process.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Authors Hrvoje Kaučić and Domagoj Kosmina received honoraria from Varian Medical Systems, Palo Alto, CA, USA, for speaking at “2nd Varian Belgian Oncology Summit”, on 13th Sep 2018 in Brussels, Belgium. The authors report no other conflicts of interest in this work.
References
1. Cellini F, Arcelli A, Simoni N, et al. Basics and frontiers on pancreatic cancer for radiation oncology: target delineation, SBRT, SIB technique, MRgRT, particle therapy, immunotherapy and clinical guidelines. Cancers. 2020;12(7):1729. PMID: 32610592; PMCID: PMC7407382. doi:10.3390/cancers12071729
2. NCCN Clinical Practice. Guidelines in oncology: pancreatic adenocarcinoma. Version 2.2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
3. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. PMID: 17227978. doi:10.1001/jama.297.3.267
4. Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019–1026. PMID: 18319412. doi:10.1001/jama.299.9.1019
5. Neoptolemos JP, Palmer DH, Ghaneh P, et al; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017; 389(10073):1011–1024. PMID: 28129987. doi:10.1016/S0140-6736(16)32409-6.
6. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73–85. PMID: 26830752. doi:10.1016/S0140-6736(16)00141-0
7. Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10(4):511–518. PMID: 16627216. doi:10.1016/j.gassur.2005.09.016
8. Hajj C, Goodman KA. Pancreatic cancer and SBRT: a new potential option? Rep Pract Oncol Radiother. 2015;20(5):377–384. PMID: 26549996; PMCID: PMC4597084. doi:10.1016/j.rpor.2015.05.008
9. Goodman KA. Stereotactic body radiation therapy for pancreatic cancer. Cancer J. 2016;22(4):290–295. doi:10.1097/PPO.0000000000000206
10. Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi:10.1016/j.ijrobp.2005.07.002
11. Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e615–e622; PMID: 21658854. doi: 10.1016/j.ijrobp.2011.04.045
12. Brunner TB, Nestle U, Grosu AL, Partridge M. SBRT in pancreatic cancer: what is the therapeutic window? Radiother Oncol. 2015;114(1):109–116. PMID: 25466369. doi:10.1016/j.radonc.2014.10.015
13. Campbell WG, Jones BL, Schefter T, Goodman KA, Miften M. An evaluation of motion mitigation techniques for pancreatic SBRT. Radiother Oncol. 2017;124(1):168–173. PMID: 28571887; PMCID: PMC5523845. doi:10.1016/j.radonc.2017.05.013
14. Rankine L, Wan H, Parikh P, et al. Cone-beam computed tomography internal motion tracking should be used to validate 4-dimensional computed tomography for abdominal radiation therapy patients. Int J Radiat Oncol Biol Phys. 2016;95(2):818–826; PMID: 27020102. doi: 10.1016/j.ijrobp.2016.01.047
15. Lens E, van der Horst A, Versteijne E, Bel A, van Tienhoven G. Considerable pancreatic tumor motion during breath-holding. Acta Oncol. 2016;55(11):1360–1368. PMID: 27583771. doi:10.1080/0284186X.2016.1221532
16. Moningi S, Abi Jaoude J, Kouzy R, et al. Impact of fiducial marker placement before stereotactic body radiation therapy on clinical outcomes in patients with pancreatic cancer. Adv Radiat Oncol. 2020;6(2):100621. PMID: 33912734; PMCID: PMC8071717. doi:10.1016/j.adro.2020.11.006
17. Scher N, Bollet M, Bouilhol G, et al. Safety and efficacy of fiducial marker implantation for robotic stereotactic body radiation therapy with fiducial tracking. Radiat Oncol. 2019;14(1):167. PMID: 31519194; PMCID: PMC6743112. doi:10.1186/s13014-019-1373-2
18. Hattel SH, Andersen PA, Wahlstedt IH, Damkjaer S, Saini A, Thomsen JB. Evaluation of setup and intrafraction motion for surface guided whole-breast cancer radiotherapy. J Appl Clin Med Phys. 2019;20(6):39–44. PMID: 31187538; PMCID: PMC6560238. doi:10.1002/acm2.12599
19. Xiao A, Crosby J, Malin M, et al. Single-institution report of setup margins of voluntary deep-inspiration breath-hold (DIBH) whole breast radiotherapy implemented with real-time surface imaging. J Appl Clin Med Phys. 2018;19(4):205–213; PMID: 29935001; PMCID: PMC6036385. doi: 10.1002/acm2.12368
20. Heinzerling JH, Hampton CJ, Robinson M, et al. Use of surface-guided radiation therapy in combination with IGRT for setup and intrafraction motion monitoring during stereotactic body radiation therapy treatments of the lung and abdomen. J Appl Clin Med Phys. 2020;21(5):48–55; PMID: 32196944; PMCID: PMC7286017. doi: 10.1002/acm2.12852
21. Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–1733. PMID: 19396496. doi:10.1245/s10434-009-0408-6
22. VisionRT/AlignRT advance. Available from: https://www.visionrt.com/alignrtadvance/.
23. Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(5):1420–1426. PMID: 20399033. doi:10.1016/j.ijrobp.2009.09.075
24. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys. 2010;37(8):4078–4101. doi:10.1118/1.3438081
25. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. PMID: 19097774. doi:10.1016/j.ejca.2008.10.026
26. Zhu X, Ju X, Cao Y, et al. Patterns of local failure after stereotactic body radiation therapy and sequential chemotherapy as initial treatment for pancreatic cancer: implications of target volume design. Int J Radiat Oncol Biol Phys. 2019;104(1):101–110; PMID: 30684663. doi: 10.1016/j.ijrobp.2019.01.075
27. Comito T, Cozzi L, Clerici E, et al. Can stereotactic body radiation therapy be a viable and efficient therapeutic option for unresectable locally advanced pancreatic adenocarcinoma? Results of a phase 2 study. Technol Cancer Res Treat. 2017;16(3):295–301; PMID: 27311310; PMCID: PMC5616043. doi: 10.1177/1533034616650778
28. Seo J. Treatment of locally advanced pancreatic cancer: comparison of stereotactic body radiation therapy to concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(2 Supplement):E187. doi:10.1016/j.ijrobp.2017.06.1048
29. Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14(1):95. PMID: 31171025; PMCID: PMC6555709. doi:10.1186/s13014-019-1309-x
30. Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation [e-pub ahead of print]. Adv Radiat Oncol. 2021;6(1):100506. doi:10.1016/j.adro.2020.06.010
31. Rwigema JC, Parikh SD, Heron DE, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34(1):63–69. PMID: 20308870. doi:10.1097/COC.0b013e3181d270b4
32. Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. PMID: 23452509; PMCID: PMC3607991. doi:10.1186/1748-717X-8-44
33. Tozzi A, Comito T, Alongi F, et al. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8:148. PMID: 23799996; PMCID: PMC3707803. doi:10.1186/1748-717X-8-148
34. Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–522; PMID: 23562768. doi: 10.1016/j.ijrobp.2013.02.022
35. Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(7):1128–1137; PMID: 25538019; PMCID: PMC4368473. doi: 10.1002/cncr.29161
36. Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015;22:2352–2358. PMID: 25564157; PMCID: PMC4459890. doi:10.1245/s10434-014-4274-5
37. Mazzola R, Fersino S, Aiello D, et al. Linac-based stereotactic body radiation therapy for unresectable locally advanced pancreatic cancer: risk-adapted dose prescription and image-guided delivery. Strahlenther Onkol. 2018; 194(9):835–842. English. PMID: 29696321. doi:10.1007/s00066-018-1306-2
38. Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2020;11(2):134–147. doi:10.1016/j.prro.2020.09.005
39. Mahadevan A, Moningi S, Grimm J, et al. Maximizing tumor control and limiting complications with stereotactic body radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2021;110(1):206–216; PMID: 33358561. doi: 10.1016/j.ijrobp.2020.11.017
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.