Back to Journals » Journal of Pain Research » Volume 15
Stem Cell Therapy for Neuropathic Pain: A Bibliometric and Visual Analysis
Authors Kan H , Fan L, Gui X, Li X , Yang S , Huang Y, Chen L, Shen W
Received 22 March 2022
Accepted for publication 14 June 2022
Published 23 June 2022 Volume 2022:15 Pages 1797—1811
DOI https://doi.org/10.2147/JPR.S365524
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sudhir Diwan
Houming Kan,1– 3,* Lijun Fan,1– 3,* Xiaodie Gui,4,* Xiaoqiang Li,4 Sen Yang,4 Yuting Huang,4 Liping Chen,4 Wen Shen4
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Jiangsu Province Key Laboratory of Anesthesia and Analgesia Application Technology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 3NMPA Key Laboratory for Research and Evaluation of Narcotic and Psychotropic Drugs, Xuzhou, Jiangsu, People’s Republic of China; 4Department of Pain, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wen Shen; Liping Chen, Tel +86 516-85609999, Email [email protected]; [email protected]
Purpose: Neuropathic pain is intractable and current treatment modalities are ineffective to cure this intractable pain, which has become a global problem. In recent years, there have been an increasing number of studies on stem cell therapy for neuropathic pain that have shown enormous potential. Using a visual analysis approach of the existing literature on stem cell therapy for neuropathic pain, we hope to understand the current research status and hot issues in this field and to provide valuable predictions for future research in this field.
Methods: We used Citespace software to visually analyze 291 articles and reviews indexed by the Web of Science Core Collection Database exploring stem cell-based treatment of neuropathic pain from 1995 to 2021. The Gunnmap online world map evaluated the number of countries and regional articles separately. Microsoft Excel 2016 was used to generate a graph of trends in annual publications.
Results: Visualization analysis revealed that the number of publications has increased yearly. The top three countries in terms of number of articles published are United States, China, and Japan. Analysis of highly co-cited articles revealed that the contents of these articles primarily involved the expression of IL-1β, IL-10, NPY, TRPA1, p-p38, p-ERK1/2, TGF-β, PKCδ, CaMKIIɑ, P2X4, P2X7 and TNF-ɑ. Keywords and citation burst analysis demonstrated that activation, regeneration, chemotherapy, and expression are likely the research hotspots and future directions of stem cell research in neuropathic pain.
Conclusion: Stem cell therapy may be a potential means of future treatment of neuropathic pain. The study of the mechanisms underlying stem cell therapy for neuropathic pain is still a focus of future research.
Keywords: neuropathic pain, stem cell, CiteSpace, Web of Science, bibliometric analysis, visualization analysis
Introduction
Neuropathic pain (NP) is pain caused by a lesion or disease of the somatosensory nervous system.1 The International Association for the Study of Pain (IASP) classifies neuropathic pain in detail according to the latest version of the World Health Organization ICD-11 classification of diseases, subdividing the condition into peripheral neuropathic pain and central neuropathic pain.2 There are multiple mechanisms to responsible for the development of neuropathic pain, but it is not clear why nerve injury transforms into a chronic inflammatory and painful state.3 Current treatments for neuropathic pain include pharmacotherapy, platelet-rich plasma therapy, and neuromodulation therapy.4–6 Despite numerous treatment options, the current treatment for neuropathic pain is not satisfactory. In recent decades, stem cells have shown remarkable efficacy in handling and repairing neuropathic pain rather than temporary analgesia.7 Stem cells promise to be effective therapies for neuropathic pain.
Bibliometrics refers to using mathematical and statistical methods to quantitatively analyze published scientific literature to help researchers quantitatively identify detailed research trends and hot spots.8 CiteSpace is an excellent bibliometric software that visualizes the relationships between the literature as a scientific knowledge map, helping to unravel past research trajectories and obtain a general idea of future research prospects.9 Citespace is used to analyze the current state of research and hot spots in a research area and is increasingly valued by researchers.
In this study, we use Citespace software to visualize and analyze research related to the use of stem cells for the treatment of neuropathic pain and anticipate future directions in this field.
Data and Methods
Retrieval Strategy
The first author extracted relevant studies addressing stem cell therapy for neuropathic pain published from 1995 to 2021.
Databases Retrieved
We retrieved the studies from databases from the Web of Science (WOS) Core Collection Database, including the Science Citation Index Expanded (SCIE), the Social Science Citation Index (SSCI), and the Emerging Sources Citation Index (ESCI).
Search Strategy
This study used stem cell and neuropathic pain for the MeSH keywords (https://www.ncbi.nlm.nih.gov/mesh) search. Further description of the specific classification of neuropathic pain was based on the International Classification of Diseases 11th Revision (https://icd.who.int/en). We selected articles from 1995 to 2021. The study included original reports and reviews as publication types. The search terms and strategies are given in Table 1.
 |
Table 1 Search Terms and Strategies |
Qualification
Full-text retrieval was performed.
Inclusion Criteria
After reading the title and abstract, all topics related to stem cells and neuropathic pain were listed. The style of the publications was limited to original research articles and reviews, but not letters, editorial materials, perspectives or abstracts from meetings.
Number of Studies Included
Based on search terms and strategies, a total of 307 records were selected. By reading the abstracts, excluding editorial materials, letters, meeting abstracts and perspectives, a total of 291 records were eventually included (Figure 1).
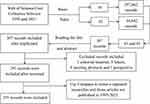 |
Figure 1 Flowchart of articles inclusion selection. |
Methodology
We analyzed included papers separately for bibliometric and network visualization using Citespace V5.8.R3, 64 bit (https://citespace.podia.com/). The Gunnmap (http://lert.co.nz/map/) online world map evaluated the number of countries and regional articles separately. Microsoft Excel 2016 was utilized to generate a graph of the trends in annual publications.
Bibliometric and Visual Analysis
All publications elements, including year of publication institutions, countries, co-cited authors, references, and keywords were extracted. Citespace software was used to visualize the relationship between literature with elements such as node circle, link between nodes, centrality, and cluster.
Results
Publication Outputs by Year
A total of 291 publications were included in this study. The annual publication rates of articles on stem cell therapy for neuropathic pain tended to increase mainly during 1995–2021 (Figure 2A). The year with the most publications was 2020, with 38, followed by 2021, with 34 publications. A total of 152 articles were published in the last five years alone, accounting for 52% of all publications. Overall, 219 original research articles were published, accounting for 75% of all publications, and 72 review articles, accounting for 25% of all publications (Figure 2B).
 |
Figure 2 Bibliometric analysis of publication output. A total of 291 publications were included from 1995 to 2021. (A) Number of publications. (B) Article type distri. |
Journals
The top 10 co-citation counts journals are presented in Table 2. The journal Pain was cited a total of 146 times and was the highest co-citation journal. Seven of the 10 journals are published in the United States. This indicates that the US has significant influence in this area. The journal Blood had the highest intermediary centrality (Centrality = 0.18, Figure 3).
 |
Table 2 The Top 10 Co-Cited Journal Information |
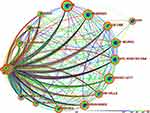 |
Figure 3 Co-citation analysis of journals. Pain was the highest co-cited journal. Blood was the journal with the highest central intermediation. |
Co-Authors’ Country and Institution Analysis
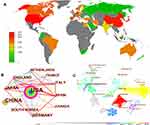
According to the bibliometric analysis via CiteSpace, 291 publications were published by research teams from 40 countries. The most prolific country was the United States (n=109, Centrality = 0.41, Figure 4A and B). The three countries that ranked after the United States were China (n=61, Centrality = 0.00), Japan (n=23, Centrality = 0.01), and Germany (n=19, Centrality = 0.10). The United States was far higher than other countries in citation and centrality.
Bibliometric and visual analysis presented a low-density map (Density = 0.0103), which indicated fewer collaborations between different research institutions. The most prolific institution was Johns Hopkins University (n=13, Centrality = 0.04) and Duke University (n=7, Centrality = 0.10) (Figure 4C).
Co-Citation References Analysis
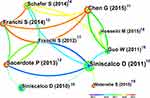
The top 10 highly cited documents are listed in Table 3.10–19 The most cited articles were from Siniscalco. Furthermore, this author had the strongest citation bursts (strength=6.19), starting in 2013 and ending in 2018.10 Top 16 references with the strongest citation bursts were showed in Figure 5.10,11,13–18,20–27 There were 4 references with citation bursts lasting until 2021, which indicated that these four references were still the hotspots of current researches.11,14,26,27 Co-citation analysis of the top 10 cited documents were showed in Figure 6.10–19 Chen, Sacerdote, Franchi and Schafer ranked second to fifth highest cited, but these nodes were circled in red, indicating that these citations had been cited recently.11–14
 |
Table 3 Top 10 Cited Articles in the Field of Stem Cell Therapy for Neuropathic Pain |
Presently, stem cell therapy for neuropathic pain is mainly focused on pre-clinical studies. The number of clinical studies on stem cell therapy for neuropathic pain is relatively limited, focusing mainly on spinal cord injuries. To understand the development of clinical studies, the clinical studies were listed in Table 4.28–38
 |
Table 4 Clinical Studies in the Field of Stem Cell Therapy for Neuropathic Pain |
Keywords
A total of 437 keywords were retrieved from 291 documents. As shown in Figure 7, the high-frequency keywords were neuropathic pain, transplantation, mesenchymal stem cell, and spinal cord injury, demonstrating that these keywords were the research hotspots. Transplantation had the highest centrality in all 437 keywords, which implied that most studies were related to transplantation.
During the past decade, therapy ranked first with the highest burst strength (3.26), followed by bone marrow stromal cells (2.72) and regeneration (2.55). Based on the end time of the bursts, we found that an increasing number of researchers focused their attention on expression, chronic pain, marrow stromal cell, regeneration, and activation in recent years (Figure 8).
To further understand different types of stem cells research trends in the last 10 years, we perform a co-occurrence analysis (Figure 9). Mesenchymal stem cells were the most studied, while neural stem cells and embryonic stem cells were less studied.
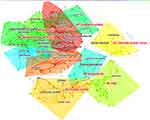
Co-occurrence cluster analysis from 1995 to 2021 revealed 437 keywords from stem cell and neuropathic pain publications. The studies clustered keywords according to the degree of connection tightness. A total of 10 cluster labels were obtained and numbered 0 to 9 (Figure 10). The smaller the number, the more keywords are contained in the clusters. Not surprisingly, the most frequent occurrence was the mechanism (cluster 0). Figure 11 showed a timeline view of the keyword clusters to visualize the keyword associations at different times. Therefore, the study of stem cells and the mechanisms involved in neuropathic pain are currently the most researched areas.
Discussion
In this study, a total of 291 articles on stem cells and neuropathic pain from 1995–2021 were visually analyzed using a bibliometric approach, mainly involving articles (75.26%) and reviews (24.74%). Stem cells and studies on neuropathic pain fluctuated in growth between 1995 and 2021 and reached a peak in 2020. This curve indicates that the field of stem cell therapy for neuropathic pain is increasing in intensity. Researchers were increasingly focused on the potential of stem cell therapy for neuropathic pain. In terms of research institutions, journals, and publications, the United States was the leader in the field of stem cell therapy for neuropathic pain. Johns Hopkins University and Duke University, two of the most cited institutions, had made outstanding contributions to the study of stem cells and neuropathic pain.
We analyzed the most highly cited journals and the top 3 journals were Pain, Journal of Neuroscience, PLoS One. The country of publication of these magazines is the United States, indicating that the United States has a leadership position in the field. We analyzed the research areas of these journals and find that most of the leading research areas focus on neuroscience. Therefore, stem cells are being studied more widely in neuroscience.
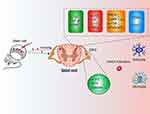
The 10 most cited publications are listed in Table 3, and 8 of these are research articles. The report published by Siniscalco et al in 2011 had the highest co-citation counts (26 times). This study confirmed that pain-like behaviors, such as mechanical allodynia and thermal hyperalgesia, could be improved by tail vein injection of hMSCs.10 The effect may be related to the ability of hAMSCs to reduce pro-inflammatory interleukin (IL-1β) levels and increase anti-inflammatory interleukin (IL-10).12 Franchi et al concluded that NSCs could decrease c-FOS, IL-1β and IL-6 expression, and increase IL-10, substance P expression.17 Siniscalco et al found that injection of hBMSCs through the lateral ventricle inhibited the over-activation of neural β-galactosidase in the prefrontal cortex and reduced IL-1β mRNA expression.10,16 Watanabe et al injected BMSCs into the spinal cord to treat spinal cord injury. BMSCs transplantation improved motor function and relieved hypersensitivities to thermal and mechanical stimulation, p-p38 and p-ERK1/2 expression were increased.19 Chen et al suggested that BMSC inhibition of neuropathic pain was associated with TGF-β but not with IL-10.11 Schafer et al concluded that the intrathecal injection of MSCs had no effect on pain and did not influence IBA-1 expression or the underlying neuroinflammatory processes in the spinal cord.14 BMSCs reduced the expression of galanin and NPY in DRG, and reduced the activation of TRPA1, PKCδ, CaMKIIα, P2X4, P2X7, which might be the mechanism of neuropathic pain improvement.39–41 Therefore, there is still controversy regarding the mechanism of stem cell therapy for neuropathic pain. The main mechanism of stem cell modulation of neuropathic pain is shown in Figure 12.
Although there are many pre-clinical studies that demonstrate the promise of stem cell therapy for neuropathic pain, clinical studies are still relatively limited. In clinical studies, stem cell therapy for neuropathic pain were mainly in the field of spinal cord injury, but the outcomes appeared to be inconsistently. The different results may be related to the type of stem cells or the route of injection. Clinical efficacy of stem cell therapy for neuropathic pain still need to be further explored in the future. The laws and policies on the use of stem cells in different countries and regions may be one of the factors affecting the application of it. The ethics and safety of stem cell applications also need to be considered seriously.
From the keyword analysis, we observe that MSCs transplantation for neuropathic pain is the current research hotspot. Among the different types of stem cells, MSCs are the most prevalent stem cell type studied. MSCs have unique advantages over other stem cells and offer a new approach to treat neuropathic pain in the future. In addition to causing motor dysfunction, spinal cord injuries can cause persistent and severe neuropathic pain.42 Stem cell transplantation for spinal cord injury has shown great potential to provide a valuable therapeutic tool to address this challenge.43
For neuropathic pain research, a keyword co-citation analysis of stem cell therapy produced ten co-citation clusters. Keyword clustering analysis finds that mechanism research is the most researched area. Furthermore, burst detection showed that expression, chronic pain, regeneration, and activation were the new hot spots within the field of stem cell and neuropathic pain. Therefore, the application of stem cells for modulating the mechanisms underlying neuropathic pain remains a hot and critical topic in current and future research.
Limitations
Although this paper systematically analyzed the current status of stem cell therapy for neuropathic pain, there are some limitations to be considered. First, although we included all relevant literature to the greatest extent possible, there are many classifications for neuropathic pain and therefore relevant literature may have been missed and not included in this study. Second, we searched only the WOS core collection, thus, any potentially missed papers were not included in the core collection. Third, stem cells contain multiple types, we only analyzed research trends in different types of stem cells, but did not further analyze their mechanisms. Fourth, the routes of injection (intrathecal, intramedullary, intracranial) also seemed to influence the therapeutic effect, however, it were not analysed in depth in this paper. Furthermore, we also did not analyze stem cell-derived exosome therapy for neuropathic pain.
Prospects
Research on the mechanism, the homing effect of different stem cell injection routes, and the efficacy of different types of stem cells are hot directions for future research. Stem cell therapy for neuropathic pain is primarily focused on animal experiments. The mechanisms of stem cell therapy for neuropathic pain are still not well understood and efficacy outcomes are inconsistent, so further research is essential. Clinical studies should be undertaken with caution until more mechanisms are explored, but this will be a promising field.
Acknowledgment
Thanks to Professor Chen Chaomei for selflessly opening up the use of CiteSpace.
Author Contributions
All authors made a significant contribution to this work in the conception, study design, execution, acquisition of data, analysis and interpretation; took part in composing the article or revising it critically; agreed to submit to this journal; reviewed and agreed on all versions of the article before submission and gave final approval of the version to be published; and agree to take responsibility and be accountable for the contents of the article.
Funding
This study was supported by the Key Project of the Natural Science Foundation of Jiangsu Education Department (16KJA320002), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_2955).
Disclosure
The authors declare no potential conflicts of interest in this research.
References
1. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. doi:10.1152/physrev.00045.2019
2. Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59. doi:10.1097/j.pain.00000000000013
3. Kuffler DP. Mechanisms for reducing neuropathic pain. Mol Neurobiol. 2020;57(1):67–87. doi:10.1007/s12035-019-01757-9
4. Nutt DJ, Phillips LD, Barnes MP, et al. A multicriteria decision analysis comparing pharmacotherapy for chronic neuropathic pain, including cannabinoids and cannabis-based medical products. Cannabis Cannabinoid Res. 2021. doi:10.1089/can.2020.0129
5. Kuffler DP. Platelet-rich plasma and the elimination of neuropathic pain. Mol Neurobiol. 2013;48(2):315–332. doi:10.1007/s12035-013-8494-7
6. Chakravarthy K, Manchikanti L, Kaye AD, Christo PJ. Reframing the role of neuromodulation therapy in the chronic pain treatment paradigm. Pain Phy. 2018;21(6):507–513.
7. Joshi HP, Jo HJ, Kim YH, An SB, Park CK, Han I. Stem cell therapy for modulating neuroinflammation in neuropathic pain. Int J Mol Sci. 2021;22(9):4853. doi:10.3390/ijms22094853
8. Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. 2019;14(10):e223994. doi:10.1371/journal.pone.0223994
9. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101 Suppl 1:5303–5310. doi:10.1073/pnas.030751
10. Siniscalco D, Giordano C, Galderisi U, et al. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci. 2011;5:79. doi:10.3389/fnint.2011.00079
11. Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin Invest. 2015;125(8):3226–3240. doi:10.3389/fimmu.2017.01
12. Sacerdote P, Niada S, Franchi S, et al. Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev. 2013;22(8):1252–1263. doi:10.1089/scd.2012.0398
13. Franchi S, Castelli M, Amodeo G, et al. Adult stem cell as new advanced therapy for experimental neuropathic pain treatment. Biomed Res Int. 2014;2014:470983. doi:10.1155/2014/470983
14. Schafer S, Berger JV, Deumens R, Goursaud S, Hanisch UK, Hermans E. Influence of intrathecal delivery of bone marrow-derived mesenchymal stem cells on spinal inflammation and pain hypersensitivity in a rat model of peripheral nerve injury. J Neuroinflammation. 2014;11:157. doi:10.1186/s12974-014-0157-8
15. Guo W, Wang H, Zou S, et al. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells. 2011;29(8):1294–1303. doi:10.1002/stem.667
16. Siniscalco D, Giordano C, Galderisi U, et al. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010;67(4):655–669. doi:10.1007/s00018-009-0202-4
17. Franchi S, Valsecchi AE, Borsani E, et al. Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain. 2012;153(4):850–861. doi:10.1016/j.pain.2012.01.008
18. Hosseini M, Yousefifard M, Aziznejad H, Nasirinezhad F. The effect of bone marrow-derived mesenchymal stem cell transplantation on allodynia and hyperalgesia in neuropathic animals: a systematic review with meta-analysis. Biol Blood Marrow Transplant. 2015;21(9):1537–1544. doi:10.1016/j.bbmt.2015.05.008
19. Watanabe S, Uchida K, Nakajima H, et al. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 2015;33(6):1902–1914. doi:10.1002/stem.2006
20. Macias MY, Syring MB, Pizzi MA, Crowe MJ, Alexanian AR, Kurpad SN. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol. 2006;201(2):335–348. PMID: 16839548. doi:10.1016/j.expneurol.2006.04.035
21. Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. Diabetes. 2008;57(11):3099–3107. doi:10.2337/db08-0031
22. Musolino PL, Coronel MF, Hökfelt T, Villar MJ. One marrow stromal cells induce changes in pain behavior after sciatic nerve constriction. Neurosci Lett. 2007;418(1):97–101. doi:10.1016/j.neulet.2007.03.001
23. Klass M, Gavrikov V, Drury D, et al. Intravenous mononuclear marrow cells reverse neuropathic pain from experimental mononeuropathy. Anesth Analg. 2007;104(4):944–948. doi:10.1213/01.ane.0000258021.03211
24. Xu Q, Zhang M, Liu J, Li W. Intrathecal transplantation of neural stem cells appears to alleviate neuropathic pain in rats through release of GDNF. Ann Clin Lab Sci. 2013;43(2):154–162. PMID: 23694790.
25. Bráz JM, Sharif-Naeini R, Vogt D, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74(4):663–675. doi:10.1016/j.neuron.2012.02.033
26. Yousefifard M, Nasirinezhad F, Shardi Manaheji H, Janzadeh A, Hosseini M, Keshavarz M. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther. 2016;7:36. doi:10.1186/s13287-016-0295-2
27. Chen C, Chen F, Yao C, et al. Intrathecal injection of human umbilical cord-derived mesenchymal stem cells ameliorates neuropathic pain in rats. Neurochem Res. 2016;41(12):3250–3260. doi:10.1007/s11064-016-2051-5
28. Vermeulen M, van Oers MH. Relapse of chronic inflammatory demyelinating polyneuropathy 5 years after autologous stem cell transplantation. J Neurol Neurosurg Psychiatry. 2007;78(10):1154. doi:10.1136/jnnp.2007.118240
29. Yoon SH, Shim YS, Park YH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25(8):2066–2073. doi:10.1634/stemcells.2006-0807
30. Kishk NA, Gabr H, Hamdy S, et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24(8):702–708. doi:10.1002/stem.2006
31. Vickers ER, Karsten E, Lilischkis R, Flood J. A preliminary report on stem cell therapy for neuropathic pain in humans. J Pain Res. 2014;7:255–263. doi:10.2147/JPR.S63361
32. Hua R, Li P, Wang X, et al. Evaluation of somatosensory evoked potential and pain rating index in a patient with spinal cord injury accepted cell therapy. Pain Phy. 2016;19(4):E659–666. PMID: 27228535.
33. Vaquero J, Zurita M, Rico MA, et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19(3):349–359. doi:10.1016/j.jcyt.2016.12.002
34. Vaquero J, Zurita M, Rico MA, et al. Intrathecal administration of autologous bone marrow stromal cells improves neuropathic pain in patients with spinal cord injury. Neurosci Lett. 2018;670:14–18. doi:10.1016/j.neulet.2018.01.035
35. Vaquero J, Zurita M, Rico MA, et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20(6):806–819. doi:10.1016/j.jcyt.2018.03.032
36. Prat S, Gallardo-Villares S, Vives M, et al. Clinical translation of a mesenchymal stromal cell-based therapy developed in a large animal model and two case studies of the treatment of atrophic pseudoarthrosis. J Tissue Eng Regen Med. 2018;12(1):532–540. doi:10.1002/term.2323
37. Levi AD, Okonkwo DO, Park P, et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery. 2018;82(4):562–575. doi:10.1093/neuros/nyx250
38. Albu S, Kumru H, Coll R, et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. 2021;23(2):146–156. doi:10.1016/j.jcyt.2020.08.008
39. Coronel MF, Musolino PL, Brumovsky PR, Hokfelt T, Villar MJ. Bone marrow stromal cells attenuate injury-induced changes in galanin, NPY and NPY Y1-receptor expression after a sciatic nerve constriction. Neuropeptides. 2009;43(2):125–132. doi:10.1016/j.npep.2008.12.003
40. Li NQ, Peng Z, Xu WW, An K, Wan L. Bone mesenchymal stem cells attenuate resiniferatoxin-induced neuralgia via inhibiting TRPA1-PKCdelta-P38/MAPK-p-P65 pathway in mice. Brain Res Bull. 2021;174:92–102. doi:10.1016/j.brainresbull.2021.06.004
41. Teng Y, Zhang Y, Yue S, et al. Intrathecal injection of bone marrow stromal cells attenuates neuropathic pain via inhibition of P2X4R in spinal cord microglia. J Neuroinflammation. 2019;16(1):271. doi:10.1186/s12974-019-1631-0
42. Hearn JH, Cotter I, Finlay KA. Efficacy of internet-delivered mindfulness for improving depression in caregivers of people with spinal cord injuries and chronic neuropathic pain: a randomized controlled feasibility trial. Arch Phys Med Rehabil. 2019;100(1):17–25. doi:10.1016/j.apmr.2018.08.182
43. Li Y, Shen PP, Wang B. Induced pluripotent stem cell technology for spinal cord injury: a promising alternative therapy. Neural Regen Res. 2021;v.16(08):54–63. doi:10.4103/1673-5374.303013
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.