Back to Journals » Infection and Drug Resistance » Volume 16
Staphylococcus aureus Induced Wound Infections Which Antimicrobial Resistance, Methicillin- and Vancomycin-Resistant: Assessment of Emergence and Cross Sectional Study
Authors Almuhayawi MS, Alruhaili MH, Gattan HS , Alharbi MT, Nagshabandi M , Al Jaouni S, Selim S , Alanazi A, Alruwaili Y, Faried OA, Elnosary ME
Received 25 April 2023
Accepted for publication 2 August 2023
Published 16 August 2023 Volume 2023:16 Pages 5335—5346
DOI https://doi.org/10.2147/IDR.S418681
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Suresh Antony
Mohammed S Almuhayawi,1 Mohammed H Alruhaili,1,2 Hattan S Gattan,2,3 Mohanned Talal Alharbi,4 Mohammed Nagshabandi,4 Soad Al Jaouni,5,6 Samy Selim,7 Awadh Alanazi,7 Yasir Alruwaili,7 Osama Ahmed Faried,8 Mohamed E Elnosary9
1Department of Clinical Microbiology and Immunology, Faculty of Medicine, King AbdulAziz University, Jeddah, Saudi Arabia; 2Special Infectious Agents Unit, King Fahad Medical Research Center, King AbdulAziz University, Jeddah, Saudi Arabia; 3Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia; 4Department of Medical Microbiology and Parasitology, Faculty of Medicine, University of Jeddah, Jeddah, 23218, Saudi Arabia; 5Department of Hematology/Oncology, Faculty of Medicine, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 6Yousef Abdulatif Jameel Scientific Chair of Prophetic Medicine Application, Faculty of Medicine, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 7Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Jouf University, Sakaka, Saudi Arabia; 8Medical Microbiology and Immunology Department, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt; 9Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
Correspondence: Mohammed S Almuhayawi; Samy Selim, Email [email protected]; [email protected]
Background: Wound infection is a prevalent concern in the medical field, being is a multi-step process involving several biological processes. Methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA) infections often occur in areas of damaged skin, such as abrasions and open wounds.
Methods: This research aims to light the incidence of MRSA and VRSA in wound swabs, the antimicrobial susceptibility configuration of isolated S. aureus patterns in pus/wound samples collected from Saudi Arabian tertiary hospital. The cross section study, β- lactamase detection, VRSA genotyping, MAR index, D-test and VRSA genotyping are methods, which used for completed this research.
Results: Patients of several ages and genders delivered specimens from two hospitals in the Al jouf area, in the northern province of Saudi Arabia. S. aureus was found in 188 (34.7%) of the 542 wounds. The traumatized wounds provided 71 isolates (38.8%), surgical wound provided 49 isolates (26.8%) and abscess were represented 16 by isolates (8.7%). In the study, 123 (65.4%) out of 188 were MRSA, 60 (31.9%) were MSSA, and five (2.7%) were VRSA. Linezolid and rifampin were found to be the most effective antimicrobials with 100% in vitro antibacterial activity against S. aureus isolates. The Multiple antimicrobials resistance (MAR) index revealed 73 isolates (38.9%) with a MAR index greater than 0.2, and 115 (61.1%) less than 0.2. The D-test showed that of MLSb phenotypes among S. aureus, 22 (11.7%) strains were D-test positive (MLSbi phenotype), 53 (28.2%) strains were constitutive MLSc phenotypes, and 17 (9%) strains were shown to have MSb phenotypes. All VRSA isolates (n=5) were found to be positive for vanA, and no vanB positive isolates were detected in the study.
Conclusion: Regular monitoring and an antimicrobials stewardship program should be in place to provide critical information that can be utilized for empirical therapy and future prevention strategies.
Keywords: methicillin, vancomycin, resistant, Staphylococcus aureus, emergence, virulent, wound infection, MAR index, D-test, tertiary hospital, Saudi Arabia
Introduction
Human skin serves as an effective barrier to infection, protecting the underlying tissues, bones, and organs.1–3 Wounds are defined as a breach in the skin or tissues’ structural integrity that affects the skin’s ability to defend itself.4,5 As one the most common causes of death and morbidity in surgical patients, wound infection accounts for 70% to 80% of deaths after burn injuries.6–8 Out of all surgical deaths, around 70–80% deaths are caused by wound infection.9–11 Bacteria that cause pus production or wound infection include S. aureus, Clostridium spp., Actinomyces spp., E. coli, Proteus spp., Neisseria spp., Vibrio vulnificus and Candida spp.12
S. aureus is a versatile pathogen capable of infecting humans with a broad spectrum of illnesses causing both infection and soft tissue infection.13–16 Skin and soft tissue infections caused by S. aureus, as life-threatening systemic illnesses, are a significant hospital-acquired and community-acquired infections.17–20 Methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA) represent a global public health hazard because of their risk and spread.21 A lack of adequate containment and treatment options for MRSA and VRSA has the potential to cause significant global mortality.22–24 VRSA is also known to be resistant to a wide range of commonly used antimicrobial treatments. As the final line of defense against MRSA and other drug-resistant, Gram-positive pathogens, glycopeptide vancomycin has long been considered a lifesaver.25–27 Antimicrobial abuse and overuse may contribute to the rise of antimicrobial resistance, which is a major cause of illness and death around the world.28–30 The most prevalent place for MRSA and VRSA to co-infect and co-colonize is in a wound, making it the most typical place where VRSA is isolated. Treatment for a VRSA infection often includes prompt attention to the wound. The eradication of VRSA is aided by wound treatment, which also eliminates a conducive environment for co-colonization, thereby preventing the spread of plasmids.30
Since the emergence of antimicrobial-resistant bacteria, treating infectious diseases has become more difficult.28 In order to be classified as multidrug resistant (MDR), an organism must be resistant to three or more categories of antimicrobial agents; extremely (extensive) drug resistant (XDR) means that an organism is resistant to all antimicrobial agents except for two or fewer categories, and pan drug resistant (PDR) means that an organism is resistant to all antimicrobial agents.31–33 MRSA refers to any S. aureus strain that has evolved or acquired a multiple drug-resistance to beta-lactam drugs. Treatment of serious MRSA infections with glycopeptide vancomycin is still the preferred option. Vancomycin suppresses cell wall manufacturing. VRSA is a phrase used to describe S. aureus isolates that are completely resistant to vancomycin (MIC≥16 µg/mL).22,25 There has been a significant rise in the incidence of antimicrobial resistance in hospital and community infections during the last decade. MRSA and VRSA have been brought to the attention of the medical profession and the general public, along with their full effect on health and economic consequences.34 Therefore, both the development of MRSA and VRSA as well as their prospective cost-effectiveness estimations are influenced by their clinical and economic consequences.28 Surgical wound infections are classified as followed by the Centers for Disease Control and Prevention (CDC):
Superficial incisional infection that only involves the skin and subcutaneous tissues. One of the following criteria has to be met: purulent discharge from the wound, isolated organism, at least one symptom of infection, and diagnosis by the surgeon. These infections account for more than 50% of all surgical infections.
Deep incisional infections involve deeper tissues, including muscles and fascial planes. One of the following criteria has to be met: purulent discharge from the wound, dehiscence, or deliberate re-opening of deep incision by the surgeon after suspecting an infection, evidence of abscess formation, or other deep infection diagnoses by the surgeon.
Organ/space infection may involve any organ apart from the incision site but must be related to the surgical procedure. One of the following criteria has to be met: purulent discharge from the drain placed in the organ, isolated organism from the organ, abscess, or other infection involving the organ.
This study sheds light on the current prevalence of MRSA and VRSA in wound swabs, the antimicrobial susceptibility pattern of the isolated S. aureus, and the presence of multidrug resistant strains among the isolates. Therefore, the study aims to assess the pattern of S. aureus isolated from pus/wound samples in Saudi Arabian tertiary hospitals.
Materials and Methods
Study Design
This cross-sectional descriptive study was carried out during the period from February 2022 to September 2022. Fisher’s formula was used to estimate the proper sample size which is 500 samples in our research. Data and specimens were collected from patients of all sexes and ages who visited the hospital throughout the research period. A total of 188 different S. aureus were isolated from 542 wound specimens in the Prince Mutaib Bin Abdulaziz Hospital (339) and Swair General Hospital in Sakaka, Al Jouf, Saudi Arabia (203). Table 1 exhibits the distribution of wound specimens by gender, age, and location. Specimens that indicated any evidence of contamination were discarded from the examination. It is important to note that patients were only included in the study if they had a sample labeled with a combination of the following keywords: a wound swab, abscess, wound, drain, culture, or discharge.
 |
Table 1 Distribution of Wound Specimens of Patients by Gender, Age and Locations |
Collection of Specimens and Bacterial Categorization
Aseptic dry swab samples were taken from the pus and wounds. The samples were properly labeled before being transferred to the lab, where they were quickly processed. Specimens were subsequently cultured on Blood Agar and incubated at 37°C for 24 hours. Staphylococcal isolates were identified using biochemical and morphological approaches.35 Multiple biochemical tests for the confirmation of S. aureus were performed on the Gram-positive cocci in cluster detected under the microscope. Identification of S. aureus based on the presence of catalase and oxidase as well as coagulase activity and DNase activity in the S. aureus colonies on mannitol salt agar. Presumptive MRSA was confirmed by Vitek 2 identification card (bioMerieux, Marcy l’Etoile, France) was used for automated strain identification according to the manufacturer’s instructions, and the quality control (QC) strain tested with each run was S. aureus ATCC25923. According to current EUCAST guidelines, methicillin susceptibility was evaluated using oxacillin discs (30 µg, Oxoid) and the mecA gene (F: GATCTGTACTGGGTTAATCA and R: CATATGACGTCTATCCATTT was identified using the PCR approach.36
Antimicrobial Resistance Testing
Stock cultures of S. aureus were employed in all assays to avoid the possible loss of antimicrobial resistance that could occur while frequently subculturing. Sterile normal saline was used to make 0.5 McFarland suspensions from the S. aureus culture. The antimicrobial discs we utilized were from (Oxoid, UK) (Table 2). The antibiotics were selected because of their widespread application in the treatment of S. aureus infections. After that, a modified Kirby–Bauer disk-diffusion susceptibility assay was performed using the suspension on Müeller–Hinton agar (MHA). It was carried out according to the CLSI protocol for antimicrobial susceptibility testing (AST).37 It was determined that the MRSA was present utilizing the 30 µg oxacillin disc diffusion test, which serves as a surrogate test for oxacillin resistance. Following incubation at 35°C for 24 hours, the data were analyzed in accordance with CLSI recommendations.38 Oxacillin Resistance Screening Agar Base is used for the screening of oxacillin-resistant microorganisms. It is recommended for the detection of methicillin-resistant Staphylococcus aureus (MRSA). These strains are resistant to penicillinase-resistant penicillins (PRPs), such as methicillin, oxacillin, and nafcillin, The bacteria is not resistance to oxacillin test when a zone diameter of approximately 24 mm. As a result, only MRSA cases were screened for VRSA/VISA; nevertheless, the estimate is based on the entire enrolled population. A pure colony of MRSA isolates was obtained and injected on MHA supplemented by vancomycin to detect the presence of VRSA. Using sterile forceps, the E-test (Epsilometer test) strips were placed on the inoculated agar surface. Results were obtained after 18 hours of incubation at 35–37°C when the MIC breakpoints were determined. In general, MICs; ≤ 2 µg/mL is considered as sensitive, 4–8 µg/mL as VISA and ≥ 16 µg/mL as vancomycin resistant S. aureus (VRSA). For the final step in the procedure of the oxacillin disc diffusion test for MRSA, S. aureus ATCC25923 was utilized as a control strain. The control strain, S. aureus ATCC 29213 with a MIC of vancomycin broth value 0.5–2.0 µg/mL, was utilized to evaluate the efficacy of vancomycin in this study.39,40
 |
Table 2 Incidence of Antimicrobial Susceptible Pattern of S. Aureus (N= 188) |
β-. Lactamase Detection
As explained by Stokes and Ridgway,41 this test was conceded. Sterilized starch paper strips of seven centimeters long were cut and sterilized in 70% ethanol. A benzylpenicillin (1000 units) in phosphate buffer solution was then added to the strips, and they were left to soak for 10 minutes. They were distributed in sterile Petri dishes. As a final step, the test paper was saturated with S. aureus cultures that were 18 to 24 hours old and spread across an area of 2 to 3 mm. After 30 minutes of incubation at 37°C in the Petri dishes, it was flooded with Gram iodine solution. In less than a minute after using this, the starch paper had turned pitch-black. Colonies with decolorized zones later demonstrated the synthesis of β -lactamase.
Calculation of MAR Index
In order to trace the cause of antimicrobial-resistant, the MAR index (MAR index is calculated as the ratio between the number of antimicrobials that an isolate is resistant to and the total number of antimicrobials the organism is exposed to) is an effective, valid, and cost-efficient tool. If an organism has been exposed to a certain number of antimicrobials and developed resistance to any of those antimicrobials, then the MAR index is great. A MAR larger than 0.2 indicates that antimicrobials are often utilized as a source of infection.42 According to Davis,43 MAR index was calculated.
D-Test
D-test was achieved by expending the erythromycin disc (15 µg) and clindamycin disc (2 µg). The antimicrobial discs were engaged on Müller-Hinton agar plate at 15 mm apart and was incubated at 37° C at 18–24 hours.44–46 The organisms that revealed devastation zone of clindamycin adjacent to the erythromycin disc were deliberated as: MSb (The macrolide-streptogramin B class) phenotype (S. aureus displaying resistance to erythromycin (zone size ≤ 13 mm) and susceptible to clindamycin (zone size ≥ 21 mm) and generous circular zone of inhibition around clindamycin disc), inducible MLSbi (The macrolide-lincosamide-streptogramin B class) phenotype (revealing resistance to erythromycin (zone size ≤ 13 mm) and susceptible to clindamycin (zone size ≥ 21 mm) and generous D shaped zone of inhibition around clindamycin disc), and constitutive MLSbc phenotype (S. aureus indicating resistance to both erythromycins (zone size ≤ 13 mm) and clindamycin (zone size ≤ 14 mm) with the generous circular shape of zone of inhibition if any around clindamycin disc).47,48
VRSA Genotyping
To distinguish the vanA and vanB genes in VRSA, PCR was achieved on a BioRad thermocycler (USA), in reaction volumes of 12.5 μL, using formerly issued primers37,38 5′- F: CATGAATAGAATAAAAGTTGC AATA & R: CCCCTTTAACGCTAATACGATCAA (forvanA); F:GTGACAAACCGGAGGCGAGGA & R: CCGCCATCCTCCTGCAAAAAA (for vanB). Each reaction enclosed 5 μL of 2xTaq master mix (Sigma Co.), 1 μL of forward and reverse primers, 3 μL nuclease-free water, and 2.5 μL of crude VRSA DNA extract (template). The cycling conditions were as follows: Initial denaturation at 94 °C, 4 min followed by 31 cycles of 94 °C 1 min, 50 °C 1 min, and 68 °C 1 min, and a final extension step at 68 °C, 10 min. The PCR products were examined by, agarose gel electrophoresis, with ethidium bromide staining (0.5 μg/mL). Repetitive elements-based PCR (Rep-PCR) genotyping, for insight into genetic relatedness of the isolates, was accomplished as earlier designated.49
Ethical Considerations
Approval was obtained from the Research Ethics Committee, Jouf University (Ethical Approval No. 3-04-43) and Research Ethics Committee, Qurayyat Health Affairs, Registered with NCBE, Reg NO: H-13-S-071; Saudi Arabia (Project No. 111). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. In this study, written consent was obtained from each patient.
Statistical Analysis
All the data were entered and evaluated by exhausting Statistical Platform for Social Science (SPSS) version 24.
Results
We examined the skin of 542 participants aged between ten years and 79 years. The majority of participants were male, p value < 0.0001.
As of this study, S. aureus was found in 188 (34.7%) of the 542 wounds, of which 129 (70.5%) were men, with a male-to-female ratio of 2.3:1 among infectious participants. S. aureus Participants varied in age from 1 to 79 years old. The 31–45-year-old age group made up 27.9% of the participants. The traumatized wounds provided 71 isolates (38.8% of the total), followed by surgical wounds providing 49 isolates (26.8%), and the abscesses revealed the lowest number of isolates representing 16 isolates (8.7%) (Table 3). In the study, 123 (65.4%) out of 188 were MRSA, 60 (31.9%) were MSSA, and 5 (2.7%) were VRSA (Figure 1). All of the VRSA strains were isolated from the traumatized wounds. Overall, MRSA (62.8%) was more common than MSSA (37.2%) through entirely demographic features, p value < 0.001 (Table 3).
 |
Table 3 The Sociodemographics, Clinical Characteristics, Numbers and Percentages of Methicillin-Resistant (MRSA) and Methicillin-Susceptible S. Aureus (MSSA) Isolates of the Participants |
 |
Figure 1 Distribution of vancomycin-resistant S. aureus (VRSA), methicillin-susceptible S. aureus (MSSA) and Methicillin-resistant S. aureus (MRSA) among wound infection. |
Out of 24 premeditated different antimicrobial drugs, linezolid and rifampin were found to be the most effective antimicrobial drugs with 100% efficiency to S. aureus, followed by daptomycin and tigecycline (99.5%) and teicoplanin (98.4%) (Table 2).
The highest resistance was similarly found in ampicillin and penicillin G (86.7%) and cefotaxime (77.1%). Out of 188 S. aureus, 86 (45.7%) were found to be MDR (Table 4). Only four staphylococcal isolates (2.1%) were found to be susceptible to all of the antimicrobial drugs tested, and none of the staphylococcal isolates tested were found to be resistant to all of the antimicrobial medicines tested. Only five isolates were revealed to be XDR (2.7%). Table 5 displays the MAR indices of S. aureus. Equally, 38.9% of S. aureus isolates accomplished a MAR index of more than 0.2 (Table 5). The MAR index revealed 73 isolates (38.9%) with a MAR index greater than 0.2, and 115 (61.1%) less than 0.2. However, no isolates displayed a MAR index of one (ie resistance to all the antimicrobials agents experienced), while four isolates showed a MAR index of zero, out of which two convalesced from a traumatized wound and two from burn wound. Isolates with a MAR index greater than 0.2 were mainly isolated from traumatized wound patients.
 |
Table 4 Frequency of Antimicrobial-Resistant Phenotypes of S. Aureus Isolates (N= 188) |
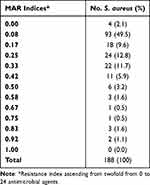 |
Table 5 Frequency of Multiple Antimicrobial Resistance (MAR) Index of S. Aureus Isolates (N = 188) Against 24 Antimicrobial Agents |
All the S. aureus isolates were subjected to recognition of methicillin resistance, β-lactamase, and MLSb phenotypes (Figure 2). In the current study, out of 188 S. aureus isolates, 92 (18.9%) were erythromycin-resistant; these were subjected to a D-test. The D-test revealed MLSb phenotypes among S. aureus, 22 (11.7%) strains were D-test positive, indicating inducible clindamycin-resistant strains of S. aureus (MLSbi phenotype), 53 (28.2%) strains were constitutive MLSc phenotypes, and 17 (9%) strains were shown to have MSb phenotypes (Figure 2). The dominance of MLSb phenotypes among MRSA and MSSA was evaluated, and it was detected that inducible and constitutive clindamycin-resistant strains of S. aureus were higher among MRSA isolates as compared to MSSA isolates.
Based on the results of the molecular study for vancomycin among 5 VRSA isolates, five VRSA-positive isolates, five isolates were found to be positive for the vanA gene, whereas the vanB gene was not detected in this study (Figure 3).
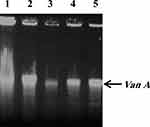 |
Figure 3 Molecular analysis of the vanA genes in the vancomycin-resistant S. aureus (VRSA) (n= 5). The vanA gene was found in all five isolates. |
Discussion
S. aureus is the major bacterial cause of skin, soft tissue and bone infections, and one of the commonest causes of healthcare-associated bacteremia. Methicillin resistance is defined as the strains of S. aureus that are resistant to the isoxazoyl penicillins, such as methicillin, oxacillin and flucloxacillin. MRSA are cross-resistant to all currently licensed β-lactam antibiotics.
The expression of methicillin resistance by S. aureus strains is by virtue of acquired penicillin-binding proteinPBP2a, encoded by mec A gene.50 Community and hospital-acquired infections caused by S. aureus have a significant fatality rate. Although it is a typical element of the human body’s microbiota, S. aureus has the potential to cause a broad variety of illnesses, from minor skin infections to more severe illnesses affecting the whole body. If left untreated, many of these illnesses may quickly become life-threatening.51–53 S. aureus was found in 34.7% of the wound swab samples examined in this investigation. The percentage of MRSA in the isolates was less than the western region of Saudi Arabia prevalence estimate of 39.5%.54 It is clear that MRSA is a global health threat, as shown by the high rates of antimicrobial resistance reported in countries, such as Eritrea (72%), Turkey (21%), the Gaza Strip (82.3%), Iran (71.9%), and a tertiary care facility in Lahore (76%).55–57 The incidence rates of VRSA strains are diverse all over the world: 16% in Africa, 5% in Asia, and 1% in Europe, 4% in North America, and 3% in South America.56 The VRSA in the current investigation is much lower related to reports from other countries.23,58
S. aureus was shown to be the most common pathogen in the research, maybe because it is a typical microbiota of the skin, glands, nails, and other parts of the body, as well as possessing diverse virulent characteristics.50 Colonization by patient microbiota, transmission via staff hands and air, surgical techniques, inanimate items, and extended hospital stays, may be responsible for the high frequency of S. aureus in wound specimens.59–61 For S. aureus, linezolid (100%) was shown to be the most effective antimicrobial, followed closely by daptomycin (99.5%). Linezolid and vancomycin were shown to be 100% susceptible in the research done by other studies.62,63 There was also a strain among the MDR that was resistant to all the antimicrobial drugs that have been investigated (extremely drug-resistant). More than one gene may lead to MDR, as well as drug expulsion and mutagenesis in the target protein.64–66
MRSA acquires a staphylococcal chromosomal cassette mec, it may acquire a mec A gene that facilitates methicillin resistance by a penicillin-binding protein (PBP-2a).67 Even when the organism has the mecA gene, methicillin resistance cannot be proven. In our study, vancomycin was shown to be completely effective against an isolated strain of S. aureus. As a result, MIC testing is required to verify the presence of a VRSA strain. van A and van B genes may be activated, causing vancomycin resistance. While doing MIC tests, the organism was discovered to be susceptible to vancomycin.68
Only 11.7% of the isolates had a positive D-test, while 9% had a negative D-test, according to the findings. This may be related to erm genes, which encode for resistance to erythromycin and clindamycin. The organism’s resistance to all macrolides, lincosamides, and type B streptogramin antimicrobials might explain a constitutive expression, according to Mama et al.69 To put it simply, the correlation between the D-test and MRSA was found, which suggests that the number of MRSA cases may rise as the number of D-test cases rise. A clindamycin-resistant phenotype should be established at each medical laboratory investigation. The gold standard for assessing a bacterium’s antimicrobial susceptibility is phenotypic testing, but it is restricted by the number of antimicrobials that can be tested in the laboratory.70,71 Genomic analysis is good for detecting resistance to many antimicrobials, discovering new resistance genes, mutations, or new synergistic interactions impacting resistance genes and identifying new resistance genes, mutations, or synergistic links affecting resistance genes.72,73
Studied mecA and vanA genes were shown to have phenotype-genotype conformity and miscellaneous among S. aureus. Extra penicillin-binding protein (PBP2a) is encrypted by the mecA determinant. Deliberate acylation of PBP2a and low enzyme empathy for β-lactams74 permit resistance to be expressed. PBP2a’s low acylation rate when exposed to β-lactams is unsettled, conferring to organizational studies,75 to a deformed active site assumed by the non-binding (NB) domain and zones round the dynamic spot channel in the transpeptidase (TP) domain. Moreover, Ser403’s position is necessary for the nucleophilic beating of the β-lactam ring, which outcomes in protein acylation.7 vanA gene cluster enclosed two agents convoluted in the transcription of the seven open reading frames. The regulatory apparatus is encoded by a two-component system consisting of the vanR (response regulator) and vanS (sensing kinase) genes, which are transcribed from a shared promoter together with the rest of the genes. vanA is example of gene products that selectively alter peptidoglycan precursor synthesis (ligase that forms D-Ala-D-Llac dipeptide). For empirical therapy and future prevention, the antimicrobial susceptibility test information gained from a deep wound infection is crucial. In order to fully grasp the magnitude and scope of the MRSA and VRSA epidemics, more molecular and epidemiological investigations are required.76 As a result, those at high risk for developing MRSA should have timely access to preventative and control measures. In addition, regular monitoring and an antimicrobials stewardship program should be in place to provide critical information that can be utilized for empirical therapy and future preventions strategies.
Conclusion
In this investigation, the percentage of MRSA was greater than VRSA. In addition, a number of characteristics, including wound infection, expanded hospital stay, contemporary use of antimicrobials, and persistent deep chronic wounds were shown to be associated with MRSA. A result of vanA in strains in our investigation indicates that the vancomycin-resistant gene is spreading among S. aureus and other bacterial pathogens. There is also a severe difficulty in therapeutic management with regard to vancomycin resistance, one of the few medications available for the treatment of MRSA infections. MRSA strains were more likely to have MDR than MSSA strains, and MRSA patients were observed to have a greater rate of D-test positives. Clindamycin resistance must be screened before prescribing the medicine in order to ensure that the infection can be effectively treated, though, linezolid may also be a possibility for treating infections like these.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
Approval was obtained from the Research Ethics Committee, Jouf University (Ethical Approval No. 3-04-43) and Research Ethics Committee, Qurayyat Health Affairs, Registered with NCBE, Reg NO: H-13-S-071; Saudi Arabia (Project No. 111). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the participants. Additionally, informed written consents from guardians or parents and assents from each study participants under 15 years were obtained in accordance with the Declaration of Helsinki. The confidentiality of study participant was kept and identification of study participant by name was avoided.
Funding
This research work was funded by Institutional Fund Projects under grant no. (IFPIP: 614-140-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabi.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Greaves P. Integumentary System. In: Histopathology of Preclinical Toxicity Studies.
2. Nguyen AV, Soulika AM. The dynamics of the skin’s immune system. Int J Mol Sci. 2019;20:1811. doi:10.3390/ijms20081811
3. Mou K, Abdalla M, Wei DQ, et al. Emerging mutations in envelope protein of SARS-CoV-2 and their effect on thermodynamic properties. Inform Med Unlocked. 2021;25:100675. doi:10.1016/j.imu.2021.100675
4. Herman TF, Bordoni B. Wound Classification. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
5. Arif M, Sharaf M, Khan S, et al. Chitosan-based nanoparticles as delivery-carrier for promising antimicrobial glycolipid biosurfactant to improve the eradication rate of Helicobacter pylori biofilm. J Biomater Sci Polym Ed. 2021;32:813–832. doi:10.1080/09205063.2020.1870323
6. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn Wound Infections. Clin Microbiol Rev. 2006;19:403–434. doi:10.1128/CMR.19.2.403-434.2006
7. Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13:R183. doi:10.1186/cc8170
8. Hadi AM, Mohammed Al-Alwany SH, Al-Khafaji ZA, Sharaf M, Mofed D, Khan TU. Molecular diagnosis of herpes virus type 1 by glycoprotein receptor primers. Gene Rep. 2022;26:101479. doi:10.1016/j.genrep.2021.101479
9. World Health Organization. Regional Office for South-East Asia. In: Regional Health Forum. Vol. 14. New Delhi: World Health Organization; 2010.
10. Wound infection: background, pathophysiology, etiology. 2021. https://emedicine.medscape.com/article/188988-overview.
11. Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections – an overview. Infect Drug Resist. 2018;11:2321–2333. doi:10.2147/IDR.S177247
12. Crum-Cianflone NF. Bacterial, Fungal, Parasitic, and Viral Myositis. Clin Microbiol Rev. 2008;21:473–494. doi:10.1128/CMR.00001-08
13. Gaupp R, Ledala N, Somerville G. Staphylococcal response to oxidative stress. Front Cell Infect Microbiol. 2012;2. doi:10.3389/fcimb.2012.00002
14. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203–218. doi:10.1038/s41579-018-0147-4
15. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:S350–S359. doi:10.1086/533591
16. Hamad AA, Sharaf M, Hamza MA, Selim S, Hetta HF, El-Kazzaz W. Investigation of the bacterial contamination and antibiotic susceptibility profile of bacteria isolated from bottled drinking water. Microbiol Spectr. 2022;10:e01516–21. doi:10.1128/spectrum.01516-21
17. Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis. 2013;13:252. doi:10.1186/1471-2334-13-252
18. Ramakrishnan K, Salinas RC, Higuita NIA. Skin and Soft Tissue Infections. Am Fam Physician. 2015;92:474–483.
19. Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–661. doi:10.1128/CMR.00134-14
20. Sharaf M, Hamouda HI, Shabana S, et al. Design of lipid-based nanocarrier for drug delivery has a double therapy for six common pathogens eradication. Colloids Surf a Physicochem Eng Asp. 2021;625:126662. doi:10.1016/j.colsurfa.2021.126662
21. Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46:S368–S377. doi:10.1086/533593
22. Choo EJ, Chambers HF. Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect Chemother. 2016;48:267–273. doi:10.3947/ic.2016.48.4.267
23. Wu Q, Sabokroo N, Wang Y, Hashemian M, Karamollahi S, Kouhsari E. Systematic review and meta-analysis of the epidemiology of vancomycin-resistance Staphylococcus aureus isolates. Antimicrob Resist Infect Control. 2021;10:101. doi:10.1186/s13756-021-00967-y
24. Helen K, Ashlesha K. Vancomycin-Resistant Staphylococcus aureus: formidable threat or silence before the storm? J Infect Dis Epidemiol. 2019;5. doi:10.23937/2474-3658/1510093
25. Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10:107. doi:10.3389/fcimb.2020.00107
26. Szymanek-Majchrzak K, Mlynarczyk A, Mlynarczyk G. Characteristics of glycopeptide-resistant Staphylococcus aureus strains isolated from inpatients of three teaching hospitals in Warsaw, Poland. Antimicrob Resist Infect Control. 2018;7:105. doi:10.1186/s13756-018-0397-y
27. Gardete S, Tomasz A. Mechanisms of Vancomycin Resistance in Staphylococcus aureus. J Clin Invest. 2014;124:2836–2840. doi:10.1172/JCI68834
28. Ventola CL. The Antibiotic Resistance Crisis: part 1: causes and Threats. Pharm Ther. 2015;40:277–283.
29. CDC what exactly is antibiotic resistance? Available From: https://www.cdc.gov/drugresistance/about.html.
30. Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi:10.1016/S0140-6736(21)02724-0
31. Basak S, Singh P, Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: a study. Journal Pathog. 2016;2016:4065603. doi:10.1155/2016/4065603
32. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi:10.1111/j.1469-0691.2011.03570.x
33. Sweeney MT, Lubbers BV, Schwarz S, Watts JL. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J Antimicrob Chemother. 2018;73:1460–1463. doi:10.1093/jac/dky043
34. Lee AS, de Lencastre H, Garau J, et al. Methicillin-Resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4:1–23. doi:10.1038/nrdp.2018.33
35. Raut S, Rijal KR, Khatiwada S, et al. Trend and Characteristics of Acinetobacter baumannii infections in patients attending universal college of medical sciences, Bhairahawa, Western Nepal: a longitudinal study of 2018. Infect Drug Resist. 2020;13:1631–1641. doi:10.2147/IDR.S257851
36. Pillai MM, Latha R, Sarkar G. Detection of methicillin resistance in Staphylococcus aureus by polymerase Chain reaction and conventional methods: a comparative study. J Lab Physicians. 2012;4:83. doi:10.4103/0974-2727.105587
37. Weinstein MP. Clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing; 2021.
38. Weinstein MP, Lewis JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol. 2020;58:e01864–19. doi:10.1128/JCM.01864-19
39. Tiwari HK, Sen MR. Emergence of Vancomycin Resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis. 2006;6:156. doi:10.1186/1471-2334-6-156
40. Sy CL, Huang T-S, Chen CS, et al. Synergy of β-lactams with vancomycin against methicillin-resistant Staphylococcus aureus: correlation of disk diffusion and checkerboard methods. J Clin Microbiol. 2016;54:565–568. doi:10.1128/JCM.01779-15
41. Stokes EJ, Ridgeway GL, Wren MWD. Clinical Microbiology.
42. Sandhu R, Dahiya S, Sayal P. Evaluation of multiple antibiotic resistance (MAR) index and doxycycline susceptibility of Acinetobacter species among inpatients. Ind Jour of Microb Res. 2016;3:299. doi:10.5958/2394-5478.2016.00064.9
43. Davis R, Brown PD. Multiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J Med Microbiol. 2016;65:261–271. doi:10.1099/jmm.0.000229
44. Kejela T, Bacha K. Prevalence and antibiotic susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) among primary school children and prisoners in Jimma Town, Southwest Ethiopia. Ann Clin Microbiol Antimicrob. 2013;12:11. doi:10.1186/1476-0711-12-11
45. Osundiya OO, Oladele RO, Oduyebo OO. Multiple antibiotic resistance (MAR) Indices of Pseudomonas and Klebsiella species isolates in Lagos university teaching hospital. African J Clin Exp Microbiol. 2013;14:164–168. doi:10.4314/ajcem.v14i3.8
46. Tefera S, Awoke T, Mekonnen D. Methicillin and vancomycin resistant Staphylococcus aureus and associated factors from surgical ward inpatients at debre markos referral hospital, Northwest Ethiopia. Infect Drug Resist. 2021;14:3053–3062. doi:10.2147/IDR.S324042
47. Raut S, Bajracharya K, Adhikari J, Pant SS, Adhikari B. Prevalence of methicillin resistant Staphylococcus aureus in Lumbini medical college and teaching hospital, Palpa, Western Nepal. BMC Res Notes. 2017;10:187. doi:10.1186/s13104-017-2515-y
48. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin Treatment of Methicillin-Resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37:1257–1260. doi:10.1086/377501
49. Maharjan B, Karki ST, Maharjan R. Antibiotic susceptibility pattern of Staphylococcus aureus isolated from pus/wound swab from children attending international friendship children’s hospital. Nepal J Biotechnol. 2021;9:8–17. doi:10.3126/njb.v9i1.38645
50. Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10(4):781–791. doi:10.1128/CMR.10.4.781
51. Taylor TA, Unakal CG. Staphylococcus aureus. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
52. Almuhayawi MS, Gattan HS, Alruhaili MH, et al. Molecular Profile and the effectiveness of antimicrobials drugs against staphylococcus aureus and pseudomonas aeruginosa in the diagnostic approaches of otitis infection. Infect Drug Resist. 2023;Volume 16:4397–4408. doi:10.2147/IDR.S418685
53. Liu GY. Molecular Pathogenesis of Staphylococcus aureus Infection. Pediatr Res. 2009;65:71R–77R. doi:10.1203/PDR.0b013e31819dc44d
54. El Amin NM, Faidah HS. Methicillin-resistant Staphylococcus aureus in the western region of Saudi Arabia: prevalence and antibiotic susceptibility pattern. Ann Saudi Med. 2012;32:513–516. doi:10.5144/0256-4947.2012.513
55. Almuhayawi MS, Alruhaili MH, Gattan HS, et al. In silico molecular modeling of cold pressed garden cress (Lepidium sativum L.) seed oil toward the binding pocket of antimicrobial resistance Staphylococcus aureus DNA-gyrase complexes. Eur Rev Med Pharmacol Sci. 2023;27:4.
56. Alkhodari SA, Elmanama AA. Multidrug resistance of uropathogens at governmental hospitals in the gaza strip/Palestine. Int Arab J Antimicrob Agents. 2021;11. doi:10.3823/855
57. Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10:12689. doi:10.1038/s41598-020-69058-z
58. Hasan R, Acharjee M, Noor R. Prevalence of vancomycin resistant Staphylococcus aureus (VRSA) in methicillin resistant S. aureus (MRSA) strains isolated from burn wound infections. Tzu Chi Med J. 2016;28:49–53. doi:10.1016/j.tcmj.2016.03.002
59. Moremi N, Claus H, Vogel U, Mshana SE. The role of patients and healthcare workers Staphylococcus aureus nasal colonization in occurrence of surgical site infection among patients admitted in two centers in Tanzania. Antimicrob Resist Infect Control. 2019;8:102. doi:10.1186/s13756-019-0554-y
60. Elnosary M, Aboelmagd H, Sofy MR, Sofy A, Elshazly E. Antiviral and antibacterial properties of synthesis silver nanoparticles with nigella arvensis aqueous extract. Egypt J Chem. 2022;1:1. doi:10.21608/ejchem.2022.159976.6894
61. Collins AS. Preventing Health Care–Associated Infections. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Advances in Patient Safety; Agency for Healthcare Research and Quality (US); 2008.
62. Kishore S, Verma D, Siddique M. Comparison of in-vitro activities of linezolid and vancomycin against Staphylococcus aureus isolated from a tertiary care hospital. J Clin Diagn Res. 2014;8:DC12–DC15. doi:10.7860/JCDR/2014/7751.4338
63. Çomoğlu Ş. Determination of in vitro activity of linezolid in resistance gram positive bacteria by E-test method. Haydarpasa Numune Med J. 2018. doi:10.14744/hnhj.2018.30085
64. Smith T, Wolff KA, Nguyen L. Molecular biology of drug resistance in Mycobacterium tuberculosis. Curr Top Microbiol Immunol. 2013;374:53–80. doi:10.1007/82_2012_279
65. Elnosary ME, Aboelmagd HA, Habaka MA, Salem SR, El-Naggar ME. Synthesis of bee venom loaded chitosan nanoparticles for anti-MERS-COV and multi-drug resistance bacteria. Int J Biol Macromol. 2023;224:871–880. doi:10.1016/j.ijbiomac.2022.10.173
66. Dookie N, Rambaran S, Padayatchi N, Mahomed S, Naidoo K. Evolution of drug resistance in Mycobacterium tuberculosis: a review on the molecular determinants of resistance and implications for personalized care. J Antimicrob Chemother. 2018;73:1138–1151. doi:10.1093/jac/dkx506
67. Miragaia M, Zhou M, Wang W, Sun X, Yarden O, Li S. Factors contributing to the evolution of meca-mediated β-lactam resistance in staphylococci: update and new insights from whole genome sequencing (WGS). Front Microbiol. 2018;9:9. doi:10.3389/fmicb.2018.00009
68. Howden BP, Davies JK, Johnson PDR, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–139. doi:10.1128/CMR.00042-09
69. Mama M, Aklilu A, Misgna K, Tadesse M, Alemayehu E. Methicillin- and inducible clindamycin-resistant staphylococcus aureus among patients with wound infection attending arba minch hospital, South Ethiopia. Int J Microbiol. 2019;2019:e2965490. doi:10.1155/2019/2965490
70. Baltekin Ö, Boucharin A, Tano E, Andersson DI, Elf J. Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging. Proc Natl Acad Sci. 2017;114:9170–9175. doi:10.1073/pnas.1708558114
71. Khan ZA, Siddiqui MF, Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics. 2019;9:49. doi:10.3390/diagnostics9020049
72. Su M, Satola SW, Read TD. Genome-based prediction of bacterial antibiotic resistance. J Clin Microbiol. 2019;57:e01405–18. doi:10.1128/JCM.01405-18
73. van Hoek A, Mevius D, Guerra B, Mullany P, Roberts A, Aarts H. Acquired Antibiotic Resistance Genes: an Overview. Front Microbiol. 2011;2:2. doi:10.3389/fmicb.2011.00002
74. Fuda C, Suvorov M, Vakulenko SB, Mobashery S. The basis for resistance to beta-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J Biol Chem. 2004;279(39):40802–40806. doi:10.1074/jbc.M403589200
75. Lim D, Strynadka NC. Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat Struct Biol. 2002;9(11):870–876. doi:10.1038/nsb858
76. Lakhundi S, Zhang K. Methicillin-resistant staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31:e00020–18. doi:10.1128/CMR.00020-18
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

