Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Stachydrine Relieved the Inflammation and Promoted the Autophagy in Diabetes Retinopathy Through Activating the AMPK/SIRT1 Signaling Pathway
Authors Yu J, Ke L, Zhou J, Ding C, Yang H, Yan D, Yu C
Received 6 May 2023
Accepted for publication 10 August 2023
Published 25 August 2023 Volume 2023:16 Pages 2593—2604
DOI https://doi.org/10.2147/DMSO.S420253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Jiewei Yu,1 Lingling Ke,1 Jingjing Zhou,2 Chunyan Ding,1 Hui Yang,1 Dongbiao Yan,3 Chengbi Yu3
1Department of Ophthalmology, Jiujiang Hospital of Traditional Chinese Medicine, Jiujiang, Jiangxi, 332000, People’s Republic of China; 2Image Center, Jiujiang Hospital of Traditional Chinese Medicine, Jiujiang, Jiangxi, 332000, People’s Republic of China; 3Department of Endocrinology, Jiujiang Hospital of Traditional Chinese Medicine, Jiujiang, Jiangxi, 332000, People’s Republic of China
Correspondence: Chengbi Yu, Department of Endocrinology, Jiujiang Hospital of Traditional Chinese Medicine, No. 555 Dehua Road, Lianxi District, Jiujiang, Jiangxi, 332000, People’s Republic of China, Tel +86 792-8188311, Email [email protected]
Background: Diabetes retinopathy (DR) is a chronic, progressive, and potentially harmful retinal disease associated with persistent hyperglycemia. Autophagy is a lysosome-dependent degradation pathway that widely exists in eukaryotic cells, which has recently been demonstrated to participate in the DR development. Stachydrine (STA) is a water-soluble alkaloid extracted from Leonurus heterophyllus. This study aimed to explore the effects of STA on the autophagy in DR progression in vivo and in vitro.
Methods: High glucose-treated human retinal microvascular endothelial cells (HRMECs) and STA-treated rats were used to establish DR model. The reactive oxygen species (ROS) and inflammatory factor levels (TNF-α, IL-1β, and IL-6) were determined using corresponding kits. Additionally, the cell growth was analyzed using CCK-8 and EdU assays. Besides, LC3BII, p62, p-AMPKα, AMPKα, and SIRT1 protein levels were measured using Western blot. The LC3BII and SIRT1 expressions were also determined using immunofluorescence.
Results: The results showed that STZ decreased the ROS and inflammatory factor levels in the HG-treated HRMECs. Besides, after STA treatment, the beclin-1, LC3BII, p-AMPKα, and SIRT1 levels were increased, and p62 was decreased in the HG-treated HRMECs and the retinal tissue of STZ-treated rats.
Conclusion: In conclusion, this study demonstrated that STA effectively relieved the inflammation and promoted the autophagy in DR progression in vivo and in vitro through activating the AMPK/SIRT1 signaling pathway.
Keywords: diabetes retinopathy, stachydrine, autophagy, AMPK/SIRT1
Introduction
Diabetes retinopathy (DR) is a chronic, progressive, and potentially harmful retinal disease associated with persistent hyperglycemia.1 In western countries, DR is the main cause of blindness among working-age people (20–64 years old).2 In China, with the increase of the incidence rate of diabetes, the prevalence of DR and the blindness caused by DR have also increased year by year.3 In the past, DR was considered to be a micro-vascular disease. In fact, in recent years, the American Diabetes Association (ADA) has defined DR as a highly tissue-specific neurovascular complication.4 A recent study found that DR occurrence may be related to the abnormal polyol metabolic pathway, the accumulation of protein nonenzymatic glycosylation products, the activation of protein kinase C (PKC), and the role of angiotensin-converting enzyme system.5 However, recent studies have found that autophagy exists in pathological neovascularization and neurodegeneration, and thus participates in the occurrence and development of DR.
Autophagy is a lysosome-dependent degradation pathway that widely exists in eukaryotic cells. Damaged proteins and organelles in cells are degraded by autophagy, and the degradation products such as amino acids and fatty acids are reused by cells.6,7 Autophagy mainly includes the formation of autophagosomes, the fusion of autophagosomes and lysosomes to form autophagolysomes. Then the autophagolysomes degrade of phagocytic substances by proteolytic enzymes.8 Autophagy is a double-edged sword in the growth process of eukaryotic cells, which runs through the normal growth, proliferation, and pathophysiological process.9 For example, in the physiological state or at the early stage of some diseases, autophagy helps to clear senescent and damaged cells or organelles, while when autophagy is too strong, it has a pathogenic effect on cells, tissues, and organs.10 Recently, many researches demonstrated that activating autophagy might be a promising therapeutic strategy in DR.11,12 Adenosine monophosphate-activated protein kinase (AMPK) participates in the regulation of energy metabolism, plays a protective role in many diseases including DR.13 Sirtuin 1 (SIRT1), one of the sirtuins family members, is a nicotinamide adenine dinucleotide (NAD +)-dependent histone deacetylase. SIRT1 is activated by the increase of intracellular adenosine monophosphate and NAD + levels.14 Previous studies have demonstrated that the phosphorylation of AMPK can activate SIRT1 and thus induce autophagy.15,16 However, the role of AMPK/SIRT1 mediated autophagy in DR remains unclear.
Traditional Chinese Medicine has made certain progress in preventing and treating DR.17 Leonurus heterophyllus has been applied in the clinical treatment of gynecological and obstetric diseases.18 Stachydrine (STA) is a water-soluble alkaloid extracted from Leonurus heterophyllus, which can inhibit cell apoptosis, maintain myocardial cell homeostasis, and reduce endothelial dysfunction.19,20 Recently, research found that STA was an effective compound of Tangwang Mingmu granule, which was a traditional Chinese medicine and widely used in the DR treatment.9 However, the potential mechanism of STA in DR and the relationship between STA and AMPK/SIRT1 mediated autophagy have not been reported.
However, there are still some limitations in this study. This study is a preliminary study on the relationship between STA and autophagy in DR. The target of STA in DR remains unclear, and more in vivo experiment showed be performed to explore the specific mechanisms of STA in DR progression.
Therefore, our research was carried out to explore the role of STA in the DR progression. We hypothesized that STA might inhibit the DR development via activating the autophagy through the AMPK/SIRT1 signaling pathway.
Materials and Methods
Cell Culture and Treatment
Human retinal microvascular endothelial cells (HRMECs) were purchased from Procell Life Science&Technology Co., Ltd. (CP-H130, Wuhan, China). The cells were cultured in endothelial cell medium (ECM) containing 5% fetal bovine serum, 1% endothelial cell growth supplement, and 1% penicillin/streptomycin solution (Thermo Fisher Scientific Co., Ltd, USA). Then the cells were transferred into the incubator, and the culture conditions were set at 37 ° C and 5% CO2. After 24 h, the HRMECs were randomly divided into control group, high glucose (HG) group, and HG+STA group.
The cells in the control group were cultured in the medium containing 5.5mmol/L glucose for 24 h; the cells in HG group were cultured in a medium containing 30 mmol/L glucose for 24 h; the cells in HG+STA group were cultured in a medium containing 30 mmol/L glucose and different concentration of STA (3.125, 6.25, 12.5, 25, or 50 μM) for 24 h.21 The STA was purchased from Sigma-Aldrich (99 atom % 13C, 97% (CP)). Additionally, for the SIRT1 inhibition, the cells in the HG+STA group were treated with EX527 (10 μM, a SIRT1 inhibitor) for 24 h. After culture, the cells were collected for the next experiments.
CCK-8 Assay
The cells of each group after treatment were seeded in 96 well plates with a cell density of 4 × 103/well. After 24 h of culture, 10 μL CCK-8 solution (Beyotime, Beijing, China) was added to each well. After incubation at 37 °C for 1.5 h, the absorbance of the cells at the wavelength of 450 nm was detected by a microplate reader.
EdU Assay
According to the instructions of EdU kit (RibBio Biotechnology Co., Ltd, Guangzhou, China), the cells were inoculated in a 96-well plate (2 × 103 cells/well) for 24 h, and incubated with diluted EdU solution for 2h. Then the cells were fixed with 50 µL 4% paraformaldehyde at room temperature for 10 min and neutralized using 50 µL 2 mg/mL glycine. After permeabilization with 0.5% Triton X-100 for 20 min, the cells were stained for DAPI with 100 µL 1× Apollo solution and stained for Edu with 100 µL 1× Hoechst 33342 solution, both at room temperature for 30 min. Finally, the cells were observed under the fluorescence microscope. Five visual fields were randomly selected from each group. The images of cells stained differently in the same visual field were superimposed with ImageJ software to calculate the number of two kinds of stained cells. Cell proliferation capacity = number of green fluorescent cells/number of blue fluorescent cells × 100%.
Lactate Dehydrogenase (LDH) Release
After treatment, the cells medium in each group were collected and centrifuged at 800 g/min for 10min. After that, the supernatant was collected. At the same time, the cells were resuspended with the same amount of culture medium and freeze thawing for 3 times. After that, the supernatant of freeze-thaw solution was centrifuged. Next, the LDH activity of cell medium and freeze-thaw solution were determined using LDH cytotoxicity assay kit (Sigma, CA, USA) at 440 nm. The LDH release rate was calculated as follows: LDH release rate = LDH activity of culture medium/(LDH activity of culture medium + LDH activity of cell freeze-thaw solution) × 100%.
Intracellular Reactive Oxygen Species (ROS) Levels Determination
After treatment, the cells were collected and re-suspended. Then the cells were stained with 10 μM DCFH-DA fluorescent probe for 30 min in the dark Next, after washing three times with PBS, the cells were analyzed using flow cytometry with 488 nm excitation wavelength and 525 nm emission wavelength. The relative average fluorescence intensity value was used to indicate the intracellular ROS level.
Immunofluorescence
The cells were spread onto glass coverslips coated with 0.1% collagen. When the cell density reached about 60%, they were fixed with precooled mixed solution of methanol and acetone (1:1), permeabilized in 1% Triton X-100/PBS. Then, after washing and drying, the cells were incubated with LC3B and SIRT1 antibodies overnight at 4 °C. Then the cell nuclei were stained with 10 ug/mL DAPI for 5 min at room temperature. After that, the slides were fixed with 4% paraformaldehyde for 4 h, incubated with PBS containing 15% sucrose for 4 h, washed with deionized water, and analyzed by laser confocal scanning.
DR Rat Model Establishment
Twenty-four 6-week-old male SD rats without specific pathogen free (SPF), with a body mass of (200 ± 20) g, were provided by Beijing Charles River Experimental Animal Technology Co., Ltd. (Beijing, China). This study was approved by the animal ethics committee of Jiujiang Hospital of Traditional Chinese medicine. Protocols were also in compliance with Basel Declaration guidelines. Twenty-four rats were randomly divided into sham group, DR group, and DR+STA group, with 8 rats in each group. All rats were fed adaptively for 1 week. Then the rats were fasted for 8 hours before modeling. In addition to the control group, the rats in the other two groups were injected with 1% STZ solution (60 mg/kg-1, STZ was dissolved in 0.1 mol/L, pH 4.2 sodium citrate buffer) intraperitoneally to establish the rat model of diabetes. The rats in the control group were injected with the same amount of normal saline. The bedding, water, and feed were changed every day during the feeding period. One week later, blood was collected by tail vein needle to detect the fasting blood glucose level. Fasting blood glucose ≥ 16.7 mmol/L and the urine glucose is positive indicated that the modeling is successful. If the modeling is not successful, the rats shall be supplemented to ensure that there are 8 rats in each group. According to a previous study,22 after 4 weeks, fundus fluorescein angiography was performed, and it was considered that DR model rats were successfully established if there were vascular tortuous expansion, background fluorescence enhancement, and neovascular fluorescence leakage. Then, rats in the DR+STA group were subcutaneously injected with 40 mg/kg STA, once a day, for 2 weeks. The Sham group and DR group were injected with an equal volume of normal saline. Rats had free access to water and food during the treatment period. On the second day of the last administration, the rats were anesthetized by intraperitoneal injection of chloral hydrate, and the blood and retinal tissues of the rats were collected.
Hematoxylin-Eosin (HE) Staining
The retinal tissues were fixed in 4% paraformaldehyde for 48 h. After washing and dehydrated, the tissues were embedded and made into paraffin sections. Next, the sections were dewaxed and hydrated, and stained with HE. After dehydration, sections were sealed with neutral gum, and observed and photographed under an upright microscope.
Determination of Inflammatory Factor
The TNF-α, IL-1β, and IL-6 levels in the cells or serum were detected using ELISA kits, which were purchased from the Elabscience Biotechnology Co., Ltd. (Wuhan, China). All experimental procedures were carried out according to the instructions.
Western Blot
For protein extraction, cells transfected as aforementioned or retinal tissues were lysed in RIPA lysis buffer (Beyotime) with freshly added protease inhibitor PMSF (Beyotime). The samples were lysed at 4 ° C for 15 min, centrifuged at 12000r/min at 4 ° C for 15 min. The protein concentration was measured by BCA kit (Beyotime), and the proteins were denatured at 97 ° C for 10 min. Next, SDS-PAGE electrophoresis was performed and the proteins were transferred to PVDF membrane. 50 g/L skim milk powder was used to block the membranes for 60 min at room temperature. Then the membranes were incubated with the primary antibodies in a shaker at 4 ° C overnight (LC3B, 1:600 dilution; p62, 1:800 dilution; p-AMPKα, 1:1000 dilution; AMPKα, 1:1500 dilution; SIRT1, 1:1200 dilution; Abcam, USA). The next day, the membranes were washed three times and incubated with the secondary antibody in a shaker at 4 °C for 2 h. Finally, the membranes were treated with ECL luminescent solution (Beyotime) in the dark. ImageJ (version 1.48; National Institutes of Health) software was used to quantify protein expression.
Statistical Analysis
The statistical software SPSS 26.0 was used for statistical analysis. The measurement data were expressed as mean ± SD, and the comparison between the means of multiple samples adopted one-way analysis of variance followed by Tukey’s test, with P<0.05 indicating a statistically significant difference.
Results
STA Concentration Selection
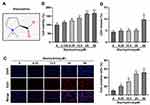
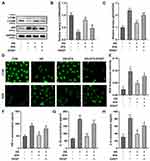
First, we performed the CCK-8, EdU, and LDH release assays to select the appropriate STA concentration. 3.125, 6.25, 12.5, 25, and 50 μM of STA were used to treat HRMECs according to the previous study.21 The molecular structural formula of STA is shown in Figure 1A. We found that after STA treatment, the cell viability was significantly increased. However, 3.125 μM of STA showed no difference in cell viability (Figure 1B). Then 6.25, 12.5, 25, and 50 μM of STA were used for the EdU assay. The EdU assay results showed that STA treatment significantly elevated the cell proliferation in a concentration-dependent manner and 25 and 50 μM of STA showed the best effect (Figure 1C). Furthermore, we found that 50 μM of STA treatment significantly increased the LDH release (Figure 1D). Therefore, according to these findings, 6.25, 12.5, and 25 μM of STA was selected for the next experiments.
STA Inhibited the ROS Production and Inflammation in the HG-Stimulated HRMECs
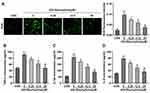
Then, HRMECs were treated with HG to establish a DR model in vitro according to previous study.23 We found that the HG treatment significantly elevated the ROS (Figure 2A), TNF-α (Figure 2B), IL-1β (Figure 2C) and IL-6 (Figure 2D) levels in the HRMECs. After 6.25, 12.5, and 25 μM of STA treatment, the ROS, TNF-α, IL-1β, and IL-6 levels in the HG-stimulated HRMECs were significantly decreased. These results indicated that STA could effectively relieve the injury of HRMECs induced by HG.
STA Induced Autophagy in the HG-Stimulated HRMECs
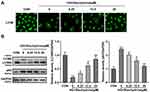
Autophagy has been demonstrated to contribute to the treatment of DR.24 However, whether STA alleviated HG-induced HRMECs damage by promoting autophagy remains unknown. Here, immunofluorescence results showed that the LC3BII levels were significantly decreased in the HG-treated HRMECs, while STA significantly increased in a dose-dependent manner (Figure 3A). The Western blot assay showed that the protein levels of LC3BII were significantly downregulated in the HG-treated HRMECs, and p62 was upregulated; after STA treatment, the protein levels of LC3BII were significantly upregulated in the HG-treated HRMECs, and p62 was downregulated (Figure 3B). These results indicated that STA promoted the autophagy in the HG-treated HRMECs.
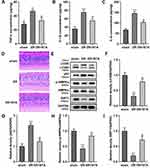
STA Activated the AMPK/SIRT1 Signaling Pathway in the HG-Treated HRMECs
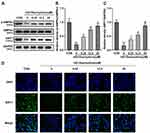
AMPK/SIRT1 signaling pathway plays an important role in energy metabolism, which was also demonstrated to induce autophagy in many diseases.25 Here, we found that HG treatment significantly decreased the p-AMPKα (Figure 4A and B) and SIRT1 (Figure 4A and C) protein levels in the HRMECs, while they were significantly elevated after STA treatment in a dose-dependent manner (Figure 4A-C). Additionally, the immunofluorescence results showed that the SIRT1 levels were significantly decreased in the HG-treated HRMECs, while STA significantly increased it in a dose-dependent manner (Figure 4D). These results indicated that STA treatment might promote autophagy by activating the AMPK/SIRT1 signaling pathway in the HG-treated HRMECs.
Inhibiting the SIRT1 Expression Reversed the Role of STA in the HG-Treated HRMECs
Phosphorylation of AMPK plays a role in cell biological behavior by activating SIRT1. Therefore, in order to further explore the role of AMPK/SIRT1 signaling pathway in DR, the HRMECs were treated with the EX527, a SIRT1 inhibitor, to suppress the SIRT1 expression. Additionally, according to our previous experimental results, 25 μM of STA was used in this experiment. The results showed that after EX527 treatment, the increase of LC3BII (Figure 5A and B) protein expressions, and the decrease of p62 (Figure 5A and C) protein expressions in the HG-treated HRMECs induced by STA were significantly reversed (Figure 5A-C). Besides, the immunofluorescence results showed that the LC3BII levels were significantly decreased after EX527 treatment (Figure 5D). Furthermore, the decrease of the ROS (Figure 5E), TNF-α (Figure 5F), IL-1β (Figure 5G), and IL-6 (Figure 5H) levels in the HG-stimulated HRMECs induced by STA was reversed after EX527 treatment. These results show that the inhibition of SIRT1 reversed the effects of STA on the autophagy in the HG-treated HRMEC, which demonstrated that STA promoted the autophagy in the HG-treated HRMECs through the AMPK/SIRT1 signaling pathway.
STA Relieved the DR Progression in vivo
Finally, we establish a DR rat model to further explore the STA roles. We found that the TNF-α, IL-1β, and IL-6 levels were significantly increased in the retinal tissue of DR rats. After STA treatment, the TNF-α, IL-1β, and IL-6 levels were significantly decreased (Figure 6A-C). Furthermore, HE staining showed that compared with the sham group, the retinal nerve fiber layer in DR group was broken, the cells in each layer were disordered, the inner and outer nuclear layers were thinner, and the cell density was reduced. The treatment of STA alleviated these symptoms (Figure 6D). Besides, the LC3BII (Figure 6E and F), p-AMPKα (Figure 6E and H) and SIRT1 (Figure 6E and I), protein levels in the retinal tissue of DR rats were significantly decreased, while p62 (Figure 6E and G) was increased; after STA treatment, the p-AMPKα, SIRT1, and LC3BII protein levels in the retinal tissue of DR rats were significantly increased, while p62 was decreased (Figure 6E-I). These results indicated that STA also induced the autophagy through the AMPK/SIRT1 signaling pathway in vivo.
Discussion
DR is the main cause of visual impairment and blindness in young adults.26 It is estimated that the number of DR patients will increase to 247.3 million by 2030.27 At present, more and more studies are aimed at finding new targets for DR drug therapy, and further exploring the therapeutic effects and mechanisms of new drugs.28,29 Here, we demonstrated that STA effectively relieved the inflammation and promoted the autophagy in DR progression in vivo and in vitro through activating the AMPK/SIRT1 signaling pathway.
Recent studies have found that Leonurus heterophyllus has a certain therapeutic and protective effect on the systemic diseases of human body.30,31 STA was an alkaloid in Leonurus, which is used as the detection index for the quality control of Leonurus. The effects of STA on the female reproductive system, cardiovascular system, and other systems have received more attention and research recently.32,33 For example, Chen et al34 found that STA exhibited a therapeutic effect against heart failure via suppressing the phosphorylation of Ca2+/calmodulin-dependent kinase II. Besides, Zhou et al21 elucidated the molecular mechanism of STA in the sunitinib-injured human umbilical vein endothelial cells, which suggested that STA improved the angiogenesis through inhibiting the mitochondrial-mediated cell apoptosis and promoting the VEGFR2/MEK/ERK signaling. Thus, it can be seen that STA has different regulatory mechanisms for various diseases. However, the role of STA in DR has not been reported currently. Tangwang Mingmu granule is a traditional Chinese medicine, which has been widely applied in the DR treatment. Wang et al9 performed a pharmacokinetic study and found that STA was the main compound of Tangwang Mingmu granule. This aroused our interest to explore whether STA can play a therapeutic effect on DR? What is its potential mechanism? In this study, we found that STA treatment inhibited the ROS, TNF-α, IL-1β, and IL-6 levels in the HG-treated HRMECs, which preliminarily indicated that STA has a certain protective effect on the development of DR.
Autophagy is a double-edged sword in the various diseases.35 Many researches have reported that the regulation of autophagy is a key factor in the treatment of DR.36,37 However, the effect of autophagy on the cell biological behavior in DR is quite complicated. It was demonstrated that moderate amount autophagy promoted cell survival in DR, whereas excessive autophagy induced cell necrosis.24 For example, Li et al38 demonstrated that artesunate has therapeutic potential in DR treatment through inducing autophagy in DR. On the contrary, Wang et al39 found that knockdown of histone HIST1H1C suppressed the HG induced reduced inflammation and cell toxicity through inhibiting the excessive autophagy, which offered a novel therapeutic target for DR treatment. LC3B is a marker of autophagy. When autophagy occurs, LC3BI is esterified to form LC3BII and localized on the membrane of autophagosome. The level of LC3BII can reflect the degree of autophagy.40 P62 is an important indicator to evaluate autophagosome degradation. When autophagy is activated, p62 will combine with ubiquitinated protein and LC3BII on autophagosome membrane to form a complex, and complete the degradation process in autophagy lysosome.41 The expression level of p62 is negatively correlated with the degree of autophagy activation. In this study, we found that STA treatment increased the LC3BII and Beclin-1 levels, and decreased the p62 levels in the HG-treated HRMECs. From the immunofluorescence observation, the LC3B levels were also significantly enhanced in the HG-treated HRMECs after STA treatment. These findings intuitively demonstrated that STA can promote autophagy to achieve the purpose of treating DR.
As reported by previous study, the activation of AMPK/SIRT1 signaling pathway can induce the autophagy in many diseases.42 For instance, Ren et al43 confirmed that metformin effectively relieved the disorders of glycolipid metabolism, oxidative stress, and renal function injury, and promoted the autophagy in diabetic rats via activating the AMPK/SIRT1 pathway. Yu et al25 demonstrated that dexmedetomidine elevated autophagy and alleviated inflammatory responses in cecal ligation and puncture-induced liver injury through activating the AMPK/SIRT1 pathway. However, the role of AMPK/SIRT1 signaling pathway mediated autophagy in DR, and whether STA enhances autophagy by activating AMPK/SIRT1 signaling pathways remains unknown. Here, we found that STA treatment significantly enhanced the p- AMPKα and SIRT1 in vivo and in vitro. Interestingly, after EX527 treatment, a SIRT1 inhibitor, the increase of autophagy, and the decrease of ROS, TNF-α, IL-1β, and IL-6 levels induced by STA were reversed. These results implied that STA might participate in the DR alleviation through activating the AMPK/SIRT1 signaling pathway.
In conclusion, this study found that STA treatment decreased inflammatory factor levels in the HG-treated HRMECs and DR rats. Mechanistically, STA enhanced the autophagy through activating the AMPK/SIRT1 signaling pathway. Our research enriched the research on DR drug therapy and also provided a new direction for the promotion and application of STA.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study protocol was approved by the Ethics Committee of Jiujiang Hospital of Traditional Chinese medicine (Data: May 18, 2023; No: 360403198009250612).
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi:10.1016/j.redox.2020.101799
2. Teo ZL, Tham YC, Yu M, et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580–1591. doi:10.1016/j.ophtha.2021.04.027
3. Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol. 2022;13:1077669. doi:10.3389/fendo.2022.1077669
4. Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr Pract. 2022;28:923. doi:10.1016/j.eprac.2022.08.002
5. Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Invest. 2021;12:1322–1325. doi:10.1111/jdi.13480
6. Levine B, Kroemer G. Biological Functions of Autophagy Genes: a Disease Perspective. Cell. 2019;176:11–42. doi:10.1016/j.cell.2018.09.048
7. Yamamoto H, Zhang S, Mizushima N. Autophagy genes in biology and disease. Nat Rev Genet. 2023;24:382–400 10.1038/s41576-022-00562–w.
8. Hollenstein DM, Kraft C. Autophagosomes are formed at a distinct cellular structure. Curr Opin Cell Biol. 2020;65:50–57. doi:10.1016/j.ceb.2020.02.012
9. Wang Y, Xue B, Wang X, Wang Q, Liu E, Chen X. Pharmacokinetic study of Tangwang Mingmu granule for the management of diabetic retinopathy based on network pharmacology. Pharm Biol. 2021;59:1332–1348. doi:10.1080/13880209.2021.1979051
10. Zhang J, Chen L, Xiong F, et al. Autophagy in regulatory T cells: a double-edged sword in disease settings. Mol Immunol. 2019;109:43–50. doi:10.1016/j.molimm.2019.02.004
11. Huang C, Qi P, Cui H, Lu Q, Gao X. CircFAT1 regulates retinal pigment epithelial cell pyroptosis and autophagy via mediating m6A reader protein YTHDF2 expression in diabetic retinopathy. Exp Eye Res. 2022;222:109152. doi:10.1016/j.exer.2022.109152
12. Yang X, Huang Z, Xu M, et al. Autophagy in the retinal neurovascular unit: new perspectives into diabetic retinopathy. J Diabetes. 2023;15:382–396. doi:10.1111/1753-0407.13373
13. Song S, Bao S, Zhang C, et al. Stimulation of AMPK Prevents Diabetes-Induced Photoreceptor Cell Degeneration. Oxid Med Cell Longev. 2021;2021:5587340. doi:10.1155/2021/5587340
14. Wang W, Sun W, Cheng Y, Xu Z, Cai L. Role of sirtuin-1 in diabetic nephropathy. J Mol Med. 2019;97:291–309. doi:10.1007/s00109-019-01743-7
15. Li K, Liu TX, Li JF, et al. rhEPO inhibited cell apoptosis to alleviate acute kidney injury in sepsis by AMPK/SIRT1 activated autophagy. Biochem Bioph Res Co. 2019;517:557–565. doi:10.1016/j.bbrc.2019.07.027
16. Wang Y, Zhao H, Li X, et al. Tangshen Formula Alleviates Hepatic Steatosis by Inducing Autophagy Through the AMPK/SIRT1 Pathway. Front Physiol. 2019;10:494. doi:10.3389/fphys.2019.00494
17. Pang B, Li QW, Qin YL, et al. Traditional Chinese medicine for diabetic retinopathy: a systematic review and meta-analysis. Medicine. 2020;99:e19102. doi:10.1097/MD.0000000000019102
18. Fierascu IC, Fierascu I, Baroi AM, et al. Phytosynthesis of Silver Nanoparticles Using Leonurus cardiaca L. Extracts. Materials. 2023;16. doi:10.3390/ma16093472
19. Liao L, Tang Y, Li B, et al. Stachydrine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Biomed Pharmacother. 2023;161:114489. doi:10.1016/j.biopha.2023.114489
20. Meng J, Zhou C, Zhang W, et al. Stachydrine prevents LPS-induced bone loss by inhibiting osteoclastogenesis via NF-kappaB and Akt signalling. J Cell Mol Med. 2019;23:6730–6743. doi:10.1111/jcmm.14551
21. Zhou F, Liu F, Liu J, et al. Stachydrine promotes angiogenesis by regulating the VEGFR2/MEK/ERK and mitochondrial-mediated apoptosis signaling pathways in human umbilical vein endothelial cells. Biomed Pharmacother. 2020;131:110724. doi:10.1016/j.biopha.2020.110724
22. Liu K, Gao X, Hu C, et al. Capsaicin ameliorates diabetic retinopathy by inhibiting poldip2-induced oxidative stress. Redox Biol. 2022;56:102460. doi:10.1016/j.redox.2022.102460
23. Zhao H, He Y. MiR-124-3p Suppresses the Dysfunction of High Glucose-Stimulated Endothelial Cells by Targeting G3BP2. Front Genet. 2021;12:723625. doi:10.3389/fgene.2021.723625
24. Feng L, Liang L, Zhang S, Yang J, Yue Y, Zhang X. HMGB1 downregulation in retinal pigment epithelial cells protects against diabetic retinopathy through the autophagy-lysosome pathway. Autophagy. 2022;18:320–339. doi:10.1080/15548627.2021.1926655
25. Yu Q, Zou L, Yuan X, Fang F, Xu F. Dexmedetomidine Protects Against Septic Liver Injury by Enhancing Autophagy Through Activation of the AMPK/SIRT1 Signaling Pathway. Front Pharmacol. 2021;12:658677. doi:10.3389/fphar.2021.658677
26. Forster RB, Garcia ES, Sluiman AJ, et al. Retinal venular tortuosity and fractal dimension predict incident retinopathy in adults with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetologia. 2021;64:1103–1112. doi:10.1007/s00125-021-05388-5
27. Xiao H, Tang J, Zhang F, et al. Global trends and performances in diabetic retinopathy studies: a bibliometric analysis. Front Public Health. 2023;11:1128008. doi:10.3389/fpubh.2023.1128008
28. Daldal H, Naziroglu M. Selenium and Resveratrol Attenuated Diabetes Mellitus-Mediated Oxidative Retinopathy and Apoptosis via the Modulation of TRPM2 Activity in Mice. Biol Trace Elem Res. 2022;200:2283–2297. doi:10.1007/s12011-022-03203-9
29. Zhai J, Li Z, Zhang H, et al. Berberine protects against diabetic retinopathy by inhibiting cell apoptosis via deactivation of the NFkappaB signaling pathway. Mol Med Rep. 2020;22:4227–4235. doi:10.3892/mmr.2020.11505
30. Shi X, Chen G, Tan J, et al. Total alkaloid fraction of Leonurus japonicus Houtt. Promotes angiogenesis and wound healing through SRC/MEK/ERK signaling pathway. J Ethnopharmacol. 2022;295:115396. doi:10.1016/j.jep.2022.115396
31. Wei Q, Cao X, Xu D, Wang S, Zhang J, Zhang H. Anti-inflammatory labdane diterpenoids from the aerial parts of Leonurus japonicus. Phytochemistry. 2023;210:113646. doi:10.1016/j.phytochem.2023.113646
32. Cheng F, Zhou Y, Wang M, et al. A Review of Pharmacological and Pharmacokinetic Properties of Stachydrine. Pharmacol Res. 2020; 155:104755. doi:10.1016/j.phrs.2020.104755
33. Li F, Zhu S, Jiang Q, et al. Novel Stachydrine-Leonurine Conjugate SL06 as a Potent Neuroprotective Agent for Cerebral Ischemic Stroke. Acs Chem Neurosci. 2021;12:2478–2490. doi:10.1021/acschemneuro.1c00200
34. Chen H, Wang S, Cao T, et al. Stachydrine hydrochloride alleviates pressure overload-induced heart failure and calcium mishandling on mice. J Ethnopharmacol. 2020;248:112306. doi:10.1016/j.jep.2019.112306
35. Wang S, Zhang K, Yao Y, Li J. Autophagy and Mitochondrial Homeostasis During Infection: a Double-Edged Sword. Front Cell Dev Biol. 2021;9:738932. doi:10.3389/fcell.2021.738932
36. Adornetto A, Gesualdo C, Laganà ML, Trotta MC, Rossi S, Russo R. Autophagy: a Novel Pharmacological Target in Diabetic Retinopathy. Front Pharmacol. 2021;12:695267. doi:10.3389/fphar.2021.695267
37. Ye S, Zhang Y, Wang X, et al. Autophagy positively regulates Wnt signaling in mice with diabetic retinopathy. Exp Ther Med. 2021;22:1164. doi:10.3892/etm.2021.10598
38. Li L, Chen J, Zhou Y, Zhang J, Chen L. Artesunate alleviates diabetic retinopathy by activating autophagy via the regulation of AMPK/SIRT1 pathway. Arch Physiol Biochem. 2021;1–8. doi:10.1080/13813455.2021.1887266
39. Wang W, Wang Q, Wan D, et al. Histone HIST1H1C/H1.2 regulates autophagy in the development of diabetic retinopathy. Autophagy. 2017;13:941–954. doi:10.1080/15548627.2017.1293768
40. Hwang HJ, Kim YK. The role of LC3B in autophagy as an RNA-binding protein. Autophagy. 2023;19:1028–1030. doi:10.1080/15548627.2022.2111083
41. Lorusso B, Cerasoli G, Falco A, et al. Β-blockers activate autophagy on infantile hemangioma-derived endothelial cells in vitro. Vasc Pharmacol. 2022;146:107110. doi:10.1016/j.vph.2022.107110
42. Siddhi J, Sherkhane B, Kalavala AK, Arruri V, Velayutham R, Kumar A. Melatonin prevents diabetes-induced nephropathy by modulating the AMPK/SIRT1 axis: focus on autophagy and mitochondrial dysfunction. Cell Biol Int. 2022;46:2142–2157. doi:10.1002/cbin.11899
43. Ren H, Shao Y, Wu C, Ma X, Lv C, Wang Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol. 2020;500:110628. doi:10.1016/j.mce.2019.110628
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.