Back to Journals » International Journal of General Medicine » Volume 16
sST2 and Big ET-1 as Alternatives of Multi-Biomarkers Strategies for Prognosis Evaluation in Patients Hospitalized with Heart Failure
Authors Chen Y, Zhao X, Liang L, Tian P, Feng J, Huang L, Huang B , Wu Y, Wang J, Guan J, Li X, Zhang J, Zhang Y
Received 15 August 2023
Accepted for publication 24 October 2023
Published 1 November 2023 Volume 2023:16 Pages 5003—5016
DOI https://doi.org/10.2147/IJGM.S435552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuyi Chen,1 Xuemei Zhao,1 Lin Liang,1 Pengchao Tian,1 Jiayu Feng,1 Liyan Huang,1 Boping Huang,1 Yihang Wu,1 Jing Wang,1 Jingyuan Guan,1 Xinqing Li,1 Jian Zhang,1,2 Yuhui Zhang1
1Heart Failure Center, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 2Key Laboratory of Clinical Research for Cardiovascular Medications, National Health Committee, Beijing, People’s Republic of China
Correspondence: Yuhui Zhang, Heart Failure Center, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China, Tel +8615901314243, Email [email protected] Jian Zhang, Heart Failure Center, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China, Tel +8613911102015, Email [email protected]
Objective: To identify biomarkers with independent prognostic value and investigate the prognostic value of multiple biomarkers in combination in patients hospitalized with heart failure.
Methods: A total of 884 consecutive patients hospitalized with heart failure from 2015 to 2017 were enrolled. Twelve biomarkers were measured on admission, and the relationships between biomarkers and outcomes were assessed.
Results: During the median follow-up of 913 days, 291 patients (32.9%) suffered from primary endpoint events. Soluble suppression of tumorigenicity-2 (sST2) (per log [unit] increase, adjusted HR [95% CI]: 1.39 [1.13,1.72], P = 0.002) and big endothelin-1 (big ET-1) (per log [unit] increase, adjusted HR [95% CI]: 1.56 [1.23,1.97], P < 0.001) remained independent predictors of primary endpoint event after adjusting for other predictors including N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTnT). Both sST2 (C-statistic: 0.810 vs 0.801, P = 0.005, and 0.832 vs 0.826, P = 0.024, respectively) and big ET-1 (C-statistic: 0.829 vs 0.801, P = 0.001, and 0.843 vs 0.826, P < 0.001, respectively) significantly improved the predictive value for primary endpoint event at 1 year and 3 years. However, only big ET-1 (C-statistic: 0.852 vs 0.846, P = 0.014) significantly improved the predictive value at 3 months when added to clinical predictors and known biomarkers. According to the number of elevated biomarkers (including NT-proBNP, hs-cTnT, sST2, and big ET-1), patients with three or more elevated biomarkers had a higher risk of primary endpoint event compared to those with two elevated biomarkers (P = 0.001), as well as in patients with two elevated biomarkers compared to those with one elevated biomarker (P = 0.004). However, the risk of primary endpoint event was comparable between patients with one elevated biomarker and those with no elevated biomarker (P = 0.582).
Conclusion: Multiple biomarkers in combination could provide a better prognostic value than a single biomarker. sST2 and big ET-1 could act as alternatives of multi-biomarkers strategies for prognosis evaluation beyond NT-proBNP and hs-cTnT in patients hospitalized with heart failure.
Keywords: heart failure, prognostic value, multiple biomarkers, sST2, big ET-1
Introduction
Heart failure is a rapidly growing public health issue, associated with worse quality of life and increased mortality. Approximately 64.3 million people present with heart failure worldwide.1 One meta-analysis reported that 1-year, 2-year, 5-year, and 10-year survival rates of heart failure were 86.5%, 72.6%, 56.7%, and 34.9%, respectively.2 Risk stratification is advocated to help guide treatment and follow-up in patients with heart failure, possibly contributing to a better quality of life and longer survival time. Clinical predictors are still fundamental for predicting adverse events in patients with heart failure. Meanwhile, the majority of biomarkers are associated with the pathophysiological mechanisms of heart failure, including neurohumoral activation, myocardial stretch, myocyte injury, matrix remodeling, inflammation, oxidative stress, and renal dysfunction,3 with the advantages of being low-risk, low-cost, quick, accurate, and repeatable, which have become one of the most promising risk stratification and prognosis evaluation tools in patients with heart failure.
Up to now, natriuretic peptide and cardiac troponin have the most supportive evidence, which have been recommended to apply for prognosis evaluation in patients with heart failure.4–6 Other biomarkers have also shown prognostic value for heart failure.7–10 However, whether these biomarkers remain independent predictors of adverse events in patients with heart failure after adjusting for clinical predictors and known biomarkers (including natriuretic peptide and cardiac troponin) is unknown. Notably, this is one reason why these biomarkers are not recommended for routine clinical application. Combining multiple biomarkers may provide a better predictive value for risk stratification and prognosis evaluation in patients with heart failure compared to a single biomarker.11–13 However, the optimal biomarkers for candidates of multiple biomarkers in combination are still under investigation. Thus, there is much room for improvement in prognosis in patients with heart failure.
Studies on the independent prognostic value of novel biomarkers and the association of multiple biomarkers in combination with heart failure are scarce. This retrospective study aimed to identify related biomarkers with independent prognostic value beyond other predictors, including N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTnT). Additionally, we aimed to investigate the prognostic value of multiple biomarkers in combination in patients hospitalized with heart failure.
Methods
Study Population
Consecutive patients hospitalized with heart failure in the heart failure center of Fuwai Hospital (Beijing, China) from July 2015 to December 2017 were enrolled. The diagnosis of heart failure included typical symptoms (eg, breathlessness, reduced exercise tolerance, and ankle edema) and/or specific signs (eg, pulmonary crackles, elevated jugular venous pressure, and displaced apical impulse), objective evidence of cardiac structural and/or functional abnormalities (eg, ventricular hypertrophy, cardiac chamber enlargement, or reduced left ventricular ejection fraction [LVEF]), and exclusion of other non-heart failure factors, which two cardiac specialists confirmed according to Chinese heart failure management guideline.4 Exclusion criteria included (1) patients with non-heart failure; (2) lacking stored blood samples; (3) lost to follow-up. Patients were classified into controls (patients without adverse events) and cases (patients with adverse events) according to whether the patients suffered from primary endpoint events.
Clinical characteristics were collected for every patient. These included age, sex, body mass index (BMI), heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), hospitalization duration, hypertension, diabetes mellitus, atrial fibrillation, aetiology of heart failure (including ischemic heart disease, dilated cardiomyopathy, valvular heart disease, hypertensive heart disease, and other reasons), classification of heart failure (including heart failure with reduced ejection fraction [HFrEF], heart failure with mildly reduced ejection fraction [HFmrEF], and heart failure with preserved ejection fraction [HFpEF]), NYHA functional class, echocardiographic data (including left atrial diameter [LAD], left ventricular end-diastolic diameter [LVEDD], and LVEF), laboratory data (including white blood cell, hemoglobin, albumin, alanine aminotransferase, sodium, potassium, triglyceride, total cholesterol, low-density lipoprotein cholesterol [LDL-C], D-dimer, estimated glomerular filtration rate [eGFR], creatinine, blood urea nitrogen [BUN], neutrophil gelatinase-associated lipocalin [NGAL], NT-proBNP, hs-cTnT, high-sensitivity cardiac troponin I [hs-cTnI], heart-type fatty acid-binding protein [H-FABP], soluble suppression of tumorigenicity-2 [sST2], big endothelin-1 [big ET-1], uric acid, serum amyloid A [SAA], and C-reactive protein [CRP]), and prescribed medication (including angiotensin converting enzyme inhibitor [ACEI]/angiotensin receptor blocker [ARB], beta blocker, mineralocorticoid receptor antagonist [MRA], diuretic, and digoxin).
Follow-Up and Primary Endpoint Event
After patients were discharged from hospital, clinical visits or telephone interviews were performed for every patient at 3-month, 6-month, 1-year, and every 6-month interval after that. Primary endpoint event was the composite of all-cause death, heart transplantation, or left ventricular assist device (LVAD). Follow-up time was calculated from admission time to the time of the primary endpoint event or the last follow-up time.
Biomarkers Analyses
Altogether 12 biomarkers were analyzed, including reflecting myocardial stretch (NT-proBNP [Roche Diagnostics]), myocyte injury (hs-cTnT [Vazyme], hs-cTnI [Vazyme], and H-FABP [Upper]), matrix remodeling (sST2 [Boditech]), neurohumoral activation (big ET-1 [Biomedica]), oxidative stress (uric acid [Chemclin]), inflammation (CRP [Orion Diagnostica] and SAA [Boditech]), and renal dysfunction (creatinine [BioSino], BUN [BioSino], and NGAL [Vazyme]). All blood samples were collected within 24h after patients were admitted to the heart failure center. Biomarkers were either measured on admission at the clinical laboratory of Fuwai Hospital (including NT-proBNP, big ET-1, uric acid, CRP, creatinine, and BUN) or measured on the stored serum samples at −80°C (including hs-cTnT, hs-cTnI, H-FABP, sST2, SAA, and NGAL).
Statistical Analysis
Data were presented with mean and standard deviation or median and interquartile range (IQR) for continuous variables and n and percentage for categorical variables. Independent sample t-tests were performed for comparisons of normal distributed data. Comparisons for non-normal distributed data were performed with Mann–Whitney U-tests. Categorical data were compared with Chi-Square tests. Associations between different biomarkers were assessed with Spearman correlation analyses and presented as a heatmap. Adjusted analyses for the primary endpoint event were performed with multivariate Cox regression analyses. Restricted cubic spline curves based on Cox proportional hazards models were performed to assess the relationships between biomarkers and primary endpoint event with 5 knots at the 5th, 35th, 50th, 65th, and 95th percentiles of biomarkers. C-statistic, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated to assess whether the prognostic value for patients hospitalized with heart failure would improve after adding biomarkers into the base model, including clinical predictors and known biomarkers (including NT-proBNP and hs-cTnT). For time-to-event data, the relationships of biomarkers with primary endpoint event were performed using Kaplan–Meier analyses and Log rank tests. The cut-off value of elevated NT-proBNP level was set as 1000 ng/L according to previous studies,14–16 and the cut-off value of elevated hs-cTnT level was set as 14 ng/L according to previous studies.17–19 P values of less than 0.05 were considered statistically significant. All analyses were performed with SPSS version 25.0 and R version 4.2.1.
Results
From July 2015 to December 2017, 884 consecutive patients hospitalized with heart failure in the heart failure center of Fuwai Hospital were enrolled. During the median follow-up duration of 913 days (IQR: 405–1183 days), 291 patients (32.9%) suffered from primary endpoint events, including 251 all-cause deaths, 38 heart transplantations, and 2 LVADs, and 593 patients did not suffer from primary endpoint event. Among the case group, 18 patients suffered from all-cause deaths, and 10 patients underwent heart transplantations during hospitalization. (Figure 1)
 |
Figure 1 Study flow diagram. This chart showed patients flow regarding enrollment and clinical outcomes assessed for the study. |
Compared to those without adverse events, patients with adverse events were older and had more atrial fibrillation and ischemic heart disease, less hypertension, lower BMI, heart rate, SBP, and DBP, and longer hospitalization duration. HFrEF and NYHA functional class IV were more common in patients with adverse events than those without. On echocardiographic characteristics, patients with adverse events had larger LAD and LVEDD and lower LVEF than those without adverse events. On laboratory characteristics, patients with adverse events had lower levels of hemoglobin, albumin, alanine aminotransferase, sodium, triglyceride, total cholesterol, LDL-C, and eGFR, and higher levels of potassium, D-dimer, creatinine, BUN, NGAL, NT-proBNP, hs-cTnT, hs-cTnI, H-FABP, sST2, big ET-1, uric acid, SAA, and CRP compared to those without adverse events. Furthermore, on prescribed medication, patients with adverse events had a lower rate of ACEI/ARB than those without adverse events. (Table 1)
 |
Table 1 Baseline Clinical Characteristics of the Study Population |
Correlations Between Different Biomarkers
Spearman correlation analyses showed that other biomarkers were positively correlated except that sST2 and big ET-1 were not correlated with NGAL. The correlations between different biomarkers were presented as a heatmap, and the correlation values between different biomarkers were presented with color depth. (Figure 2)
sST2 and Big ET-1 as Independent Predictors of Adverse Events
Adjusted for age, sex, BMI, hypertension, diabetes mellitus, ischemic heart disease, NYHA functional class, LVEF, ACEI/ARB, beta blocker, NT-proBNP, and hs-cTnT, multivariate Cox regression analyses showed that sST2 (per log [unit] increase, adjusted HR [95% CI]: 1.39 [1.13,1.72], P = 0.002) and big ET-1 (per log [unit] increase, adjusted HR [95% CI]: 1.56 [1.23,1.97], P < 0.001) were independent predictors of primary endpoint event in patients hospitalized with heart failure (Table 2).
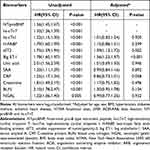 |
Table 2 Multivariate Cox Regression Analyses for Primary Endpoint Event in Patients Hospitalized with Heart Failure |
Restricted cubic spline curve showed that the risk of primary endpoint event increased significantly with the increase of sST2 levels when sST2 concentrations were more than 22 ng/mL after adjusting for age, sex, BMI, hypertension, diabetes mellitus, ischemic heart disease, NYHA functional class, LVEF, ACEI/ARB, beta blocker, NT-proBNP, and hs-cTnT (Figure 3A). The risk of primary endpoint event increased significantly with the increase of big ET-1 levels when big ET-1 concentrations were more than 0.5 pmol/L after adjusting for the above covariates (Figure 3B).
According to the cut-off value of sST2 in restricted cubic spline curve, patients were divided into high sST2 group (≥22.0 ng/mL) and low sST2 group (<22.0 ng/mL). Kaplan–Meier analysis further demonstrated that patients with high sST2 levels had a higher rate of primary endpoint event than those with low sST2 levels (P < 0.001) (Figure 4A). According to the cut-off value of big ET-1 in restricted cubic spline curve, patients were divided into high big ET-1 group (≥0.5 pmol/L) and low big ET-1 group (<0.5 pmol/L). Kaplan–Meier analysis further demonstrated that patients with high big ET-1 levels had a higher rate of primary endpoint event than those with low big ET-1 levels (P < 0.001) (Figure 4B).
Additional Predictive Value of sST2 and Big ET-1 for Adverse Events
The rates of primary endpoint event were 9.2% at 3 months, 18.6% at 1 year, and 31.1% at 3 years, respectively. Based on the base model including age, sex, BMI, hypertension, diabetes mellitus, ischemic heart disease, NYHA functional class, LVEF, ACEI/ARB, beta blocker, NT-proBNP, and hs-cTnT, adding sST2 did not improve the predictive value for primary endpoint event at 3 months in patients hospitalized with heart failure (C-statistic: 0.847 vs 0.846, P = 0.252). Adding sST2 did not improve NRI (−0.052, P = 0.062) and IDI (0.004, P = 0.344) for prognostic value at 3 months, either. However, adding sST2 significantly improved the predictive value for primary endpoint event at both 1 year (C-statistic: 0.810 vs 0.801, P = 0.005) and 3 years (C-statistic: 0.832 vs 0.826, P = 0.024). Adding sST2 also improved NRI (0.064, P = 0.020, and 0.068, P = 0.006, respectively) and IDI (0.011, P = 0.049, and 0.008, P = 0.055, respectively) for prognostic value at 1 year and 3 years. However, the discrimination of IDI for prognostic value at 3 years only reached borderline significance. All models had good calibrations (all Hosmer–Lemeshow P > 0.05).
Based on the above base model, adding big ET-1 significantly improved the predictive value for primary endpoint event at 3 months (C-statistic: 0.852 vs 0.846, P = 0.014), 1 year (C-statistic: 0.829 vs 0.801, P = 0.001), and 3 years (C-statistic: 0.843 vs 0.826, P < 0.001). Adding big ET-1 also significantly improved NRI (0.051, P = 0.022, and 0.109, P = 0.014, respectively) and IDI (0.018, P = 0.024, and 0.048, P < 0.001, respectively) for prognostic value at 1 year and 3 years. Furthermore, adding big ET-1 improved NRI (0.065, P = 0.096) and IDI (0.021, P = 0.053) for prognostic value at 3 months. However, they only reached borderline significance. All models had good calibrations (all Hosmer–Lemeshow P > 0.05). (Table 3)
 |
Table 3 Evaluation of Predictive Models for Primary Endpoint Event in Patients Hospitalized with Heart Failure |
Multiple Biomarkers in Combination and Prognostic Value
According to the number of elevated biomarkers (including NT-proBNP ≥1000 ng/L, hs-cTnT ≥14 ng/L, sST2 ≥22 ng/mL, and big ET-1 ≥0.5 pmol/L), patients with heart failure were classified into patients with no elevated biomarker, patients with one elevated biomarker, patients with two elevated biomarkers, and patients with three or more elevated biomarkers. Kaplan–Meier analysis showed that the rate of primary endpoint event increased significantly with increasing numbers of elevated biomarkers (P < 0.001). The rate of primary endpoint event was comparable between patients with one elevated biomarker and those with no elevated biomarker (HR [95% CI]: 3.19 [0.92,11.11], P = 0.121). However, patients with two elevated biomarkers had a higher rate of primary endpoint event than those with one elevated biomarker (HR [95% CI]: 3.36 [1.75,6.47], P = 0.001), and patients with three or more elevated biomarkers had a higher rate of primary endpoint event than those with two elevated biomarkers (HR [95% CI]: 2.78 [2.09,3.70], P < 0.001). (Figure 4C) Adjusted for age, sex, BMI, hypertension, diabetes mellitus, ischemic heart disease, NYHA functional class, LVEF, ACEI/ARB, and beta blocker, multivariate Cox regression analyses showed that patients with three or more elevated biomarkers had a higher risk of primary endpoint event compared to those with two elevated biomarkers (adjusted HR [95% CI]: 2.51 [1.45,4.34], P = 0.001). Likewise, patients with two elevated biomarkers had a higher risk of primary endpoint event compared to those with one elevated biomarker (adjusted HR [95% CI]: 3.73 [1.54,9.04], P = 0.004). However, the risk of primary endpoint event was comparable between patients with one elevated biomarker and those with no elevated biomarker (adjusted HR [95% CI]: 1.96 [0.18,21.26], P = 0.582). (Table 4)
 |
Table 4 Associations of Different Numbers of Elevated Biomarkers with Primary Endpoint Event in Patients Hospitalized with Heart Failure |
Discussion
The main findings of this study are as follows: (1) both baseline sST2 and baseline big ET-1 remained independent predictors of adverse events in patients hospitalized with heart failure after adjusting for other predictors, including NT-proBNP and hs-cTnT; (2) baseline sST2 could provide incremental long-term prognostic value for patients hospitalized with heart failure when added to clinical predictors and known biomarkers (including NT-proBNP and hs-cTnT); (3) baseline big ET-1 could provide incremental short-term prognostic value, and could provide incremental long-term prognostic value for patients hospitalized with heart failure when added to clinical predictors and known biomarkers; (4) multiple biomarkers in combination could provide a better prognostic value for patients hospitalized with heart failure compared to a single biomarker. Furthermore, the risk of adverse events increased with increasing numbers of elevated biomarkers.
sST2 is a biomarker reflecting myocardial fibrosis and cardiac hypertrophy, associated with the development and progress of heart failure.3 Studies reported that sST2 remained an independent predictor of heart failure rehospitalization, all-cause death, and cardiovascular death in patients with chronic heart failure after adjusting for other predictors, including NT-proBNP and hs-cTnT.20,21 However, the prognostic value of sST2 in patients hospitalized with heart failure has not been evaluated in a model including natriuretic peptide and cardiac troponin. To our knowledge, this study is the first to show that baseline sST2 remained an independent predictor of all-cause death, heart transplantation, or LVAD in patients hospitalized with heart failure after adjusting for other predictors, including NT-proBNP and hs-cTnT. And baseline sST2 significantly improved the long-term prognostic value for patients hospitalized with heart failure when added to the base model, including clinical variables, NT-proBNP, and hs-cTnT. However, baseline sST2 did not significantly improve the short-term prognostic value for patients hospitalized with heart failure when added to the base model, which may be associated with different pathophysiological mechanisms of sST2, NT-proBNP, and hs-cTnT in heart failure. Further study is needed to clarify the association between sST2 and clinical outcomes of patients hospitalized with heart failure based on other predictors, including natriuretic peptide and cardiac troponin.
The international ST2 consensus panel recommended 35 ng/mL as the cut-off value of sST2 for risk stratification and prognosis evaluation in patients with acute heart failure.22 However, the best cut-off value of sST2 for prognosis evaluation in patients with heart failure remains controversial. Pascual-Figal et al reported that 65 ng/mL was the cut-off value of sST2 for predicting adverse events in patients with acute heart failure.23 Another study reported that high sST2 concentration (>17.3 ng/mL) was associated with adverse prognosis in patients with acute heart failure.24 Our study showed that the risk of primary endpoint event increased significantly with the increase of sST2 levels when sST2 concentrations were more than 22 ng/mL, which may be associated with adjusting for more predictors in our study. Interestingly, the cut-off value of sST2 for prognosis evaluation in Chinese patients with heart failure may be lower than the value recommended in the international ST2 consensus panel.22 Further study is needed to verify the best cut-off value of sST2 for risk stratification and prognosis evaluation in patients with heart failure.
Big ET-1 is a biomarker reflecting neurohumoral activation, produced by vascular endothelium in response to shear stress, neuronal stimulation, and inflammation, associated with vasoconstriction, proinflammation, prooxidative action, and cardiac remodeling.10 Studies reported that baseline big ET-1 was an independent predictor of adverse events in patients with acute heart failure and provided additional prognostic value when combined with NT-proBNP.25,26 However, studies on the association between big ET-1 and clinical outcomes of patients hospitalized with heart failure are scarce. Additionally, the prognostic value of big ET-1 in patients hospitalized with heart failure has not been evaluated in a model including natriuretic peptide and cardiac troponin. To our knowledge, this study is the first to show that baseline big ET-1 remained an independent predictor of all-cause death, heart transplantation, or LVAD in patients hospitalized with heart failure after adjusting for other predictors, including NT-proBNP and hs-cTnT. Big ET-1 significantly improved the short-term and long-term prognostic value for patients hospitalized with heart failure when added to the base model, including clinical variables, NT-proBNP, and hs-cTnT. The improvement in big ET-1 was greater than in sST2 regardless of short-term and long-term prognosis, which may be associated with different pathophysiological mechanisms of big ET-1 and sST2, and most patients (73.9%) with reduced LVEF in our study. Further study is needed to clarify the association between big ET-1 and clinical outcomes of patients hospitalized with heart failure based on other predictors, including natriuretic peptide and cardiac troponin.
The development and progress of heart failure usually involves several pathophysiological mechanisms simultaneously. Combining multiple non-strongly correlated biomarkers from different pathophysiological mechanisms may provide superior prognostic value for patients with heart failure compared to a single biomarker. Studies reported that multiple biomarkers in combination could provide superior prognostic value for patients with acute heart failure, and the risk of adverse events increased significantly with increasing numbers of elevated biomarkers.23,27,28 Our study also demonstrated that multiple biomarkers in combination improved the prognostic value for patients hospitalized with heart failure. Furthermore, we found that the risk of adverse events increased significantly with increasing numbers of elevated biomarkers, which was consistent with previous studies. However, the risk of adverse events was comparable between patients with one elevated biomarker and patients with no elevated biomarker in our study, which may be associated with small sample, short follow-up duration, and low rate of adverse events (Supplementary Table 1). It should be evaluated appropriately before recommending a novel biomarker into a multi-biomarkers approach. However, most biomarkers only use simple discrimination analysis in previous studies, which do not analyze the inter-biomarker correlations, include the statistical analyses of NRI and IDI calculation, and adjust for clinical predictors and known biomarkers (including natriuretic peptide and cardiac troponin). Thus, proper evaluation of a novel biomarker or multiple biomarkers in combination is required in future investigations.
There are several limitations to our study. First, most of the data were complete, and only missing data of big ET-1 were more than 10%. However, the main clinical characteristics were comparable between patients with big ET-1 and patients without big ET-1 (Supplementary Table 2). Furthermore, the analyzed numbers of big ET-1 were not too small, and the analysis in this study was rigorous, which would not significantly influence the conclusion. Second, only baseline biomarkers were available in this study. Longitudinal monitoring of these biomarkers may provide better prognostic value. Third, the comparisons of different combination patterns of elevated biomarkers were lacking in this study, because the samples of different categories of elevated biomarkers were too small. Additionally, patients who lacked stored blood samples or were lost to follow-up were excluded from this study, leading to a selection bias. Finally, this is a retrospective and single-center study, which may decrease the confidence of the conclusion. These findings remain to be tested with prospective, multi-center, and large sample trials.
Conclusion
Multiple biomarkers in combination could provide a better prognostic value than a single biomarker. sST2 and big ET-1 could act as alternatives of multi-biomarkers strategies for prognosis evaluation beyond NT-proBNP and hs-cTnT in patients hospitalized with heart failure.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
This study complied with the principles contained in the Declaration of Helsinki and was approved by the ethics committee of Fuwai Hospital (No. 2014-501). Written informed consent has been obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Key Research and Development Program of China (2017YFC1308300) and the National Natural Science Foundation of China (81873472).
Disclosure
All authors declare no conflicts of interest in this work.
References
1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
2. Jones NR, Roalfe AK, Adoki I, et al. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. 2019;21(11):1306–1325. doi:10.1002/ejhf.1594
3. Ibrahim NE, Januzzi JL. Established and emerging roles of biomarkers in heart failure. Circ Res. 2018;123(5):614–629. doi:10.1161/CIRCRESAHA.118.312706
4. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Chin J Heart Fail Cardiomyop. 2018;2(4):196–225. doi:10.3760/cma.j.issn.2096-3076.2018.12.002
5. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
6. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a Report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e895–e1032. doi:10.1161/CIR.0000000000001063
7. Aimo A, Vergaro G, Ripoli A, et al. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart Fail. 2017;5(4):287–296. doi:10.1016/j.jchf.2016.12.016
8. Huang H, Huang B, Li Y, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16(1):15–24. doi:10.1093/eurjhf/hft132
9. Ter Maaten JM, Kremer D, Demissei BG, et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur J Heart Fail. 2019;21(6):732–743. doi:10.1002/ejhf.1437
10. Castiglione V, Aimo A, Vergaro G, et al. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev. 2022;27(2):625–643. doi:10.1007/s10741-021-10105-w
11. Demissei BG, Cotter G, Prescott MF, et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur J Heart Fail. 2017;19(8):1001–1010. doi:10.1002/ejhf.749
12. De Buyzere ML. Multi-biomarker risk stratification in heart failure: a story of diminished marginal returns after Herculean efforts? Eur J Heart Fail. 2018;20(2):278–280. doi:10.1002/ejhf.1035
13. Bayes-Genis A, Ordonez-Llanos J. Multiple biomarker strategies for risk stratification in heart failure. Clin Chim Acta. 2015;443:120–125. doi:10.1016/j.cca.2014.10.023
14. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–731. doi:10.1002/ejhf.1494
15. Lam CSP, Li YH, Bayes-Genis A, et al. The role of N-terminal pro-B-type natriuretic peptide in prognostic evaluation of heart failure. J Chin Med Assoc. 2019;82(6):447–451. doi:10.1097/JCMA.0000000000000102
16. Miller WL, Hartman KA, Grill DE, et al. Only large reductions in concentrations of natriuretic peptides (BNP and NT-proBNP) are associated with improved outcome in ambulatory patients with chronic heart failure. Clin Chem. 2009;55(1):78–84. doi:10.1373/clinchem.2008.108928
17. Peacock WFT, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358(20):2117–2126. doi:10.1056/NEJMoa0706824
18. Tentzeris I, Jarai R, Farhan S, et al. Complementary role of copeptin and high-sensitivity troponin in predicting outcome in patients with stable chronic heart failure. Eur J Heart Fail. 2011;13(7):726–733. doi:10.1093/eurjhf/hfr049
19. Arcari L, Luciani M, Cacciotti L, et al. Coronavirus disease 2019 in patients with cardiovascular disease: clinical features and implications on cardiac biomarkers assessment. J Cardiovasc Med. 2021;22(11):832–839. doi:10.2459/JCM.0000000000001252
20. O’Meara E, Prescott MF, Claggett B, et al. Independent prognostic value of serum soluble ST2 measurements in patients with heart failure and a reduced ejection fraction in the PARADIGM-HF Trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. 2018;11(5):e004446. doi:10.1161/CIRCHEARTFAILURE.117.004446
21. Emdin M, Aimo A, Vergaro G, et al. sST2 predicts outcome in chronic heart failure beyond NT-proBNP and high-sensitivity troponin T. J Am Coll Cardiol. 2018;72(19):2309–2320. doi:10.1016/j.jacc.2018.08.2165
22. Januzzi JL, Mebazaa A, Di Somma S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. Am J Cardiol. 2015;115(7 Suppl):26b–31b. doi:10.1016/j.amjcard.2015.01.037
23. Pascual-Figal DA, Manzano-Fernández S, Boronat M, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13(7):718–725. doi:10.1093/eurjhf/hfr047
24. Wang Z, Pan X, Xu H, et al. Serum soluble ST2 is a valuable prognostic biomarker in patients with acute heart failure. Front Cardiovasc Med. 2022;9:812654. doi:10.3389/fcvm.2022.812654
25. Zhang CL, Xie S, Qiao X, et al. Plasma endothelin-1-related peptides as the prognostic biomarkers for heart failure: a PRISMA-compliant meta-analysis. Medicine. 2017;96(50):e9342. doi:10.1097/MD.0000000000009342
26. Mo R, Yang YM, Yu LT, et al. Elevated plasma big endothelin-1 at admission is associated with poor short-term outcomes in patients with acute decompensated heart failure. Front Cardiovasc Med. 2021;8:629268. doi:10.3389/fcvm.2021.629268
27. Lassus J, Gayat E, Mueller C, et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168(3):2186–2194. doi:10.1016/j.ijcard.2013.01.228
28. Jackson CE, Haig C, Welsh P, et al. The incremental prognostic and clinical value of multiple novel biomarkers in heart failure. Eur J Heart Fail. 2016;18(12):1491–1498. doi:10.1002/ejhf.543
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



