Back to Journals » Lung Cancer: Targets and Therapy » Volume 13
Spotlight on Furmonertinib (Alflutinib, AST2818). The Swiss Army Knife (del19, L858R, T790M, Exon 20 Insertions, “uncommon-G719X, S768I, L861Q”) Among the Third-Generation EGFR TKIs?
Received 16 August 2022
Accepted for publication 30 September 2022
Published 25 October 2022 Volume 2022:13 Pages 67—73
DOI https://doi.org/10.2147/LCTT.S385437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Fengying Wu
Shannon S Zhang,1 Sai-hong Ignatius Ou1,2
1University of California Irvine School of Medicine, Department of Medicine, Department of Medicine, Orange, CA, USA; 2Chao Family Comprehensive Cancer Center, Orange, CA, USA
Correspondence: Sai-hong Ignatius Ou, Chao Family Comprehensive Cancer Center, Division of Hematology and Oncology, Department of Medicine, University of California Irvine School of Medicine, 200 South Manchester Ave, Suite 400, Orange, CA, 92868, USA, Email [email protected]
Abstract: Osimertinib, a third-generation (3G) epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) is now considered the standard of care for the first-line (1L) treatment of advanced EGFR+ NSCLC due to statistically significant improved progression-free survival (PFS) and overall survival (OS) compared with first-generation (1G) treatment from the FLAURA trial. Recently two other 3G EGFR TKIs (aumolertinib and furmonertinib) have been approved in China for treatment of EGFR T790M+ NSCLC. Randomized Phase 3 trials of these two 3G EGFR TKIs have also demonstrated PFS over gefitinib respectively. Among these two Chinese home-grown, 3G EGFR TKIs, furmonertinib seems to most closely resemble osimertinib in terms of dosing regimen, efficacy and adverse events profile. In this article, we reviewed the clinical activity and adverse events of furmonertinib at 80 mg daily (approved dose), potential usage of 160 mg daily for CNS metastasis in EGFR+ NSCLC, and usage of 160 mg or 240 mg daily in EGFR exon20 insertion positive (EGFRex20ins+) NSCLC patients.
Keywords: furmonertinib, Alflutinib, third generation EGFR TKI, non-small cell lung cancer, EGFR T790M mutation, exon 20 insertions, uncommon EGFR mutations
Introduction
Historically, epidermal growth factor receptor (EGFR+) positive non-small cell lung cancer (NSCLC) has been optimally treated using first-generation (1G) (gefitinib, erlotinib, icotinib) and second-generation (2G) TKIs (afatinib, dacomitinib) based on statistically improved progression-free survival (PFS) over standard platinum-doublet chemotherapy.1 Invariably resistance develops, most commonly due to acquiring the EGFR gatekeeper mutation T790M which alters drug binding and enzymatic activity of the EGFR protein and abolishes the binding of 1G and 2G EGFR TKIs inside the EGFR kinase domain pocket.2 Additionally, adverse events of 1G and 2G from EGFR TKIs are common and can be severe such as rash, diarrhea, mucositis, and stomatitis that limit the quality of life of patients. Hence, many 3G EGFR TKIs have been developed from multiple global pharmaceutical companies to overcome these resistance mutations3 but only osimertinib has been successfully approved for second-line (2L) EGFR T790M+ NSCLC based on AURA-3 trial4 and then 1L approved based on improved PFS and OS in FLAURA.5,6 By September 2019, osimertinib has been approved for 1L treatment of EGFR+ NSCLC in over 75 countries and in more than 80 countries for EGFR T790M+ NSCLC.7 More recently, at least seven 3G EGFR TKIs that are structurally similar to osimertinib were being developed from essentially China and Republic of Korea.8 The Phase 2 safety and clinical efficacy trials of these 3G EGFR TKIs in EGFR T790M+ NSCLC patients have been published and summarized.8 Among these seven 3G EGFR TKIs, furmonertinib and aumolertinib have been approved in China and lazertinib has been approved in Republic of Korea for treatment of EGFR T790M+ NSCLC.3,7 Additionally, head to head trials of furmonertinib and aumolertinib against 1G EGFR TKIs have been published.8–10
Furmonertinib (AST2818), previously known as alflutinib, is a novel 3G TKI that received approval in China in March 2021 to treat locally advanced or metastatic EGFR T790M+ NSCLC that developed after progression on treatment with 1G EGFR TKIs.10 Much of the Chinese prescribing information is summarized here.11 To date, clinical trial results have indicated the efficacy and adverse events of furmonertinib are similar to osimertinib.10 Here, we review the efficacy and adverse events of furmonertinib as a strong competitor of osimertinib in treatment of EGFR+ NSCLC.
Furmonertinib Pre-Clinical Data
Furmonertinib (AST2818, Alflutinib) is manufactured by Shanghai Allist Pharmaceuticals Co., Ltd. in Shanghai, China. Furmonertinib is designed with a trifluoro-ethoxypyridine-based modification of osimertinib (Figure 1). Essentially the methyl-group on the methoypyridine ring of osimertinib is replaced with three fluorine groups. The adjacent benzene ring is replaced with one nitrogen to form an azabenzene ring. The binding of trifluoroethoxyl in a hydrophobic pocket to an unexplored binding pocket of EGFR protein may improve its activity and stability.11
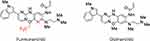 |
Figure 1 Comparison of the structure of furmonertinib and osimertinib. |
While the structure of furmonertinib is published,12 comprehensive pre-clinical data on the in vitro biochemical kinase, cellular proliferation, and xenograft inhibition potency against various EGFR mutations especially against EGFR T790M or EGFR exon20 insertions (ex20ins) has not been published by the manufacturer although there were selectively presented in poster format in the 2022 North America Conference on Lung Cancer held in Chicago, USA from September 23-25, 2022 13. Neither was any pre-clinical central nervous system (CNS) potency of furmonertinib published. Finally, comparison of pre-clinical activities of furmonertinib to osimertinib has not been published although they have been cited indirectly.12
Furmonertinib Pharmacokinetic (PK) Data
Furmonertinib is metabolized by CYP3A4 to its active metabolite AST5902 in human hepatocytes experiments.14 Furmonertinib is also a potent inducer of CYP3A4 similar to rifampin in its potency to induce CYP3A4.14 AST5902 is potentially a CYP3A4 inducer also. Furmonertinib is not an inducer of CYP1A2 or CPY2B6.14 Hence, the steady state of the furmonertinib may be affected by auto-induction and lead to subtherapeutic levels. Indeed itraconazole, a potent CYP3A4 inhibitor increased the level of furmonertinib in healthy volunteers. At a steady 80 mg once daily dose, the median Tmax of furmonertinib on empty stomach is about 3 hours with a half-life of about 40.6 hours.15 The administration of itraconazole, a potent CYP3A4, increased steady state furmonertinib’s Tmax to 6 hours and half-life to 70.3 hours.15 Single dose high-fat food effect study drug absorption indicated the peak concentration of furmonertinib increased by approximately 53%, and the AUC0-24 increased by approximately 32%.16 However, the peak concentration of its metabolite AST5902 decreased by approximately 20%, and the AUC decreased by approximately 8%.16 Overall from population PK studies, while auto-induction of CYP3A4 and food effect decreased and increased the level of furmonertinib respectively, the corresponding inverse increase and decrease of level of AST5902 essentially balanced out with the total active ingredients remaining the same.17
Determination of Furmonertinib Phase 2 Dose
The Phase 1 dose escalation of furmonertinib investigated the safety of furmonertinib from 20 mg to 240 mg daily (20 mg, 40 mg, 80 mg, 160 mg, and 240 mg) with dose expansion with 40 mg, 80 mg, 160 mg, 240 mg in EGFR T790M+ NSCLC patients. Overall, no dose limiting toxicity (DLT) was observed and thus the maximum tolerated dose (MTD) was not determined. The steady concentration AUC0-24 (ng·h/mL) of furmonertinib increased less than proportional with increased dose: about 100% from 40 mg to 80 mg, about 50% increase from 80 mg to 160 mg and about 25% increase from 160 mg to 240 mg. The overall response rate (ORR) in the 40 mg, 80 mg, 160 mg, and 240 mg groups were 83.3% (5/6), 77.8% (35/45), 78.0% (39/50), and 66.7% (10 of 15), respectively. The CNS ORR in the 40 mg, 80 mg, 160 mg, and 240 mg groups were 50.0% (1/2), 100.0% (4/4), 66.7% (6/9), and 50.0% (1/2), respectively. Importantly, the median PFS and duration of response (DOR) were similar among 80 mg, 160 mg, and 240 mg patients and much longer than the 40 mg. In terms of adverse events, grade 1–2 QTc prolongation was observed in eight (6%) patients: two patients at 20 mg, 40 mg, 80 mg and 160 mg respectively. Including QTc prolongation, all other adverse events from furmonertinib are similar in incidence and severity as osimertinib. Thus, 80 mg once daily was selected as the phase 2 dose going forward.
Furmonertinib Phase II Clinical Data (EGFR T790M+ NSCLC)
A phase 2b multicenter single arm pivotal study enrolled 220 patients investigated the efficacy and safety of furmonertinib in EGFR T790M+ NSCLC patients across 46 hospitals in China after progression on 1G or 2G EGFR TKIs. The ORR and PFS for furmonertinib were 74% (95% CI: 68–80%) and 9.6 months (95% CI: 8.2–9.7)18 compared favorably to ORR of 62% (95% CI: 54–69%) and PFS of 9.7 months from the phase 2 osimertinib AURA17 trial conducted exclusively in China. Interestingly, a higher number of patients in the trial had CNS metastases (48% versus 37% in AURA17).19 Adverse events profile was also similar between furmonertinib and osimertinib including incidence of QTc prolongation (Table 1). Grade 3 QTc prolongation is defined as > 500 milliseconds. Based on this pivotal trial, furmonertinib received approval in China on March 2021 to treat locally advanced or metastatic EGFR T790M+ NSCLC.3,11
 |
Table 1 Comparison of Incidence of QTc Prolongation Between Furmonertinib and Osimertinib |
FURLONG: Furmonertinib versus 1G EGFR (gefitinib) TKI (FURLONG) Phase 3 Trial
Furmonertinib 80 mg once daily was compared with gefitinib 250 mg once daily in a randomized phase 3 trial as 1L treatment of advanced stage activating EGFR+ NSCLC across 55 hospitals in mainland China in the FURLONG study. Stratification factors were EGFR mutation subtype and presence/absence of CNS metastasis. Like FLAURA, FURLONG was a placebo-controlled, double-blind, double dummy study. Furthermore, unlike FLAURA, FURLONG’s primary endpoint is independent review committee (IRC)-assessed PFS. Median PFS in patients receiving furmonertinib was significantly higher at 20.8 months (95% CI: 17.8–23.5) compared with those receiving gefitinib at 11.1 months (95% CI: 9.7–12.5) (HR = 0.44; 95% CI: 0.34–0.58; p<0.0001).9 These efficacy results compare favorably to the overall FLAURA results and Asian subgroup analysis of FLAURA as assessed by the investigators (osimertinib: 16.5m (95% CI: 13.8–20.7) versus gefitinib/erlotinib: 11.0 months (95% CI: 9.5–12.6) (HR = 0.54; 95% CI 0.41, 0.72; p<0.0001). Given the potential differences in the composition of the patient population, only comparison of IRC-assessed and investigator-assessed hazard ratios were plotted for comparison (Figure 2A).20 Adverse events among furmonertinib and osimertinib were similar in nature and incidences.
CNS Efficacy of Furmonertinib (FURLONG)
CNS metastases occur frequently in EGFR+ NSCLC patients. Close to 34% (121/357) of the patients enrolled in FURLONG and 36% of the patients enrolled onto FLAURA had baseline metastasis. Thus, the ability for a 3G EGFR TKI to penetrate the blood–brain barrier is important to treat the CNS metastasis and also theoretically to delay or even prevent the emergence of CNS metastasis in EGFR+ patients who do not have CNS metastasis at the diagnosis. Not surprisingly furmonertinib achieved superior PFS over gefitinib regardless of CNS metastasis.21 More granular analysis of the FURLONG trial focusing on the CNS PFS also demonstrated superior CNS PFS of furmonertinib over gefitinib with an HR of 0.40. Importantly, the CNS PFS advantage for furmonertinib was similar for del19 or L858R patients21 and compares favorably to the HR for CNS PFS in the overall FLAURA22 (Figure 2B). The numerical value of CNS PFS in the furmonertinib arm of FURLONG was similar to the osimertinib arm of FLAURA but given the CNS PFS are constrained by the difference in patient characteristics (sex, age, number of CNS lesions, EGFR mutation subtype: del19 versus L858R, smoking status), we are comparing HRs rather than the actual CNS PFS.
It has been demonstrated that osimertinib at 160 mg daily achieved good CNS control including patients with leptomeningeal carcinomatosis (LC). In a multi-center single arm study of osimertinb at 160 mg in the patients with brain metastasis, intracranial ORR and disease control rates were 55.0% and 77.5%, respectively.23 The median progression-free survival (PFS) was 7.6 months (95% CI: 5.0–16.6); the median overall survival (OS) was 16.9 months (95% CI: 7.9–not reached (NR)). In the LC cohort, intracranial disease control rate was 92.5% and complete response rate was 12.5%. The median OS was 13.3 months (95% CI 9.1–NR); the median PFS was 8.0 months (95% CI: 7.2–NR).23 Similar retrospective study also revealed similar efficacy of high dose (160 mg) of osimertinib in CNS metastasis and leptomeningeal carcinomatosis.24 Furmonertinib 160 mg daily is now being investigated in several single institutions study in EGFR+ patients with CNS metastasis (iFORCE, NCT05465343; NCT05379803).
Comparison of Incidence of QTc Prolongation of Furmonertinib and Osimertinib
QTc prolongation is a rare unique adverse event in furmonertinib and osimertinib. The vast majority of QTc prolongation from furmonertinib is grade 1–2 (< 500 ms). (Table 1) provides a comparison of the reported incidence of QTc prolongation.
Furmonertinib Efficacy in EGFR exon20 Insertion (EGFRex20ins+) positive NSCLC
Insertion mutations into the exon 20 of EGFR in NSCLC (EGFRex20ins+) constitute a diverse population of activating EGFR mutations.25 While currently there are two US FDA approved drugs, mobocertinib (a 3G EGFR TKI) and amivantamab (a bi-specific EGFR/MET antibody), nevertheless, the treatment of EGFRex20ins+ NSCLC still require optimization. Case reports and small-scale clinical trials have indicated that osimertinib has demonstrated clinical activity against a certain subset of EGFRex20ins+ NSCLC and mostly at the higher dose of 160 mg rather than 80 mg.26–28 At 160 mg, the ORR was 27% among 17 EGFRex20ins+ NSCLC patients treated with a median duration of response (DOR) not reached (95% CI: 4.7–NR) and median PFS of 9.6 months (95% CI: 4.1–10.7).27 At 80 mg of osimertinib, there was no objective response among 12 patients treated from a different study.28 In comparison among the 114 platinum-pretreated patients, the ORR for mobocertinib in their “pivotal” registration trial was 28% (95% CI: 20–37), median PFS of 7.3 months (95% CI: 5.5–9.2) and median DOR was (95% CI: 7.4–20.3).29
Furmonertinib has demonstrated pre-clinical activity against EGFRex20ins+ NSCLC with IC50 against EGFR20ins S768_D770dup at 11nM, 14nm against A767_V769dup, and 20nM against N771_H773dup.30 Several case reports have reported clinical activity of furmonertinib at 160 mg daily against treatment-refractory EGFR ex20ins mutations (one unknown, one EGFR P772_H773insV).31,32 A small-scale study of the clinical activity of furmonertinib at 160 mg or 240 mg dose against EGFRex20ins (FAVOUR, NCT04858958).30 Cohort 1 of FAVOUR using 240 mg of furmonertinib has completed with an IRC-assessed ORR of 60% and DRC of 100%. Of note 20% (2/10) had grade 1–2 QTc prolongation (no grade ≤ 3 QTc prolongation) not significantly higher than the incidence when furmonertinib is given at 80 mg daily. There was also 20% grade 1–2 creatinine phosphokinase (CPK) elevation.30 Hence a large single arm study of furmonertinib 240 mg daily is being planned in China (NCT05466149). Additionally, a phase 1b dose escalation study of furmonertinib in exon20 insertion in HER2 and EGFR in US is ongoing (NCT05364073, FURMO-002) sponsored by the US ArriVent Biopharma who licensed ex-China rights furmonertinib from Allist. The pre-clinical efficacy of furmonertinib against HER2 was recently presented with Ba/F3 cellular IC50 against ERBB2 A775_G776insYVMA at 118nM and 25nM cellular IC50 against ERBB2 V777_G778insGC.13
Future Development of Furmonertinib
The role of adjuvant osimertinib in resected early-stage EGFR+ NSCLC has been well established by ADAURA33 and approved by the FDA.34 Similarly, furmonertinib has commenced a similarly designed trial in China. The FORWARD trial enrolled resected stage II–IIIA EGFR+ NSCLC patients and randomized them to adjuvant furmonertinib versus placebo (NCT04853342).
Additionally, unlike the role of immune checkpoint inhibitors in driver mutation negative NSCLC, the role of neoadjuvant EGFR TKI in early stage resectable and consolidation/maintenance EGFR TKI in optimally treated locally advanced EGFR+ NSCLC remained to be determined. Clinical trials addressing these roles for EGFR TKI are being planned or on-going with furmonertinib: neoadjuvant (chemo + furmonertinib in Stage IIIA–IIIB EGFR+ NSCLC; FORESEE, NCT05430802; single agent furmonertinib in stage IIIA–IIIB EGFR+ NSCLC, FRONT, NCT04965831).
Finally, afatinib, 2G EGFR TKI, is the only EGFR TKI approved to treat the three “uncommon” EGFR mutations (G719X, S768I, L861Q).35 Osimertinib has demonstrated clinical activity against these three “uncommon” EGFR mutations in a small prospective multi-center trial with an ORR of 50% (18/36; 95% CI: 33–67%) and median PFS was 8.2 months (95% CI: 5.9–10.5).36 Furmonertinib recently published pre-clinical data against G719S (Ba/F3 cellular IC50 = 12.4nM), L861Q (Ba/F3 cellular IC50 = 3.8nM), and S768I (Ba/F3 cellular IC50 = 21.6nM),13 thus it is conceivable that furmonertinib should be investigated as an EGFR TKI against the three “uncommon” EGFR mutations with potentially better tolerability profile.
Concluding Remarks
Furmonertinib has achieved excellent clinical efficacy and safety profile in both activating EGFR+ NSCLC mutations and the acquired EGFR T790M mutation. Phase 1 dose escalation trial has indicated furmonertinib has a wide therapeutic window (from 80 mg to 240 mg). In essence, furmonertinib is as efficacious as osimertinib as 1L treatment of advanced EGFR+ NSCLC. In contrast to osimertinib, furmonertinib could theoretically be active against EGFRex20ins albeit at higher dose based on promising data at 240 mg dose. Furthermore, pre-clinical data suggests furmonertinib has potential clinical activity against the three “uncommon EGFR mutations. With the potential of lower pricing, furmonertinib can be the one drug that can be used to treat the vast majority of activating EGFR+ mutations.
Disclosure
Dr Sai-Hong Ignatius Ou previously owned stock from Turning Point Therapeutics (prior to August 17, 2022) and Elevation Oncology (prior to September 30, 2022); owns stock and stock options and is a member of the scientific advisory board of BlossomHill Therapeutics; owns stock in MBrace Therapeutics; reports personal fees from Elevation Oncology, Lilly, Pfizer, DAVA Oncology LLP, BeiGene, and JNJ/Janssen, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Lee CK, Davies L, Wu YL, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst. 2017;109(6). doi:10.1093/jnci/djw279
2. Attili I, Karachaliou N, Conte P, et al. Therapeutic approaches for T790M mutation positive non-small-cell lung cancer. Expert Rev Anticancer Ther. 2018;18(10):1021–1030. doi:10.1080/14737140.2018.1508347
3. Nagasaka M, Zhu VW, Lim SM, et al. Beyond osimertinib: the development of third-generation EGFR tyrosine kinase inhibitors for advanced EGFR+ NSCLC. J Thorac Oncol. 2021;16(5):740–763. doi:10.1016/j.jtho.2020.11.028
4. Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
5. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
6. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662
7. AstraZeneca Tagrisso approved in China as a 1st-line treatment for EGFR-mutated non-small cell lung cancer. Available from: https://www.astrazeneca.com/media-centre/press-releases/2019/tagrisso-approved-in-china-as-a-1st-line-treatment-for-egfr-mutated-non-small-cell-lung-cancer-04092019.html#.
8. Lau SCM, Ou SI; And still they come over troubled waters. Can Asia’s third-generation EGFR TKIs (furmonertinib, aumolertinib, rezivertinib, limertinib, befotertinib, SH-1028, and lazertinib) impact global treatment of EGFR+ NSCLC. J Thoracic Oncol. 2022;17(10):1144–1154. doi:10.1016/j.jtho.2022.08.016
9. Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicenter, double-blind, randomized phase 3 study. Lancet Respir Med. 2022. doi:10.1016/S2213-2600(22)00168-0
10. Lu S, Dong X, Jian H, et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastaticnon-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;17. doi:
11. Deeks ED. Furmonertinib: first Approval. Drugs. 2021;81(15):1775–1780. doi:10.1007/s40265-021-01588-w
12. Shi Y, Zhang S, Hu X, et al. Safety, clinical activity, and pharmacokinetics of alflutinib (AST2818) in patients with advanced NSCLC with EGFR T790M mutation. J Thorac Oncol. 2020;15(6):1015–1026. doi:10.1016/j.jtho.2020.01.010
13. Musib L, Kowanetz M, Li Q, et al. Furmonertinib is an oral, irreversible, highly brain-penetrant pan-EGFR mutant inhibitor with activity against classical and atypical EGFR mutations.
14. Liu XY, Guo ZT, Chen ZD, et al. Alflutinib (AST2818), primarily metabolized by CYP3A4, is a potent CYP3A4 inducer. Acta Pharmacol Sin. 2020;41(10):1366–1376. doi:10.1038/s41401-020-0389-3
15. Heng J, Tang Q, Chen X, et al. Evaluation of the pharmacokinetic effects of itraconazole on alflutinib (AST2818): an open-label, single-center, single-sequence, two-period randomized study in healthy volunteers. Eur J Pharm Sci. 2021;162:105815. doi:10.1016/j.ejps.2021.105815
16. Zhu S, Deng J, Tang Q, et al. A randomized, open, single-centre, crossed study of the effect of food on the pharmacokinetics of one oral dose of alflutinib mesylate tablets (AST2818) in healthy male subjects. Iran J Pharm Res. 2020;19(3):24–33. doi:10.22037/ijpr.2020.113112.14116
17. Zou HX, Zhang YF, Zhong DF, et al. Effect of autoinduction and food on the pharmacokinetics of furmonertinib and its active metabolite characterized by a population pharmacokinetic model. Acta Pharmacol Sin. 2022;43(7):1865–1874. doi:10.1038/s41401-021-00798-y
18. Shi Y, Hu X, Zhang S, et al. Efficacy, safety, and genetic analysis of furmonertinib (AST2818) in patients with EGFR T790M mutated non-small-cell lung cancer: a phase 2b, multicentre, single-arm, open-label study. Lancet Respir Med. 2021;9(8):829–839. doi:10.1016/S2213-2600(20)30455-0
19. Zhou C, Wang M, Cheng Y, et al. AURA17 study of osimertinib in Asia-Pacific patients (pts) with EGFR T790Mpositive advanced non-small cell lung cancer (NSCLC): updated phase II results including overall survival (OS). Ann Oncol. 2018;29(suppl 9):IX157. doi:10.1093/annonc/mdy425.022
20. Cho BC, Chewaskulyong B, Lee KH, et al. Osimertinib versus standard of care EGFR TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subset. J Thorac Oncol. 2019;14(1):99–106. doi:10.1016/j.jtho.2018.09.004
21. Shi Y, Chen G, Wang X, et al. Central nervous system efficacy of Furmonertinib (AST2818) versus gefitinib as first-line treatment for EGFR mutated non-small cell lung cancer: results from the FURLONG study. J Thorac Oncol. 2022;2022. doi:10.1016/j.jtho.2022.07.1143
22. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:3290–3297. doi:10.1200/JCO.2018.78.3118
23. Park S, Lee MH, Seong M, et al. A Phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol. 2020;31(10):1397–1404. doi:10.1016/j.annonc.2020.06.017
24. Piper-Vallillo AJ, Rotow JK, Aredo JV, et al. High-dose osimertinib for CNS progression in EGFR+ NSCLC: a multi-institutional experience. JTO Clin Res Rep. 2022;3(6):100328. doi:10.1016/j.jtocrr.2022.100328
25. Meador CB, Sequist LV, Piotrowska Z. Targeting EGFR exon 20 insertions in non-small cell lung cancer: recent advances and clinical updates. Cancer Discov. 2021;11(9):2145–2157. doi:10.1158/2159-8290.CD-21-0226
26. Piotrowska Z, Fintelmann FJ, Sequist LV, Jahagirdar B. Response to osimertinib in an EGFR exon 20 insertion-positive lung adenocarcinoma. J Thorac Oncol. 2018;13:e204–e206. doi:10.1016/j.jtho.2018.05.017
27. Piotrowska Z, Wang Y, Sequist LV, et al. ECOG-ACRIN 5162: a phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions. J Clin Oncol. 2020;38(15):9513. doi:10.1200/JCO.2020.38.15_suppl.9513
28. Yashuda H, Ichihara E, Sakakibara-Konishi J, et al. A phase I/II study of osimertinib in EGFR exon 20 insertion mutation-positive non-small cell lung cancer. Lung Cancer. 2021;162:140–146. doi:10.1016/j.lungcan.2021.10.006
29. Zhou C, Ramalingam SS, Kim TM, et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: a phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021;7(12):e214761. doi:10.1001/jamaoncol.2021.4761
30. Han B, Zhou C, Wu L, et al. Preclinical and preliminary clinical investigations of furmonertinib in NSCLC with EGFR exon 20 insertions (20ins). Ann Oncol. 2021;32:S949–S1039. doi:10.1016/j.annonc.2021.08.1815
31. Jiang W, Sha M, Chen C. Successful salvage therapy with a high dose of furmonertinib in a case of lung adenocarcinoma harboring EGFR exon 20 insertion. Am J Ther. 2022;28:10–97.
32. Jia K, Yang S, Chen B, et al. Advanced lung adenocarcinoma patient with EGFR exon 20 insertion benefits from high-dose furmonertinib for nine months after progression from mobocertinib: a case report. Ann Transl Med. 2022;10(6):386. doi:10.21037/atm-22-1167
33. Wu Y, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. NEJM. 2020;383(18):1711–1723. doi:10.1056/NEJMoa2027071
34. Koch AL, Vellanki PJ, Drezner N, et al. FDA approval summary: osimertinib for adjuvant treatment of surgically resected non-small cell lung cancer, a collaborative project orbis review. Clin Cancer Res. 2021;27(24):6638–6643. doi:10.1158/1078-0432.CCR-21-1034
35. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of Afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. doi:10.1016/S1470-2045(15)00026-1
36. Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09). J Clin Oncol. 2020;38(5):488–495. doi:10.1200/JCO.19.00931
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

