Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Spotlight on fluticasone furoate/umeclidinium/vilanterol in COPD: design, development, and potential place in therapy
Received 3 November 2016
Accepted for publication 12 December 2016
Published 30 December 2016 Volume 2017:12 Pages 135—140
DOI https://doi.org/10.2147/COPD.S114273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Chitra Lal, Charlie Strange
Division of Pulmonary, Critical Care, Allergy and Sleep Medicine, Medical University of South Carolina, Charleston, SC, USA
Abstract: COPD is characterized by persistent airflow obstruction caused by exposure to irritants including cigarette smoke, dust, and fumes. According to the latest GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines, a combination of inhaled corticosteroids, long-acting β2 agonists, and long-acting muscarinic receptor antagonists can be used for group D COPD patients who are at high risk for exacerbations. Umeclidinium/fluticasone furoate/vilanterol is one such triple-combination therapy currently under development with some completed and several ongoing clinical trials. This review paper summarizes the pharmacologic profiles of these medications and highlights findings from clinical trials, including safety and efficacy data, while speculating on the role of this therapy in current treatment for COPD.
Keywords: COPD, umeclidinium, fluticasone furoate, vilanterol, triple therapy
Introduction
COPD is characterized by persistent airflow obstruction1 caused by exposure to irritants including cigarette smoke, dust, and fumes. To decrease the associated morbidity and mortality,2 there has been considerable interest in developing new medications for COPD. Given the regulatory barriers that are easiest to meet by improvement in lung function, the bulk of research is being directed towards the development of new bronchodilator and inhaled corticosteroid (ICS) medications.
According to the latest GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines,3 a combination of ICS, long-acting β2 agonist (LABA), and long-acting muscarinic receptor antagonist (LAMA) can be used for group D COPD patients who are at high risk for exacerbations. Group D patients are symptomatic and usually have a forced expiratory volume in 1 second (FEV1) of <50% of the predicted value, two or more exacerbations within the last year, and/or one or more hospitalizations for COPD exacerbations. The recommendation is based on a few studies showing the superiority of the ICS/LAMA/LABA combinations4,5 as compared to monotherapy alone, in the level of bronchodilation, dyspnea score change, and use of rescue medications.
The concept of combining different bronchodilators in a single inhaler is an attractive one as it minimizes the number of COPD medications that the patient takes on a daily basis and thus may boost patient compliance. However, the challenge in combining medications of different classes in a single drug delivery device is ensuring the bio-stability of the individual compounds. Thus, while “open” triple-combination therapies such as tiotropium added to salmeterol/fluticasone and glycopyrronium added to salmeterol/fluticasone6 have been evaluated, there are few combined or “closed triple” therapies which are currently in development/available in the world as shown in Table 1.
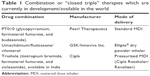  | Table 1 Combination or “closed triple” therapies which are currently in development/available in the world |
While LABA/LAMA combinations for COPD have become available in the US, there are currently no fixed-dose “closed triple” therapies for COPD. Thus, there has been longstanding interest in the development of triple-combination therapies for severe and very severe COPD in the US, in keeping with the current GOLD guidelines.
Umeclidinium/fluticasone furoate/vilanterol (UMEC/FF/VI) is one such triple-combination therapy currently under development with some completed and several ongoing clinical trials. The purpose of this review paper is to highlight findings from these studies, including safety and efficacy data, while speculating on the role of this therapy in current treatment for COPD.
Pharmacologic profiles
UMEC is an LAMA, VI is an LABA, and FF is an ICS. UMEC/VI and FF/VI are already being marketed as Anoro Ellipta and Breo Ellipta, respectively, by GlaxoSmithKline (GSK).
In isolated human bronchial strips, UMEC produces competitive antagonism of the muscarinic receptors versus carbachol. This antagonism is slowly reversible in a concentration-dependent manner.7 The time to 50% restoration of contraction at 10 nM is about 381 versus 413 minutes for tiotropium bromide. Thus, UMEC is a potent anticholinergic agent that shows slow functional reversibility at the human M3 muscarinic receptor. This kinetics translates into a long duration of bronchodilation and once-daily dosing.
Inhaled VI is recommended at a dose of 25 μg. Studies of oral VI have shown it to be well tolerated up to doses of 500 μg as a single administration in healthy men.8 A study9 of inhaled VI in asthma at a dose of 25 μg once a day has shown it to be well tolerated with the commonest side effects reported being nasopharyngitis, headache, upper respiratory tract infection, and oropharyngitis. In yet another study conducted in healthy Japanese men,10 single inhaled doses of VI of 50 μg were found to be safe and well tolerated with no deaths or serious adverse events reported. Thus, the low dose of inhaled VI which is used therapeutically is unlikely to produce unexpected systemic toxicity in COPD patients.
FF is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. It is structurally related to fluticasone propionate, but with a distinct chemical and pharmacological profile. FF was found to be more potent than other glucocorticoids for preserving epithelial integrity and reducing epithelial permeability in response to protease- and mechanical-induced cell damage11 and was also found to have greater tissue retention than other glucocorticoids.
Pharmacokinetics of the triple therapy of UMEC/FF/VI12 has been studied in healthy volunteers. This triple therapy also called the “closed triple” is being developed by GSK and will be administered in a single inhaler (Ellipta® dry powder device).
Two single-center studies12 assessed the systemic exposure, systemic pharmacodynamics (PD), and safety profile of the closed triple therapy of UMEC/FF/VI compared with dual therapies. Healthy nonsmokers aged 18–65 years and with normal lung function were enrolled. Both studies were randomized, single-dose, crossover trials with study 1 being double-blind and study 2 being open-label. Participants were randomized to receive four consecutive inhalations (each administered as a single dose) via an Ellipta® dry powder inhaler in the following ways.
In study 1 (CTT116415/NCT01691547), FF/UMEC/VI at total doses of 400/500/100 μg, FF/UMEC 400/500 μg, UMEC/VI 500/100 μg, and FF/VI 400/100 μg were compared.
In study 2 (200587/NCT01894386), FF/UMEC/VI at total doses of 400/500/100 μg or 400/250/100 μg, FF/VI 400/100 μg, and UMEC/VI 250/100 μg were compared.
Of 88 subjects, 95% completed both studies. Total systemic exposure was similar for the triple therapy of UMEC/FF/VI compared with FF/VI and UMEC/VI. No clinically significant systemic PD findings were detected. The incidence of adverse events was low and similar across all treatment arms.
Thus, these studies showed that the closed triple therapy of UMEC/FF/VI was as safe as the currently marketed therapies of UMEC/VI (marketed as Anoro Ellipta) and FF/VI (marketed as Breo Ellipta). In addition, the systemic exposure to the individual components showed no clinically significant differences when administered as triple versus dual therapy.
Clinical efficacy
Two randomized studies have been conducted to study the safety and efficacy of UMEC added to FF/VI (NCT01957163; NCT02119286). Both of these were 12-week, double-blind, placebo-controlled, parallel-group, multicenter studies and evaluated the safety and efficacy of UMEC (62.5 and 125 μg) added to FF/VI (100/25 μg) in COPD.13 Patients (N=1,238) were randomized 1:1:1 to treatment with once-daily blinded UMEC 62.5 μg, UMEC 125 μg, or placebo (PBO) added to open-label FF/VI 100/25 μg. Inclusion criteria were age ≥40 years, diagnosis of COPD, at least a 10 pack-years smoking history, pre- and post-salbutamol (albuterol), FEV1/forced vital capacity ratio of <0.7 and predicted FEV1 ≤70%, and a modified Medical Research Council dyspnea scale score ≥2. Subjects with asthma or other known respiratory disease, hospitalization in the prior 12 weeks for COPD or pneumonia, pregnancy, or use of long-term oxygen therapy were excluded.
The primary end point was trough FEV1 on Day 85. The secondary end point was 0–6 hours post-dose weighted mean FEV1 at Day 84. Quality of life was reported using St George’s Respiratory Questionnaire (SGRQ). In both studies, the primary and secondary end points were met; the trough FEV1 was significantly improved on Day 85 with UMEC + FF/VI (62.5 and 125 μg) versus PBO + FF/VI (range: 0.111–0.128 L, P<0.001) as was 0–6 hours post-dose weighted mean FEV1 on Day 84 (range: 0.135–0.153 L, P<0.001). Improvements in SGRQ were not seen consistently across both studies or with both doses of UMEC.
The overall incidence of adverse events was similar across all groups. Further details about adverse effects with the triple-combination therapy are included in the “Safety and tolerability” section.
There are some additional studies which are currently underway evaluating the safety and efficacy of the triple therapy of UMEC/FF/VI. These are listed in Table 2.
  | Table 2 Trials which are currently underway evaluating the safety and efficacy of the triple therapy of UMEC/FF/VI |
Safety and tolerability
The triple combination of UMEC/FF/VI has been found to be generally well tolerated. The commonest side effects reported include nasopharyngitis, headache, and back pain. Six total deaths were reported in both studies, NCT01957163 and NCT02119286, but were not considered related to the study treatment. Of note, all treatment regimens had both inhaled β agonists and corticosteroids, and therefore, side effect differences would only be expected for the variance in UMEC use and dose that were evaluated in primary licensing studies.14,15
Regulatory affairs
Based on these successful clinical trials, GSK and Innoviva Inc. plan to file a new drug application in the US for the once-daily closed triple-combination therapy, FF/UMEC/VI, for patients with COPD by the end of 2016. The companies expect an EU regulatory submission of the closed triple-combination therapy for COPD by the end of 2016 as well.16
Expert opinion
One of the benefits of a triple combination of an ICS/LABA/LAMA is the potential improvement in patient compliance due to the need to take fewer inhalers. As triple therapy with ICS/LABA/LAMA is recommended for COPD patients at high risk for exacerbations, this should in turn translate into fewer COPD exacerbations, and consequently fewer COPD-related hospitalizations. This is particularly important since the number of inhalers filled on average in the US is <40% of that prescribed.17,18
On the other hand, the need for continued steroid use in severe COPD patients with a history of exacerbations, on LABA and LAMA therapy, has been called into question. In a recent study, in patients with severe COPD on tiotropium + salmeterol, the risk of moderate or severe exacerbations was similar among those who discontinued ICS and those who continued ICS.19 There was, however, a greater decrease in lung function during the final step of glucocorticoid withdrawal.
Moreover, LABA/LAMA combination inhalers have been shown to be superior to LABA/ICS in one recent clinical trial,20 further calling into question the necessity of an inhaled ICS in COPD treatment regimens. Thus, while the initiation of triple therapy with ICS/LABA/LAMA in COPD patients at high risk for exacerbations is appropriate, the landscape of clinical use is changing.
The clinical explosion in use of the first long LABA/ICS combination salmeterol/fluticasone fumarate (Advair) occurred at a time of limited competition. Although some physicians at the time predicted its failure since monotherapy of LABA and ICS was already available, the primary care physician community drove use. A medication that could define if dyspnea was likely of pulmonary airways origin was welcomed. In contrast, airways disease patients are almost always responsive to combinations of any two components of an LABA/LAMA/ICS regimen. Therefore, today many alternatives can give the same diagnostic value.
Because these drugs and their devices have been required to enter the US Food and Drug Administration clinical trials arena together, there still remains no generic long-acting bronchodilator on the US market. The major reason for non-compliance with inhaled medications for COPD is the cost of medication and/or copayments. With scheduled arrival of generic long-acting bronchodilators that will likely be less expensive within the life cycle of UMEC/FF/VI, few individuals believe that uptake will be rapid unless an aggressive price reduction from other combination bronchodilators accompanies the product launch.
Most of the current focus in drug development for COPD has been on producing newer bronchodilators or combining bronchodilators in the same drug delivery device. With failure of large trials to significantly change mortality in COPD despite enhancement of populations for cardiovascular disease, the use of LAMA/LABA/ICS medications is viewed at one level as an expenditure for quality-of-life improvements, particularly for dyspnea. Therefore, compliance with medication that is used for improving quality of life may fit within the domain of the patient.
The second indication that is outside of the patient domain is to decrease exacerbations that drive cost to both patients and hospital systems. As more health systems generate vertical models that require cost sharing for poor outcomes, the cost of triple-combination medications may be small in the relative cost of COPD. Therefore, the success of UMEC/FF/VI likely resides within health systems. For that reason, the upcoming studies on triple therapy for COPD exacerbation reduction compared to dual therapies are important. Until we can develop disease-modifying therapies for COPD, the inhaler world keeps getting more crowded with combinations of LABA, LAMA, and ICS preparations.
Conclusion
Current studies of the closed triple therapy of UMEC/FF/VI reveal it to be a promising new treatment for COPD patients who are at high risk for exacerbations. Additional studies are underway to further delineate the impact of this therapy on lung function, exacerbation frequency, and quality of life. This drug combination also appears to be safe and well tolerated. If approved for marketing, it will be an important addition to the treatment regimen for COPD, will boost patient compliance, and may decrease the risk of hospitalizations due to COPD exacerbations.
Disclosure
Dr Charlie Strange has current, past, or pending grants in COPD from the Alpha-1 Foundation, CSL Behring, Entera Health, Grifols, Shire, and the NIH. He consults for Abeona, AstraZeneca, CSL Behring, and Grifols, on COPD. Dr Chitra Lal has received grant support from Jazz Pharmaceuticals and Invado Pharmaceuticals and is a consultant for Ikaria Pharmceuticals. The authors report no other conflicts of interest in this work.
References
Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. | ||
Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest. 2000;117(2 Suppl):1S–4S. | ||
Lee H, Kim J, Tagmazyan K. Treatment of stable chronic obstructive pulmonary disease: the GOLD guidelines. Am Fam Physician. 2013;88(10):655–663, 663B–663F. | ||
Singh D, Brooks J, Hagan G, Cahn A, O’Connor BJ. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63(7):592–598. | ||
Aaron SD, Vandemheen KL, Fergusson D, et al; Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555. | ||
Frith PA, Thompson PJ, Ratnavadivel R, et al; Glisten Study Group. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trial. Thorax. 2015;70(6):519–527. | ||
Salmon M, Luttmann MA, Foley JJ, et al. Pharmacological characterization of GSK573719 (umeclidinium): a novel, long-acting, inhaled antagonist of the muscarinic cholinergic receptors for treatment of pulmonary diseases. J Pharmacol Exp Ther. 2013;345(2):260–270. | ||
Harrell AW, Siederer SK, Bal J, et al. Metabolism and disposition of vilanterol, a long-acting β(2)-adrenoceptor agonist for inhalation use in humans. Drug Metab Dispos. 2013;41(1):89–100. | ||
Lötvall J, Bateman ED, Busse WW, et al. Comparison of vilanterol, a novel long-acting beta2 agonist, with placebo and a salmeterol reference arm in asthma uncontrolled by inhaled corticosteroids. J Negat Results Biomed. 2014;13(1):9. | ||
Kelleher DL, Mehta RS, Jean-Francois BM, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of umeclidinium and vilanterol alone and in combination: a randomized crossover trial. PLoS One. 2012;7(12):e50716. | ||
Salter M, Biggadike K, Matthews JL, et al. Pharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L660–L667. | ||
Brealey N, Gupta A, Renaux J, Mehta R, Allen A, Henderson A. Pharmacokinetics of fluticasone furoate, umeclidinium, and vilanterol as a triple therapy in healthy volunteers. Int J Clin Pharmacol Ther. 2015;53(9):753–764. | ||
Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studies. Respir Med. 2015;109(9):1155–1163. | ||
Feldman G, Maltais F, Khindri S, et al. A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 μg compared with tiotropium 18 μg in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:719–730. | ||
Church A, Beerahee M, Brooks J, Mehta R, Shah P. Dose response of umeclidinium administered once or twice daily in patients with COPD: a randomised cross-over study. BMC Pulm Med. 2014;14:2. | ||
GSK. Regulatory update on US filing plans for closed triple combination therapy FF/UMEC/VI in patients with COPD. 2016. Available from: http://www.gsk.com/en-gb/media/press-releases/2016/regulatory-update-on-us-filing-plans-for-closed-triple-combination-therapy-ffumecvi-in-patients-with-copd/. Accessed October 29, 2016. | ||
Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf. 2007;16(4):441–448. | ||
Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831–838. | ||
Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. | ||
Wedzicha JA, Banerji D, Chapman KR, et al; FLAME Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
