Back to Journals » OncoTargets and Therapy » Volume 11
Spotlight on copanlisib and its potential in the treatment of relapsed/refractory follicular lymphoma: evidence to date
Authors Mensah FA, Blaize JP , Bryan LJ
Received 28 February 2018
Accepted for publication 19 June 2018
Published 13 August 2018 Volume 2018:11 Pages 4817—4827
DOI https://doi.org/10.2147/OTT.S142264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Felix A Mensah,1,* Jean-Pierre Blaize,2,* Locke J Bryan1,*
1Division of Hematology/Oncology, Augusta University, Augusta, GA, USA; 2Department of Medicine, Augusta University, Augusta, GA, USA
*These authors contributed equally to this work
Abstract: The importance of the phosphatidylinositol-3-kinase (PI3K) pathway in cell survival and proliferation has made it an attractive target in cancer therapy. The development of small molecule inhibitors for the PI3K pathway continues to provide treatment alternatives across a range of malignancy types. Several agents, including idelalisib, copanlisib and duvelisib, not only inhibit the PI3K pathway, but also have effects on associated mechanisms including the ATK and mTOR pathways. The advent of PI3K-specific small molecular inhibitors has led to increased efficacy with avoidance of an excessive toxicity profile. Key enzymes of the PI3K pathway exhibit differing expression in tissue types and roles in tumor pathogenesis. Copanlisib (BAY 80-6946) is a pan-specific PI3K small molecule inhibitor for four key isoforms with increased activity against PI3Kα and PI3Kδ, both important in B-cell malignancies. Follicular lymphoma is one of the most common indolent B-cell non-Hodgkin lymphomas worldwide. Follicular lymphoma like other indolent B-cell non-Hodgkin lymphomas is beleaguered by high relapse rates and the need for subsequent therapy options. Based on efficacy and a limited toxicity profile, copanlisib received accelerated US Food and Drug Administration approval for the treatment of adult patients with relapsed follicular lymphoma following two lines of therapy. Here, we review the development of copanlisib and the role of this agent in the treatment of follicular lymphoma.
Keywords: copanlisib, follicular lymphoma, PI3K, non-Hodgkin lymphoma, kinase inhibitor
Introduction
Follicular lymphoma (FL) is an indolent non-Hodgkin lymphoma (iNHL) derived from germinal center B cells of both small cleaved (centrocytes) and large non-cleaved (centroblasts) follicular center cells. FL is one of the most common forms of non-Hodgkin lymphoma (NHL) commonly affecting adults in the sixth decade of life worldwide with no specific geographic or racial predilection.1 There are no validated risk factors, though a small number of familial cases have been reported.2 Though the pathogenesis of FL is not well understood, the genetic hallmark is chromosomal translocation t (14:18) (q32;q21) resulting in overexpression of the BCL2 protein. Affected individuals typically present with a long-standing history of asymptomatic waxing and waning peripheral lymphadenopathy of the cervical, axillary, inguinal and femoral regions.3 Bone marrow involvement and widespread disease at diagnosis are a common presentation.4
FL is staged as limited (I–II) or advanced (III–IV) based on the Lugano classification. Tumor grade, which is determined by the number of centroblasts per high-power field, ranges from 1 to 3b based on the World Health Organization classification. Grades 1, 2 and 3a disease is considered an indolent lymphoma, while grade 3b disease is considered a more aggressive tumor resembling diffuse large B-cell lymphoma (DLBCL).5 This evolution of disease is thought to be via clonal evolution with up to 45% of patients experiencing transformation from indolent to aggressive B-cell lymphoma during the course of their disease.6–8 Prognosis is determined by the FL International Prognostic Index (FLIPI) score and the grade of tumor at diagnosis. The FLIPI score helps to risk stratify patients into three different categories – low risk, intermediate risk and high risk – based on the patient’s individual clinical and laboratory findings. Using this index, the FLIPI database predicts a 10-year overall survival rate of 71%, 51% and 36% in low-risk, intermediate and high-risk categories, respectively.9
Treatment of FL initially includes observation until indications for therapy are present based on the Groupe d’Etude des Lymphomes Folliculaires criteria.10 Like other iNHLs, therapy for FL is not considered curative and relapses are expected; hence, observation plays an important role in delaying treatment-related toxicity. In general, therapies for limited stage disease include involved field radiotherapy or immunotherapy (anti-CD20 monoclonal antibodies) ± chemotherapy. Patients who undergo radiation therapy (RT) and chemotherapy for limited stage disease typically have long median survival. In one study, in patients who received RT or combination RT/chemotherapy, the 5-, 10- and 15-year overall survival (OS) was noted to be 93%, 75% and 62%, respectively.11 The advent of rituximab significantly changed treatment approaches in symptomatic FL, as evident by improved progression-free survival (PFS) and OS vs chemotherapy alone, bringing chemoimmunotherapy (chemotherapy plus rituximab) to the frontline.12 Induction therapy response rates as high as 74.7% have been reported.13 Rituximab monotherapy and radioimmunotherapy (an antibody labeled with a therapeutic radionuclide) both remain viable options.14 For advanced stage disease, the therapeutic options include observation or combination chemoimmunotherapy. Patients with advanced age or significant comorbidities deemed ineligible for aggressive therapy may receive immunotherapy alone or radioimmunotherapy. Of note, grade 3b disease is treated following algorithms for DLBCL based on long-term results of FOLL05 trial.15
A majority of patients present with advanced disease at diagnosis, and current guidelines recommend immediate treatment for patients who are symptomatic. Treatment with a rituximab backbone combined with chemotherapeutic agents in the form of CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone), CVP (cyclophosphamide, vincristine and prednisone), fludarabine-containing regimens or bendamustine may be considered.16–18 Initial regimens for both limited and advanced FL are typically chosen based on clinician experience in addition to patient characteristics and are usually followed by a period of interval surveillance. The BRIGHT study for patients with iNHL treated with chemoimmunotherapy showed a 5-year PFS of 65.5% for bendamustine–rituximab (B-R) and 55.8% for R-CHOP/R-CVP.19 Similarly, the median PFS from the StiL NHL1 study in patients with iNHL treated with chemoimmunotherapy was 69.5 months for B-R vs 31.2 months for R-CHOP. The median time to next treatment was not reached for B-R, compared to 56 months for R-CHOP.20 Findings confirmed treatment with B-R reduced the need for next-line therapy; however, a majority of patients went on to need subsequent treatment.
Initial therapy is not curative, and maintenance therapy with either rituximab monotherapy or radioimmunotherapy (ie, ibritumomab tiuxetan) has been shown to improve PFS, but not OS, and patients ultimately relapse.21,22 Treatment for relapsed or refractory disease is based on patient characteristics and the type of response to initial therapy. Treatment options include return to observation, immunotherapy or a chemoimmunotherapy rechallenge with the same or a similar regimen.23–25 Immunotherapy maintenance and consideration of a consolidative stem cell transplantation (either autologous or allogeneic) may be appropriate in select cases.26–28 The need for tolerable and effective treatment options has led to the development of targeted agents in the form of small molecule inhibitors. Studies that determine the mechanisms of pathogenesis in FL have identified the phosphatidylinositol-3-kinase (PI3K) pathway to be of particular interest.29
The PI3K pathway is known to promote cellular survival and play a role in developing resistance to current chemotherapy.3,30 The initial US Food and Drug Administration (FDA) approval of a small molecule PI3K inhibitor for FL was idelalisib, a potent PI3Kδ inhibitor. Idelalisib was approved as a single agent for the treatment of relapsed FL in patients who received at least two prior systemic therapies.31 In the Phase II studies, idelalisib showed an overall response rate (ORR) of 57%, with only 6% reaching a complete response (CR). The median PFS and OS were 11 and 20 months, respectively.32,33 The severe adverse event (AE; grade 3 or 4) profile for idelalisib includes troublesome neutropenia (27%), elevated aminotransferase levels (13%), diarrhea (13%) and pneumonia (7%). Due to these AEs, this drug carries boxed warnings regarding increased risk of fatal and/or serious hepatotoxicity, diarrhea, intestinal perforation, colitis and pneumonitis. Hepatic function should be evaluated prior to and during treatment. Fatal and/or serious infections occurred in 21% of patients on monotherapy. The drug is associated with an increase in opportunistic infections, prompting the manufacturer to recommend prophylaxis for Pneumocystis jirovecii pneumonia (PJP) and monitoring for cytomegalovirus (CMV) reactivation.34
Subsequent to the development of idelalisib, other small molecule inhibitors directed at the differing PI3K isoforms have emerged. Copanlisib (BAY 80-6946) is an intravenous (IV) inhibitor of the PI3K pathway currently undergoing investigation in multiple clinical trials for patients with indolent lymphomas. A Phase II clinical trial, CHRONOS-1, demonstrated promising results in the treatment of relapsed or refractory indolent lymphoma for patients who have received at least two prior systemic therapies.29 Copanlisib was approved by the FDA in September 2017 for the treatment of adult patients with relapsed FL.35 We aim to discuss the mechanism, clinical trial experience and future directions for copanlisib in the treatment of FL.
Pharmacology
Mechanism of action
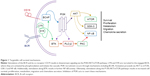
The B-cell receptor is an antigen receptor identified to play a major role in mature B-cell survival. When activated, it leads to downstream signaling pathways including PI3K, protein kinase B (AKT) and mammalian target of rapamycin (mTOR). The PI3K/AKT/mTOR pathways are key players in proliferation, cell survival and angiogenesis (Figure 1). The PI3K pathway is known as one of the most activated signaling pathways in many cancers and has been shown to rescue B-cell receptor–deficient mature B cells, leading to proliferation.36,37 The overexpression of PI3K isoforms has been shown to predict a poor prognosis and is as well a cause for relapse and cancer resistance in B-cell malignancies including FL.38,39
Copanlisib inhibits the catalytic activity of four class 1 enzymes, including the PI3K isoforms PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ with IC50 values of 0.5, 3.7, 6.4 and 0.7 nmol/L, respectively.40 PI3Kα and PI3Kβ have ubiquitous expression across cell types, while PI3Kγ and PI3Kδ are expressed mostly in hematopoietic tissues. PI3Kδ is implicated in B-cell proliferation and survival, while PI3Kα is implicated in relapsed disease.39,41,42 This presents an excellent opportunity for tumor control with PI3K inhibition. Beneficial off-target effects include upregulation of apoptotic pathways and the inhibition of CXCR12 mediated chemotaxis of malignant B cells and NFκB signaling in lymphoma cell lines.43
Pharmacokinetics and pharmacodynamics
In the first human Phase I trial by Patnaik et al, the pharmacokinetics of copanlisib was studied in patients with advanced solid tumors and NHL.44 The maximum tolerated dose (MTD) was determined to be 0.8 mg/kg IV once weekly. The maximum plasma concentration (Cmax) was reached between 0.5 and 1 hour, followed by a rapid multiphasic and then slow decline in the plasma concentration–time profiles for up to 168 hours. Terminal half-life was identified as 38.2 hours. Area under the curve from time 0 to 25 hours showed an increased dose proportionally between 0.1 and 1.2 mg/kg with moderate to high variability. In the study, 93% of patients experienced a predefined pharmacodynamics effect following the first copanlisib infusion. When evaluating for hyperglycemia, copanlisib exposure correlated positively with increasing plasma glucose levels. A second trial reported further pharmacodynamic evidence of on-target modulation along the PI3K pathway via inhibition of pAKT-S473 and p56 in tumors as well as inhibition of pAKT in platelet-rich plasma.45 A dose dependency was observed with improved inhibition using a 0.8 mg/kg dose over a 0.4 mg/kg dose. Based on these findings, a flat dose of 60 mg is the approved recommended dose. Copanlisib is infused weekly as 28-day cycles, 3 weeks on and 1 week off. Dose modifications for toxicity are guided by the drug package insert.35
Adverse events and toxicity
Drug-related AEs and toxicity of copanlisib are similar to other drugs in this pharmacologic category, which includes both hematologic and non-hematologic events as established in the initial Phase I experience.44 In the Phase II trial using copanlisib monotherapy in patients with hematologic malignancies, the most common treatment-related grade ≥3 AE occurring in >10% of patients included hyperglycemia (41%), hypertension (24%), neutropenia (24%) and lung infections (16%).29 Here, we address the common AE and toxicity profile most relevant to clinical practice.35
Hematological toxicities including grade 3 or 4 events related to direct bone marrow suppression with copanlisib therapy have been reported. Leukopenia, neutropenia, lymphopenia and anemia are not uncommon. Approximately 25% of patients receiving copanlisib may experience grade 3 or 4 neutropenia. Weekly blood counts, therapy interruption, dose reduction or discontinuation of treatment may be required to manage effectively. Infections in the range of grade 3 or higher are not uncommon. Pneumonia was the most common infection with rare reports of serious PJP. Monitoring for signs and symptoms of infection and prompt interruption of therapy for grade 3 or higher infections is prudent. PJP prophylaxis is recommended and in confirmed cases, treatment of PJP in addition to interruption of therapy is required. Copanlisib can be resumed once PJP treatment is completed and the infection is cleared.
Copanlisib-induced non-infectious pneumonitis (NIP) has been reported with therapy. Therapy interruption and diagnostic evaluation to rule out infectious causes, followed by administration of systemic corticosteroids as indicated is the preferred approach. Copanlisib may be resumed at reduced dose following resolution of NIP. If there is recurrence of grade 2 NIP or if the patient had grade 3 or higher NIP at diagnosis, therapy should be discontinued.
Hyperglycemia of grade 3 or 4 is common with patients receiving copanlisib monotherapy. Hyperglycemia observed was either immediate (peak at 5–8 hours postinfusion) or delayed (usually 1 day after infusion). In patients with HbA1c <5.7%, there was a 10% incidence of HbA1c >6.5% at the end of copanlisib therapy. Optimal glycemic control prior to and during the duration of therapy is necessary and depending on the severity and persistence of hyperglycemia, treatment interruption, dose adjustment or discontinuation should be considered. Diabetic patients can be treated with copanlisib, provided close monitoring and adequate glucose control are feasible.
Hypertension with grade 3 or higher events has been associated with copanlisib therapy. At least 25% of patients receiving copanlisib monotherapy experienced grade 3 hypertension (systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥100 mmHg). Blood pressure elevations are evident ~2 hours after the start of infusion. Optimal blood pressure control and monitoring prior to and during infusion is required. Dose reduction, therapy interruption or treatment discontinuation may be needed based on the persistence and severity of hypertension.
Dermatological toxicities with copanlisib, including grade 3 and 4, have been well documented. They include maculopapular and exfoliative rash, pruritus and exfoliative dermatitis. Severity and persistence of grade 3 or 4 toxicity should guide the decision to interrupt therapy, reduce dose or discontinue treatment.
Gastrointestinal toxicities such as abdominal pain, anorexia, constipation, diarrhea, elevated liver enzymes, mucositis, nausea and vomiting have been reported.
While it is reasonable to extrapolate the toxicity and AEs of oral PI3K inhibitors to IV copanlisib, a pulled safety analysis has shown otherwise. Combined, the AE profile in Phase I and II studies of patients with relapsed iNHL treated with copanlisib is quite different in comparison to reported data with other agents in the same pharmacologic class. In a poster presented by Zinzani et al, the safety profile of intermittent IV copanlisib was compared to continuous oral PI3K inhibitors, which revealed the most common severe AEs of hyperglycemia and hypertension are mostly self-limiting and transient.46 For copanlisib, there is a low frequency of individual severe AE and little to no evidence for late-onset AEs. Inflammatory AEs including pneumonitis, colitis, and hepatitis associated with oral PI3K inhibitors were infrequent and less severe during treatment with copanlisib.
Efficacy
Preclinical studies
The high incidence of genetic alterations in the P13K pathway across a broad range of cancers has made the development of inhibitors to this pathway of significant clinical interest. PI3K preclinical studies have attempted to identify tumors in which copanlisib therapy would potentially be most beneficial. Early investigations have also determined the pharmacologic properties of this agent.
In the first preclinical studies by Liu et al, the efficacy of copanlisib was investigated in tumor cell lines and xenograft models.36,40 Results showed a cellular selectivity to inhibition of class I PI3K isoforms with no mTOR inhibition. As a selective PI3K inhibitor, copanlisib became an agent of particular interest. Most agents have dual PI3K and mTOR inhibitors, thus copanlisib provided an opportunity to study the efficacy and tumor suppressing capabilities specific to the PI3K pathway. Of the class 1 PI3K isoforms, more selective inhibition was noted in PI3Kα and PI3Kδ.40
In a second preclinical study by Paul et al, 45 FL patients and 45 DLBCL patients were investigated. Expression analysis was used to identify PI3K isoforms in these tumor types.47 PI3Kδ was the dominant isoform in both FL and DLBCL at 87% and 96%, respectively. PI3Kα had a higher expression in DLCBL at 62%, compared to 18% in FL. Expression of PI3Kα was associated with later stage disease in FL and a high FLIPI prognostic score. These expression profiles suggest that a PI3K inhibition that targets both PI3Kα and PI3Kδ isoforms would have potential efficacy in the treatment of NHL.48 The preclinical findings translated into several completed and ongoing clinical trials in hematologic malignancies (Table 1).
Phase I trial experience
In the Phase I trial by Patnaik et al, copanlisib was administered to patients with advanced solid tumors in 28-day cycles.44 In total, 67 patients were enrolled and 57 received treatment. A dose-escalation cohort included 17 patients who received doses of 0.1, 0.2, 0.4, 0.8 and 1.2 mg/kg. Patients received treatment once weekly on days 1, 8 and 15 by 1-hour IV infusions. Multiple dose-limiting toxicities were observed in one patient who was in the 1.2 mg/kg group, resulting in the MTD being defined as 0.8 mg/kg. The study further divided the other patients into expansion cohorts including solid tumors (25 patients), NHL (9 patients) and diabetics (6 patients). For the expansion cohort phase, all patients received the MTD of 0.8 mg/kg. In the NHL expansion cohort, nine patients were followed, including six patients with FL and three with DLBCL. Of the NHL patients, 67% (6/9) had a grade 3 or grade 4 AE, with the most common including hyperglycemia (89%), nausea (78%), hypertension (33%) and diarrhea (33%). Of the six patients with FL, the ORR was 100% with one patient showing CR and five showing partial response. Two FL patients had long-term responses, with one patient on treatment for ~4 years.
A Phase I trial from Japan enrolled ten patients with advanced or refractory solid tumors.49 Patients received either a single IV dose of 0.4 mg/kg (three patients) or 0.8 mg/kg (seven patients) of copanlisib on days 1, 8 and 15 of a 28-day cycle. The mean duration of therapy was 6.2 weeks. Copanlisib was well tolerated and MTD was determined to be 0.8 mg/kg. No patients achieved CR or partial response. Four patients had stable disease and six had progressive disease. The mean time to progression was 52 days. The study did not include patients with lymphoma. The trial, however, confirmed a similar AE profile, with the most common including hyperglycemia, hypertension and constipation.
In a smaller Phase I study by Gerisch et al, six healthy volunteers were observed with similar pharmacokinetics to the above studies.50 All volunteers were healthy males and received a single dose of 12 mg of copanlisib. Using radioactivity, the primary means of elimination of the drug was in the feces (64%) and urine (22%). Half-life of the drug was observed to be 52.1 hours. Of those treated, three volunteers experienced what was defined as a treatment-emergent AE. The intensity of these treatment-emergent AEs was considered mild–moderate with mostly gastrointestinal symptoms, except for one event of myalgia and one event of oropharyngeal pain.
Phase II trial experience
The Phase II trial, CHRONOS-1 (NCT01660451), is an open-label, uncontrolled trial using copanlisib monotherapy in patients with relapsed NHLs. Two peer-reviewed journal manuscripts report maturing data from the trial.29,51 Results from the CHRONOS-1 trial ultimately resulted in FDA approval of copanlisib for relapsed FL.
The first publication is by Dreyling et al in Annals of Oncology, which reports data from Part A of the CHRONOS-1 trial.51 The trial enrolled 33 patients with indolent lymphoma and 51 patients with aggressive lymphoma. Indolent lymphoma subtypes included FL (FL grades 1–3a – 49%), chronic lymphocytic lymphoma (40%), marginal zone lymphoma (MZL – 9%) and small lymphocytic lymphoma (SLL – 3%). Aggressive lymphoma subtypes included DLBCL (30%), mantle cell lymphoma (22%), peripheral T-cell lymphoma (33%), transformed indolent FL (12%) and FL grade 3b (2%). Patients received at least 2 prior therapies, with a median number of 4 for the indolent lymphomas (range 2–10) and 3 for the aggressive lymphomas (range 2–9). The patients were ~50% male/female with a median age of 68 years in the indolent group and 63 years in the aggressive group. Copanlisib was administered IV on days 1, 8 and 15 of a 28-day cycle, with a median treatment duration of 23 weeks in the indolent group and 8 weeks in the aggressive group. The ORR was noted to be 43.7% and 27.1% in the indolent and aggressive groups, respectively. The median PFS was 294 days (0–874 days) in the indolent group and 70 days (0–897 days) in the aggressive group.28 The most common AEs as discussed earlier included hyperglycemia, hypertension, fatigue, diarrhea, neutropenia, nausea and anemia in >30% across all grades. There were ten deaths considered potential drug related, including infections in five patients (meningitis, pneumonia, lower respiratory tract infection, pyelonephritis and septic shock), deterioration in health in two patients and acute respiratory failure, circulatory collapse and progressive disease each in one patient.
The second publication is by Dreyling et al in Journal of Clinical Oncology, which reports data of an additional 142 patients with indolent lymphoma on Part B of the CHRONOS-1 trial.29 Patients enrolled had a median age of 63 years (25–82 years) with relapsed or refractory indolent lymphoma who had received a median of three lines of therapy (range 2–9). Lymphoma subtypes included FL grade 1–3a (73%), MZL (16%), SLL (6%) and lymphoplasmacytic lymphoma (4%). Patients were treated with the same dose and schedule of copanlisib as in Part A. The ORR was 58.5% including 14.1% with CR based on the February 2017 data cutoff occurring 8 months after the primary analysis. The median duration of response was 12.2 months (range 0.03–2.81), median PFS was 11.2 months and median OS had not been reached. The AE profile for all grades in >30% of patients again included hyperglycemia (50%), diarrhea (34%), neutropenia (20%), fatigue (30%) and hypertension (30%). There were two deaths reported secondary to lung infections. The robust efficacy and manageable safety profile in these heavily pretreated patients reaffirmed that copanlisib is an option for treatment of patients with relapsed or refractory iNHL.
Of interest, in the Phase II CHRONOS-1 trial, an ORR of 75.0% was seen in eight patients with SLL and 16.7% in six patients with lymphoplasmacytic lymphoma.29 In a subset analysis of 23 patients with MZL, copanlisib monotherapy showed an impressive ORR of 70% and CR of 13%.52 At 9 months from the time of analysis for this subgroup, in the 85% of patients with a clinical response, the median duration of response had not yet been reached. No mortality or nonfatal opportunistic infections attributable to copanlisib were noted in the MZL cohort. In comparison to the historical clinical experience of current treatment for MZL, further study of copanlisib in this population is warranted.
An active study of relapsed and refractory DLBCL treated with the single agent copanlisib has interim results available from 40 of the planned 67 enrolled patients.53 The median age of patients was 69 with heavily pretreated disease including a median of three prior lines (range 1–13). DLBCL subtypes included 16 activated B cells (ABC), which is historically known as more aggressive and resistant disease. The ORR was 25%, including five patients with CR. Of the ABC subtype patients, the ORR was 37.5%, including four patients with CR. Therapy was well tolerated with the most common AEs including diarrhea, nausea, fatigue and transient hyperglycemia and hypertension. The findings from the trial are encouraging for DLBCL treatment, especially in the ABC subtype.
Phase III trials
There are currently three Phase III trials using copanlisib in indolent lymphomas, including CRONOS-2 (NCT02369016), CHRONOS-3 (NCT02367040) and CHRONOS-4 (NCT02626455). The CHRONOS-2 trial is a randomized protocol evaluating copanlisib vs placebo in patients with rituximab-refractory disease. The trial has completed accrual; however, results are yet to be reported. CHRONOS-3 and CHRONOS-4 are actively recruiting patients on international protocols. The CHRONOS-3 trial is a randomized, double-blinded, placebo-controlled study evaluating copanlisib in combination with rituximab for relapsed disease. The CHRONOS-4 trial is a randomized, double-blinded, controlled study of copanlisib in combination with standard chemoimmunotherapy (R-CHOP or B-R) vs standard chemoimmunotherapy alone for relapsed disease.
Discussion
The PI3K/ATK/mTOR pathway has clear implications in cancer pathogenesis and remains an attractive target for cancer therapeutics. Copanlisib is currently the second FDA-approved PI3K inhibitor, but the first IV formulation in this group. There are a host of agents in development with several currently in Phase II and Phase III trials across a broad range of both hematologic and solid malignancies.54–56 Copanlisib represents a specific PI3K inhibitor that has effects on four isoforms of the PI3K protein. As monotherapy for the treatment of iNHL, copanlisib has shown a high ORR (>50%) including 14% of patients with a CR.29 The published results from clinical trials of copanlisib in hematologic malignancies are summarized in Table 2. Based on the expectation of a limited PFS when treating iNHL, copanlisib appears to be a valuable agent in relapsed or refractory disease. Patients with FL typically require multiple lines of therapy, with a shortened PFS with each new line of therapy. In a study that analyzed PFS in patients who received multiple lines of therapy in FL treatment, it was noted that the median PFS was 4.8, 1.6 and 1 year(s) after the first, second and third line of treatment, respectively.57 In the CHRONOS-1 trial that evaluated patients treated with copanlisib after relapsed or refractory indolent lymphoma after a median of three lines of therapy, the PFS was noted to be 11.2 months. This is equivalent to the PFS of patients treated with three lines of therapy in the study conducted by Alperovich et al.57 Results of ongoing investigations are needed to further investigate OS and support the use of copanlisib in the treatment of iNHL including FL.
The prior approved PI3K inhibitor, idelalisib, has similar clinical responses in comparison to copanlisib.32,33 The most notable difference is the toxicity profile. Idelalisib use carries a risk of inflammatory type reactions including hepatitis, pneumonitis and gastritis. Additionally, idelalisib is associated with reactivation of CMV and severe pulmonary infections related to PJP. Secondary to an increase in death rates, several trials with idelalisib were halted prior to accrual goals. The improvement in toxicity profile established for copanlisib has been consistent across the available clinical trial data. The use of PJP prophylaxis remains a recommendation and clinical vigilance for CMV reactivation. Alternatively, copanlisib is associated with more hyperglycemia and hypertension, for which both are transient.46 The difference in toxicity may be related to continuous exposure with an oral agent vs intermittent dosing with the copanlisib IV formulation.
A third PI3K inhibitor, duvelisib, is an oral agent and dual inhibitor of PI3Kδ and PI3Kγ that has shown positive Phase III results. Duvelisib has been granted priority review for the treatment of patients with relapsed refractory FL and chronic lymphocytic lymphoma/SLL based on results from the Phase II DYNAMO study and the Phase III DUO trial. In the Phase II study, subjects with refractory iNHL showed an ORR of 46% (P<0.001) including 41% in FL. The median PFS and OS for the entire cohort of iNHL were 8.4 and 18.4 months, respectively.58 AEs were mostly grade 1–2. Grade 3 or more AEs, though transient, included hematologic toxicity (neutropenia 23%, anemia 12% and thrombocytopenia 10%) and diarrhea (15%). Six patients died in the trial, including two with severe cutaneous reaction of toxic epidermal necrolysis (TEN) and drug reaction with eosinophilia and systematic symptoms (DRESS). Both FDA-approved PI3K inhibitors, idelalisib and copanlisib, together with duvelisib pending FDA approval, all have similar response expectations, but differing side effect profiles. Approval of duvelisib in this drug category will certainly give more options for combination regimens and other head-to-head trials to optimize the treatment in relapsed refractory FL utilizing the PI3K pathway.
The CHRONOS-2 trial which randomized copanlisib vs placebo in relapsed or refractory iNHL has completed accrual and the results are expected within this year. One may speculate the responses will be similar to earlier clinical trial outcomes and the toxicity profile will be unchanged. In the CHRONOS-2 trial, patients are allowed to receive copanlisib at disease progression; thus, OS will likely appear tempered secondary to crossover from the placebo arm. The CHRONOS-3 and CHRONOS-4 trials explore combinations of copanlisib with rituximab and standard chemoimmunotherapy, respectively. Both trials may represent another shift in standard treatment approaches based on clinical outcomes.
While the PI3K pathway is important in the pathogenesis of FL, this is not the single mechanism of disease. Therefore, copanlisib monotherapy is likely to have limited clinical benefit. The combination of copanlisib with other agents such as cytotoxic chemotherapy may improve clinical outcomes. Results from the CHRONOS-4 trial will provide evidence of these combinations. The high proliferation rate for aggressive lymphomas may require molecular profiling to predict response as well as a combination of cytotoxic agents with copanlisib to derive maximal clinical benefit.59
A major theoretical issue with targeting the PI3K pathway simply lies with the complexity of the cell survival and proliferation mechanisms. The PI3K pathway in association with ATK/mTOR and BTK results in several “escape routes” along the cytokine cascade. Inhibition specific to PI3K could result in alternate pathway signaling, drug resistance or upregulation of alternative driver mechanisms for cell survival.60,61 Combination inhibitors have resulted in increased toxicity profiles that may limit clinical benefit.62 Thoughtful combination of copanlisib with other small molecule inhibitors, immunotherapy and/or chemotherapy will hopefully improve upon clinical outcomes and provide durable response rates.
Future directions
Copanlisib is a small molecule inhibitor under heavy investigation across a broad range of hematologic and solid malignancies. There are currently trials in breast, endometrial, head/neck and cholangiocarcinoma. Investigations for treatment of aggressive NHL include DLBCL, mantle cell lymphoma and peripheral T-cell lymphoma. The FDA approval is currently specific to FL; however, given the high ORR (70%) on a subset analysis of MZL, we foresee a role in treatment for this lymphoma subtype.52 Based on the acceptable toxicity profile, we expect copanlisib will remain an agent of clinical significance. Further investigations as combination therapy will potentially push copanlisib to an earlier line of therapy for the iNHL. We await the results of the CHRONOS-4 clinical trial that combines copanlisib with standard chemoimmunotherapy (B-R or R-CHOP). The lack of autoimmune-type AEs on copanlisib as compared to idelalisib may allow for combination therapy with checkpoint inhibitors including nivolumab or pembrolizumab. Attempts to combine idelalisib with lenalidomide and rituximab proved excessively toxic and both studies were closed.63 Given the differing toxicity profile of copanlisib, the combination could be revisited. The combination of idelalisib with a SYK inhibitor, entospletinib, resulted in high rates of treatment-emergent pneumonitis. Thus, attempts to combine copanlisib with SYK inhibitors may also be problematic.64 Furthermore, research aimed at eliciting the correlation between PI3K expression and response to copanlisib and other PI3K inhibitors will be of immense benefit to therapeutic decision making.
Disclosure
The authors report no conflicts of interest in this work.
References
Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. | ||
Conde L, Halperin E, Akers NK, et al. Genome-wide association study of follicular lymphoma identifies a risk locus at 6p21.32. Nat Genet. 2010;42(8):661–664. | ||
Luminari S, Bellei M, Biasoli I, Federico M. Follicular lymphoma – treatment and prognostic factors. Rev Bras Hematol Hemoter. 2012;34(1):54–59. | ||
Ott G, Katzenberger T, Lohr A, et al. Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood. 2002;99(10):3806–3812. | ||
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. | ||
Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5165–5169. | ||
Bastion Y, Sebban C, Berger F, et al. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol. 1997;15(4):1587–1594. | ||
Montoto S, Davies AJ, Matthews J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25(17):2426–2433. | ||
Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–4562. | ||
Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15(3):1110–1117. | ||
Guadagnolo BA, Li S, Neuberg D, et al. Long-term outcome and mortality trends in early-stage, Grade 1-2 follicular lymphoma treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64(3):928–934. | ||
Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. | ||
Saguna C, Mut ID, Lupu AR, Tevet M, Bumbea H, Dragan C. Immunotherapy with rituximab in follicular lymphomas. Maedica (Buchar). 2011;6(2):100–108. | ||
Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised Phase 3 trial. Lancet Oncol. 2014;15(4):424–435. | ||
Luminari S, Ferrari A, Manni M, et al. Long-term results of the FOLL05 trial comparing R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage symptomatic follicular lymphoma. J Clin Oncol. 2018;36(7):689–696. | ||
Luminari S, Ferrari A, Manni M, et al. Long-term results of the FOLL05 trial comparing R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage symptomatic follicular lymphoma. J Clin Oncol. 2018;36(7):689–696. | ||
Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority Phase 3 trial. Lancet Oncol. 2016;17(1):57–66. | ||
Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, Phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. | ||
Flinn I, van der Jagt R, Chang J, et al. First-line treatment of INHL or MCL patients with BR OR R-CHOP/R-CVP: results of the bright 5-year follow-up study. Hematol Oncol. 2017;35:140–141. | ||
Rummel M, Maschmeyer G, Ganser A, et al. Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: nine-year updated results from the StiL NHL1 study. J Clin Oncol. 2017;35:7501. | ||
Morschhauser F, Radford J, van Hoof A, et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J Clin Oncol. 2013;31(16):1977–1983. | ||
Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a Phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. | ||
Cheson BD, Wendtner CM, Pieper A, et al. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk. 2010;10(1):21–27. | ||
Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a multicenter study. Cancer. 2010;116(1):106–114. | ||
Sehn LH, Goy A, Offner FC, et al. Randomized Phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS study. J Clin Oncol. 2015;33(30):3467–3474. | ||
Khouri IF, Champlin RE. Nonmyeloablative allogeneic stem cell transplantation for non-Hodgkin lymphoma. Cancer J. 2012;18(5):457–462. | ||
Khouri IF, Saliba RM, Erwin WD, et al. Nonmyeloablative allogeneic transplantation with or without 90yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood. 2012;119(26):6373–6378. | ||
Montoto S, Corradini P, Dreyling M, et al. Indications for hematopoietic stem cell transplantation in patients with follicular lymphoma: a consensus project of the EBMT-Lymphoma Working Party. Haematologica. 2013;98(7):1014–1021. | ||
Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905. | ||
Sadeghi N, Gerber DE. Targeting the PI3K pathway for cancer therapy. Future Med Chem. 2012;4(9):1153–1169. | ||
Miller BW, Przepiorka D, de Claro RA, et al. FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res. 2015;21(7):1525–1529. | ||
Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123(22):3406–3413. | ||
Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. | ||
Zydelig (idelalisib) [package insert]. Foster City, CA: Gilead Sciences; 2018. | ||
Aliqopa (copanlisib) [package insert]. Whippany, NJ: Bayer HealthCare; 2017. | ||
Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. | ||
Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. | ||
Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. | ||
Cui W, Cai Y, Wang W, et al. Frequent copy number variations of PI3K/AKT pathway and aberrant protein expressions of PI3K subunits are associated with inferior survival in diffuse large B cell lymphoma. J Transl Med. 2014;12:10. | ||
Liu N, Rowley BR, Bull CO, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319–2330. | ||
Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. | ||
Tzenaki N, Papakonstanti EA. p110δ PI3 kinase pathway: emerging roles in cancer. Front Oncol. 2013;3:40. | ||
Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. | ||
Patnaik A, Appleman LJ, Tolcher AW, et al. First-in-human Phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann Oncol. 2016;27(10):1928–1940. | ||
Morschhauser F, Awada A, Machiels J, et al. Pharmacodynamic study of copanlisib in patients with non-Hodgkin’s lymphoma and advanced solid tumors: confirmation of on-target PI3K inhibitory activity. Blood. 2017;130:1256. | ||
Zinzani PL, Patnaik A, Morschhauser F, et al. Pooled safety analysis from Phase I and II studies for patients with relapsed indolent non-Hodgkin’s lymphoma treated with intravenous copanlisib. Blood. 2017;130:4042. | ||
Paul J, Soujon M, Wengner AM, et al. Simultaneous inhibition of PI3Kδ and PI3Kα induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-κB and AKT. Cancer Cell. 2017;31(1):64–78. | ||
Pauls SD, Lafarge ST, Landego I, Zhang T, Marshall AJ. The phosphoinositide 3-kinase signaling pathway in normal and malignant B cells: activation mechanisms, regulation and impact on cellular functions. Front Immunol. 2012;3:224. | ||
Doi T, Fuse N, Yoshino T, et al. A Phase I study of intravenous PI3K inhibitor copanlisib in Japanese patients with advanced or refractory solid tumors. Cancer Chemother Pharmacol. 2017;79(1):89–98. | ||
Gerisch M, Schwarz T, Lang D, et al. Pharmacokinetics of intravenous pan-class I phosphatidylinositol 3-kinase (PI3K) inhibitor [14C]copanlisib (BAY 80-6946) in a mass balance study in healthy male volunteers. Cancer Chemother Pharmacol. 2017;80(3):535–544. | ||
Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169–2178. | ||
Dreyling M, Panayiotidis P, Egyed M, et al. Efficacy of copanlisib monotherapy in patients with relapsed or refractory marginal zone lymphoma: subset analysis from the CHRONOS-1 trial. Blood. 2017;130:4053. | ||
Lenz G, Verhoef G, Haioun C, et al. Phase II study of single-agent copanlisib in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). J Clin Oncol. 2017;35:7536. | ||
Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: from laboratory to patients. Cancer Treat Rev. 2017;59:93–101. | ||
Lampson BL, Brown JR. PI3Kδ-selective and PI3Kα/δ-combinatorial inhibitors in clinical development for B-cell non-Hodgkin lymphoma. Expert Opin Investig Drugs. 2017;26(11):1267–1279. | ||
O’Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103. | ||
Alperovich A, Batlevi C, Smith K, et al. Benchmark of progression free survival for multiple lines of therapy in follicular lymphoma treated in the rituximab era. Blood. 2016;128(22):2955. | ||
Flinn IW, Miller BW, Ardeshna KM, et al. DYNAMO: a Phase 2 study demonstrating the clinical activity of duvelisib in patients with refractory indolent non-Hodgkin lymphoma. Blood. 2016;128(22):1218. | ||
Pons-Tostivint E, Thibault B, Guillermet-Guibert J. Targeting PI3K signaling in combination cancer therapy. Trends Cancer. 2017;3(6):454–469. | ||
Le X, Antony R, Razavi P, et al. Systematic functional characterization of resistance to PI3K inhibition in breast cancer. Cancer Discov. 2016;6(10):1134–1147. | ||
Tan J, Yu Q. Molecular mechanisms of tumor resistance to PI3K-mTOR-targeted therapy. Chin J Cancer. 2013;32(7):376–379. | ||
Lorusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34(31):3803–3815. | ||
Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 Phase 1 trials. Lancet Haematol. 2017;4(4):e176–e182. | ||
Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127(20):2411–2415. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.