Back to Journals » Clinical Interventions in Aging » Volume 18
Sports Participation Promotes Beneficial Adaptations in the Erythrocyte Guanylate Nucleotide Pool in Male Athletes Aged 20–90 Years
Authors Pospieszna B , Kusy K , Slominska EM, Ciekot-Sołtysiak M , Zieliński J
Received 8 March 2023
Accepted for publication 25 May 2023
Published 22 June 2023 Volume 2023:18 Pages 987—997
DOI https://doi.org/10.2147/CIA.S406555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Barbara Pospieszna,1 Krzysztof Kusy,1 Ewa Maria Slominska,2 Monika Ciekot-Sołtysiak,1 Jacek Zieliński1
1Department of Athletics, Strength and Conditioning, Poznan University of Physical Education, Poznan, Poland; 2Department of Biochemistry, Medical University of Gdansk, Gdansk, Poland
Correspondence: Barbara Pospieszna, Department of Athletics, Strength and Conditioning, Poznan University of Physical Education, Królowej Jadwigi 27/39, Poznan, 61-871, Poland, Tel +48-618-355-270, Email [email protected]
Introduction: The guanine nucleotide pool (GTP, guanosine-5’-triphosphate; GDP, guanosine-5’-diphosphate, and GMP, guanosine-5’-monophosphate) is an essential energy donor in various biological processes (eg protein synthesis and gluconeogenesis) and secures several vital regulatory functions in the human body. The study aimed to predict the trends of age-related changes in erythrocyte guanine nucleotides and examine whether competitive sport and related physical training promote beneficial adaptations in erythrocyte guanylate concentrations.
Methods: The study included 86 elite endurance runners (EN) aged 20– 81 years, 58 sprint-trained athletes (SP) aged 21– 90 years, and 62 untrained individuals (CO) aged 20– 68 years.
Results: The concentration of erythrocyte GTP and total guanine nucleotides (TGN) were highest in the SP group, lower in the EN group, and lowest in the CO group. Both athletic groups had higher guanylate energy charge (GEC) values than the CO group (p = 0.012). Concentrations of GTP, TGN, and GEC value significantly decreased, while GDP and GMP concentrations progressively increased with age.
Conclusion: Such a profile of change suggests a deterioration of the GTP-related regulatory function in older individuals. Our study explicitly shows that lifelong sports participation, especially of sprint-oriented nature, allows for maintaining a higher erythrocyte guanylate pool concentration, supporting cells’ energy metabolism, regulatory and transcription properties, and thus more efficient overall body functioning.
Keywords: guanosine triphosphate, GTP, guanylate energy charge, GEC, purine metabolites, endurance, sprint, master athletes
Introduction
Guanylate nucleotides are one of the four purine nucleotides, key biomolecules of the cell, required for a variety of life processes, including growth and replication. Their higher level secures each cell when under stress or constantly changing environmental conditions; eg during physical activity. As the de novo synthesis of the purine nucleotides does not occur in erythrocytes (RBC), these cells depend exclusively on salvage reactions: free purine bases−guanine and hypoxanthine−attach to phosphoribosyl pyrophosphate (PRPP) and form purine nucleoside monophosphates.1 The formation of guanosine-5’-monophosphate (GMP) from guanine and inosine monophosphate (IMP) from hypoxanthine is catalyzed by hypoxanthine-guanine phosphoribosyltransferase (HGPRT).2 Unlike in other cells, in matured erythrocytes, IMP serves as a precursor only of GMP, which can later be transformed in phosphorylation reactions to guanosine diphosphates (GDP) and triphosphates (GTP).3 Under normal conditions−due to the balanced activity of guanylate kinases and catabolic enzymes−the guanine nucleotide pool (TGN = [GTP] + [GDP] + [GMP]) tends to be constant. This equilibrium assures the maintenance of a sufficiently high phosphorylation potential − the guanylate energy charge (GEC) of the cell.4
Because of the highest contribution in the erythrocytes’ free nucleotides, adenylates hold the leading role in securing the erythrocyte’s energy needs. Yet, the guanine nucleotide pool is essential as an energy donor in a more selected group of biological processes and it secures some vital regulatory functions. First of all, guanylates are used as a source of energy for protein synthesis and gluconeogenesis.5,6 They are the activated precursors of nucleic acids, and they serve as allosteric regulators and energy sources during the transcription process of RNA synthesis.7–10 GTP is also essential for the growth and functioning of the cell cytoskeleton and endoplasmic reticulum.11,12 Finally, guanylates with GTP-binding proteins are essential components of signal-transduction pathways (in second-messenger mechanisms).5,13,14
Available publications related to guanine nucleotides cover two main fields of interest. The early scientific studies on RBC guanine nucleotides focused on depicting guanylate-dependent metabolic pathways and functions15,16 and were concerned with the states of guanine nucleotide depletion. They showed that the most often causes of guanylate deficiencies are of enzymatic origin: HGPRT and purine nucleoside phosphorylase (PNP) deficiency or phosphoribosylpyrophosphate synthetase (PRPP synthetase) superactivity.1 Later, scientific interest evolved into broad clinical studies on cellular Inosine-5′-monophosphate dehydrogenase (IMPDH) activity, as it was found to be a progression-linked key enzyme in tumorigenesis.17 Other recent papers on guanine nucleotides relate rather to deeper explaining their roles in temporal and spatial determination of specific cell functions, eg gene transcription modulation, protein synthesis, vesicle trafficking, cytoskeletal organization, and nucleocytoplasmic transport.8,10,11,14 Yet, to our best knowledge, nothing is known about the concentration of guanine nucleotides and their metabolites in aging humans or under a life-long sports training regime.
Prolonged participation in sports activities is known to improve blood hemorheology, usually manifested by increased blood volume, improved blood composition (higher quantity of reticulocytes), and higher hemoglobin concentration.18–21 The sports adaptation concerning the erythrocyte count and the rate of their senescence is multifaceted. During high-intensity efforts, states of hyperthermia, hypoglycemia, local hypoxia, or elevated oxidative stress frequently occur, causing significant imbalances of internal homeostasis and, in consequence, increased post-exercise hemolysis and future elevated erythropoiesis.18,20,22–24 On the other hand, substantial sport-related modifications of erythrocyte membrane properties21,25 induce further increment in erythrocyte turnover and thus result in a higher proportion of younger erythrocytes – known for greater osmotic resistance, deformation capability, oxygen affinity, a higher glycolysis efficiency, and better antioxidant protection compared to older ones.26 Significant age distribution modifications of the RBC population20,27 lead to more efficient erythrocyte metabolism and higher energy status in trained individuals compared to recreationally trained and sedentary ones.20,28,29 All elite athletes reveal beneficial adaptations in erythrocyte profile, yet most probably, the diverse types of training influence it differently. The main distress in endurance-trained athletes is the inadequate oxygen-carrying capacity and the post-exercise hemolysis (increased erythrocyte turnover is its direct consequence), while in sprinters, it is the need for opposing to repetitive oxidative stress and ischemia-reperfusion syndrome, therefore they develop greater osmotic resistance and superior antioxidant protection.30 Nevertheless, the baseline of all adaptation changes is the energy that is required by all living cells, which eventually form tissues and organelles of our body.
Because of higher concentrations in the erythrocyte and much more pronounced influence on RBC energetics, adenylates address much more scientific interest than guanylates. In our previous study, conducted on a limited number of young adults, we showed that neither a single exercise test nor a one-year training cycle (either professional or recreational) cause significant changes in the concentration of guanine nucleotides in human erythrocytes.29 It is still not reported, however, how guanylate concentrations change over longer periods in people of different sports involvement. The results of our other study show a significant decreasing relationship between age and the concentrations of erythrocyte adenylate metabolites, except for ADP and AMP concentrations, which tend to increase with advancing age.31 In our earlier study, we showed an age-related increase in plasma concentration of hypoxanthine, xanthine, and uric acid suggesting a significant depletion of the nucleotide pool with advancing age.32 At the same time, we have shown that, in highly trained athletes, the activity of erythrocyte hypoxanthine-guanine phosphoribosyltransferase increases both in a one-year training cycle29 and an age-related manner.32 These observations indicate that participation in specialized sports training adapts the body to support the salvage pathway of the cells’ adenylate pool restoration.
Therefore, this study aimed to estimate the predicted trends of age-related changes in erythrocyte guanylate nucleotides and examine whether life-long physical training promotes adaptive changes in erythrocyte guanylate concentrations. Based on our previous findings, and that erythrocyte tends to keep the equilibrium between the concentration of adenylate and guanylate nucleotides and their energy charges5 we hypothesized that i) the direction of age-related changes of erythrocyte guanylates is similar in trained and untrained individuals, and that ii) the athletic groups–regardless of age–have higher concentrations of erythrocyte guanylate nucleotides than controls.
Materials and Methods
The study included one hundred forty-four healthy non-smoking men between 20 and 90 years of age divided into 2 athletic groups: 86 endurance runners (EN) aged 20–81 years and 58 sprint-trained athletes (SP) aged 21–90 years. Sixty-two untrained men, aged 20–68 years, were in the control group (CO). The exclusion criteria were (i) reported history of cardiovascular or cardiopulmonary-related disease, (ii) inability to run, (iii) medications affecting circulatory function, (iv) abnormal erythrocyte count and hemoglobin content, and (v) any pathological states known to be characterized by a significantly elevated adenylate or guanylate pool, eg sickle cell disease, renal insufficiency, diabetes, leukemia, tuberculosis, or sepsis. We did not include females in the study, which is a limitation of this research. Before the study, all study participants underwent an initial clinical examination.
In athletic groups, there were elite athletes of two origins. Sportsmen under 35 years of age were part of the Polish national track and field team and this study was part of a routine periodical physiological evaluation performed in our Laboratory. Older athletes (masters European and world championships athletes, ranked 1–10 in their age categories) participated voluntarily. All examinations were performed during the competition phase of the annual training cycle. There were no significant differences in athletes’ training history: EN athletes participated in competitive sport for 26.7 ± 14.4 years, ranging from 6 to 66 years depending on age (0.92, p < 0.001), and SP athletes for 28.6 ± 18.8 years, ranging from 7 to 77 years depending on age (r = 0.93, p < 0.001). There were also no significant differences in athletes’ present training volume (mean number of weekly training hours during the last year) between the athletic groups. EN trained weekly for 9.1 ± 3.5 h · week−1 (range: 5‒18.5 h · week−1 depending on age; r = −0.73, p < 0.001) and SP athletes for 8.7 ± 3.2 h · week−1 (range: 5‒15 h · week−1 depending on age; r = −0.70, p < 0.001).
Controls were not specifically trained. In line with the World Health Organization recommendations,33 they regularly implemented recreational forms of physical activity (eg, Nordic walking, swimming, or group gymnastics) in their free time, up to 150 min of moderate-intensity physical activity per week. They were recruited to the study via local mass media or through personal communications.
All testing procedures were performed in the LaBthletics Human Movement Laboratory of the Poznan University of Physical Education (Poznań, Poland). Each participant submitted his written consent to participate after he had been informed of the purpose and risks of the study, and the testing procedures. The protocols used in this study were approved by the Ethics Committee at the Karol Marcinkowski Poznan University of Medical Sciences and align with the Declaration of Helsinki. The study was registered in the clinical trials registry under the trial registration number: NCT05113914.
Participants were instructed to not participate in any intense training for at least one day before testing. Testing was performed in the morning hours after a 12-hour overnight fast and started with the anthropometric measurements using a digital stadiometer (SECA 285, Hamburg, Germany). Body mass index (BMI) was calculated by dividing body mass (kg) by height squared (cm2). During the examination, to minimize measurement error, subjects wore only their underwear.
A running incremental test was performed on a mechanical treadmill (h/p/cosmos Sports & Medical GmbH, Nussdorf–Traunstein, Germany) to determine maximal oxygen uptake (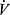 O2max). After the introductory part, comprising 3 min free-standing and 3 min walking at a constant speed of 4 km · h−1, the treadmill speed was set to 8 km · h−1 and every 3 min increased progressively by 2 km · h−1 until volitional exhaustion. An ergospirometer (MetaMax 3B-R2, Cortex Biophysics GmbH, Leipzig, Germany) was used to measure respiratory parameters. MetaSoft Studio v. 5.1.0 Software (Cortex Biophysik, Leipzig, Germany) was later used to analyze the data. Polar Bluetooth Smart H6 (Polar Electro Oy, Kempele, Finland) device was used to monitor heart rate (HR, beat · min−1) during the test. The distance completed during the test (m) was noted.
O2max). After the introductory part, comprising 3 min free-standing and 3 min walking at a constant speed of 4 km · h−1, the treadmill speed was set to 8 km · h−1 and every 3 min increased progressively by 2 km · h−1 until volitional exhaustion. An ergospirometer (MetaMax 3B-R2, Cortex Biophysics GmbH, Leipzig, Germany) was used to measure respiratory parameters. MetaSoft Studio v. 5.1.0 Software (Cortex Biophysik, Leipzig, Germany) was later used to analyze the data. Polar Bluetooth Smart H6 (Polar Electro Oy, Kempele, Finland) device was used to monitor heart rate (HR, beat · min−1) during the test. The distance completed during the test (m) was noted.  O2max was expressed in mL·kg−1·min−1 (body mass-adjusted values). At least three of the following criteria needed to be achieved: (i) a plateau in
O2max was expressed in mL·kg−1·min−1 (body mass-adjusted values). At least three of the following criteria needed to be achieved: (i) a plateau in 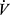 O2 despite an increase in running speed, (ii) blood lactate (LA) concentration ≥ 9 mmol/L, (iii) respiratory exchange ratio ≥ 1.10, and (iv) HR ≥ 95% of the age-predicted maximum.34
O2 despite an increase in running speed, (ii) blood lactate (LA) concentration ≥ 9 mmol/L, (iii) respiratory exchange ratio ≥ 1.10, and (iv) HR ≥ 95% of the age-predicted maximum.34
Resting venous blood samples were drawn from an antecubital vein into two separate tubes with EDTA (2.7 mL) and lithium heparinate (4.9 mL) as anticoagulants (S-monovette, Sarstedt, Nümbrecht, Germany) using the peripheral venous catheter 1.3×32 mm (BD Venflon Pro, Becton Dickinson, Helsingborg, Sweden). From the EDTA tube 10 μL of blood was aspirated for the determination of hemoglobin concentration, mean corpuscular hemoglobin concentration (MCHC), and hematocrit (HCT) value. The 18-parametric automated hematology analyzer Mythic® 18 (Orphée, Geneva, Switzerland) was used. Within 3 min after drawing a sample the whole blood in the lithium heparinate tube was centrifuged (5000 rpm, 5 min, 37°C; Universal 320 R centrifuge, Hettich Lab Technology, Tuttlingen, Germany). After centrifugation, the plasma and buffy coat were removed, and remaining erythrocytes were washed (buffered 0.9% NaCl solution) and centrifuged (3000 rpm, 5 min, 4°C) three times. Finally, the erythrocyte pellet, with a small volume of PBS, was immediately deep-frozen at −80°C until the later analysis of erythrocyte nucleotides concentration. A detailed erythrocyte purine nucleotides sampling procedure and analysis are described in our other paper.29 All measurements, according to the method described by Slominska et al,35 were performed using high-performance liquid chromatography (HPLC) with UV-VIS detection (Merck-Hitachi/Agilent, Japan/USA). The values of total guanine nucleotide pool and guanylate energy charge were later calculated according to the following formulas: TGN = GTP + GDP + GMP and GEC = ([GTP]+0.5 [GDP])/([GTP]+ [GDP]+ [GMP]).4
Directly before and three minutes after the running test, an additional 20 μL of capillary blood was taken from fingertip and used for lactate (LA) concentration assessment, performed with the use of a spectrophotometric enzymatic method (Biosen C-line, EKF Diagnostics, Barleben, Germany).
Statistica 13.3 software (StatSoft, Inc., Tulsa, OK) was used for statistical analyses. To ensure at least a medium effect size the sample size was a priori estimated using an α-level of 0.05 and the statistical power (1-β) of 0.80. It was calculated, that to detect significant differences and correlations (G*Power software; Heinrich-Heine-Universität, Düsseldorf, Germany) there is a need for at least 36 participants in a single group. All data were tested for normality of distribution using the Shapiro–Wilk test. As the requirements for normal distribution were met further analyses were performed with the use of parametric tests. The linear regression analyses were performed to estimate longitudinal relationships between erythrocyte purine metabolites and participant’s age. The slopes of regression lines (regression equations) were calculated to predict the cross-sectional rates of change of dependent variables with age. The determination of differences between groups was performed with the use of a one-way analysis of variance (ANOVA) with a post-hoc Bonferroni test. The equal slopes test with Bonferroni post-hoc test was used to determine differences between the slopes of the regression lines. Differences were considered significant with p < 0.05. The lowest statistical power of ANOVA analyses was noted for GDP/GMP ratio (0.15) and IMP and age (0.16). For other descriptive and exercise characteristics it varied from 0.61 (HCT) to 1.0 (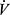 O2max, and covered distance). Similarly for other erythrocyte guanine nucleotides, the statistical power of ANOVA analyses varied from 0.57 for GTP/GDP and GDP/GMP ratios to 1.0 for GTP and TGN. Effect sizes for ANOVA were calculated using η2 and defined as small (0.01), medium (0.06), and large (0.14).
O2max, and covered distance). Similarly for other erythrocyte guanine nucleotides, the statistical power of ANOVA analyses varied from 0.57 for GTP/GDP and GDP/GMP ratios to 1.0 for GTP and TGN. Effect sizes for ANOVA were calculated using η2 and defined as small (0.01), medium (0.06), and large (0.14).
Results
All data are expressed as mean ± standard deviation (SD). Table 1 presents the somatic, hematological, and exercise characteristics of study participants. Of the somatic variables, only the mean age was not significantly different between groups. Concerning the hematological indices, there were no differences between the athletic groups, but the concentration of hemoglobin and mean corpuscular hemoglobin differed between athletes and controls. Maximal oxygen uptake and the distance covered during the exercise test differed significantly between groups.
 |
Table 1 Somatic, Hematological, and Exercise Characteristics of Endurance Runners (EN; n = 86), Sprinters (SP; n = 58), and Controls (CO; n = 62) |
The erythrocyte concentration of guanine nucleotides, inosine monophosphate, total guanine nucleotides, and guanylate energy charge is shown in Table 2. The concentration of most analyzed indices was highest in the SP group compared to EN, and CO group. Only the concentration of erythrocyte GTP differed significantly between all studied groups. Significant differences between the athletic groups were found only in guanine nucleotides (GTP, GDP, and GMP) and total guanylate pool.
 |
Table 2 Erythrocyte Concentration of Guanine Nucleotides, Inosine Monophosphate, and Guanylate Energy Charge in Endurance Runners (EN; n = 86), Sprinters (SP; n = 58), and Controls (CO; n = 62) |
In all three groups, a significant decrease with age was observed in the majority of variables. The medium effect size was observed for hemoglobin (r2 = 0.23–0.27), while the large effect size of age-related decline (p ≤ 0.001) was achieved for 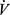 O2max and distance covered during the exercise test (r2 = 0.62–0.82).
O2max and distance covered during the exercise test (r2 = 0.62–0.82).
In both athletic groups, linear relationships between age and the resting concentrations of erythrocyte guanylates, all except the GDP/GMP ratio, were significant (Figures 1 and 2). In the control group, the age-related change in concentration of IMP and TGN was not significant. The highest coefficients of determination (r2 > 0.54) were observed for GTP, GTP/GDP ratio, and GEC in all studied groups, and additionally for GDP and IMP in sprinters. The smallest coefficients of determination (r2 < 0.25) were shown for TGN in both athletic groups, IMP in EN, and GMP in the CO group.
The predicted rates of age-related changes in concentration of erythrocyte guanylate metabolites are presented in Table 3. In all three groups, the highest value of cross-sectional decline was shown for the GTP/GDP ratio and the highest increase for erythrocyte GDP concentration. Bonferroni post-hoc test revealed the significant differences between the slopes of the regression lines only for GTP (between all groups), TGN (EN vs SP and SP vs CO), and GEC (athletes vs CO).
Discussion
The metabolic and energetic efficiency of each cell is the key element supporting effective human functioning at all ages. Yet, it still is discussed how to accelerate or at least sustain that efficiency over a lifespan. Increased rate of physical activity is one of the proven methods to achieve this goal, however, there are various uncertainties on what kind of physical training is most beneficial in this regard. Our study was designed to analyze the changes in erythrocyte guanine nucleotide concentrations and the guanylate energy charge in men aged 20‒90 years, representing different levels of physical activity and sports specialization. Across analyzed ages, the significant decrements in concentration of GTP and TGN, as well as of GEC value show a substantial similarity between athletes and controls. Such a trend confirms the lower GTP-related regulatory functions in older individuals. The significantly higher concentrations of guanylate nucleotides, along with the equal level of GEC in SP compared to EN, strongly suggests that sprint-oriented training is more efficient in preserving guanylate-related erythrocyte functions across a very wide age range.
Physiological functional capacity declines in an age-dependent manner and is connected with the deterioration in both physical and cognitive capacity.36–39 To present certain aging-related changes scientists frequently use a simple biological model – the red blood cell. It is well documented that during aging erythrocytes undergo many detrimental changes linked with their structure and functions, which directly impacts their morphology, membrane deformability, and oxidation ability.25,40–43 Recent studies on blood metabolomics41,44 showed that with advancing age several metabolic markers of oxidative stress, inflammation, and immunosenescence experience important alterations. These were especially associated with the glycolytic pathway, the nucleotide metabolism, and the glutathione metabolism of erythrocytes. When Chaleckis et al41 determined the coefficients of variation (CV30) of erythrocyte GTP and GDP in two age groups to be at low levels (<0.4) he suggested that those guanylate metabolites, not only support fundamental erythrocyte functions, but their concentration in erythrocytes might be a good health check indicator.
In this study, we reported that the concentrations of all erythrocyte guanylate metabolites–except for GDP and GMP–significantly decrease with advancing age, with no matter of sports involvement. We have previously confirmed that adenylates exhibit similar changes.31 Both of these findings support the observation of decreasing energy potential of the red blood cell during healthy aging. It appears that the most probable cause of this tendency is the age-dependent increased efflux of nucleotides from red blood cells.29,31,32 Theoretically, the observed decreased concentration of guanylate nucleotides in erythrocytes could be connected with higher levels of oxidative stress, distinctive in the elderly population.45 However, the slow progressive decline of antioxidant status in healthy free-living older society, showed in the ZENITH study, concerned European free-living elderly people, not elite athletes like in our study. Moreover, the results of our research rather exclude such a possibility. Tavazzi et al46 when studying the influence of progressing oxidative stress on energy metabolism in erythrocytes proved that while the concentration of adenosine-5’-triphosphate (ATP) and GTP gradually decreases, the IMP level significantly increases due to intensive and unexpected AMP-deaminase activation. The participants in our study were only healthy males and besides the decreased concentration of guanylate nucleotides in erythrocytes the level of IMP was unchanged in the control group and even decreased in elite runners.
However, despite the direction and magnitude of the predicted age-related changes of erythrocyte guanylate nucleotides seeming very similar in all tested groups, there are significant differences between the slopes of the regression lines for GTP, TGN, and GEC. The rate of decline of those variables is lowest in CO group, which is most likely related to the lowest baseline values in controls. We have shown that in sprinters the baseline level of erythrocyte GTP and TGN is the highest of all study participants and that the rate of their decline with aging is smaller compared to endurance runners. Therefore, it suggests that sprint-oriented training is more beneficial for maintaining high erythrocyte guanylate energy potential throughout the lifespan and that it seems more beneficial to prescribe to senior citizens shorter and more intensive exercises.
In a recent study on metabolomics Schranner et al47 based on the analysis of 151 serum metabolite concentrations, proved that sprinters, endurance athletes, bodybuilders, and untrained controls differ significantly in metabolite profiles, both at rest and after maximal exercise. Also, in our previous studies, we demonstrated that the concentration of adenylates is significantly higher in elite athletes compared to recreationally trained controls. The significantly higher levels of ATP in athletes, especially in a sprint-oriented training regime, were noted at rest, in single maximal exercise, and during recovery in all key phases of the annual training cycle, when measured in erythrocytes29 and venous plasma.48,49 This study complemented those findings, as we demonstrated that the level of GTP and erythrocyte GEC, similarly, tend to be higher in well-trained athletes compared to less active controls. In our other study, we showed an age-related increase in plasma concentration of hypoxanthine and xanthine–regardless of sports engagement level–suggesting a significant depletion of the nucleotide pool with advancing age.32 At the same time, we have shown a significant difference between more and less active study participants in the level of erythrocyte hypoxanthine-guanine phosphoribosyltransferase activity.29,32 Importantly, only in elite athletes (not in controls) did the activity of erythrocyte HGPRT increase with age32 and during the consecutive phases of the one-year training cycle.29,50,51 These observations indicate that participation in specialized sports training adapts the body to support the salvage pathway of the purine pool in the cell.
The highest levels of erythrocyte guanine nucleotides and TGN across the whole analyzed age range, observed in sprinters, suggest a higher energetic potential of the guanylate pool in speed-power athletes. This might be connected with the higher sprinters’ need for protein synthesis, as the direct source of energy for protein production is GTP, rather than ATP.11,52 It is most likely that sprint-oriented training, consisting of systematic high-intensity sprints, stimulates the greater need for cellular adaptation towards higher rapid energy requirements and later protein synthesis, and therefore, gives an advantage in hypertrophic muscle changes and muscle mass preservation in older sprinters.53,54
Conclusion
In conclusion, our study demonstrated that athletes have significantly higher concentrations of erythrocyte GTP and guanylate energy charge than control individuals across a wide age range, even though the rate of the predicted age-related changes is smallest in the CO group. Our findings suggest that sports participation supports erythrocyte energetics preservation, which secures other beneficial functional adaptations in adult and elderly humans. Moreover, it appears that sprint-oriented training is more efficient in the preservation of higher erythrocyte guanylate concentrations.
Acknowledgments
The authors thank the Polish national team coaches (Tadeusz Osik, Paweł Barszowski, Filip Przymusiński), and all athletes for effective collaboration.
Funding
National Science Centre in Poland funded this research (grant no. DEC-2013/09/B/NZ7/02556).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Giacomello A, Salerno C. Possible Metabolic Basis for GTP Depletion in Red Cells of Patients with PRPP Synthetase Superactivity. Adv Exp Med Biol. 1991;253–256. doi:10.1007/978-1-4615-7703-4_56
2. Simmonds HA, Fairbanks LD, Morris GS, Webster DR, Harley EH. Altered erythrocyte nucleotide patterns are characteristic of inherited disorders of purine or pyrimidine metabolism. Clin Chim Acta. 1988;171(2):197–210. doi:10.1016/0009-8981(88)90145-3
3. Carlucci F, Tabucchi A, Pagani R, Marinello E. Synthesis of adenine and guanine nucleotides at the ‘inosinic branch point’ in lymphocytes of leukemia patients. Biochim Biophys Acta - Mol Basis Dis. 1999;1454(1):106–114. doi:10.1016/s0925-4439(99)00032-0
4. Atkinson DE, Fall L. Adenosine triphosphate conservation in biosynthetic regulation. J Biol Chem. 1967;242(13):3241–3242. doi:10.1016/s0021-9258(18)95957-0
5. Berg JM, Tymoczko JL, Gatto GJ, Stryer L. Biochemistry.
6. Rupniak HTR, Quincey RV. Small changes in energy charge affect protein synthesis in reticulocyte lysates. FEBS Lett. 1975;58(1–2):99–101. doi:10.1016/0014-5793(75)80234-1
7. Wilden B, Savelsbergh A, Rodnina MV, Wintermeyer W. Role and timing of GTP binding and hydrolysis during EF-G-dependent tRNA translocation on the ribosome. Proc Natl Acad Sci U S A. 2006;103(37):13670–13675. doi:10.1073/pnas.0606099103
8. Li W, Liu Z, Koripella RK, Langlois R, Sanyal S, Frank J. Activation of GTP hydrolysis in mRNA-tRNA translocation by elongation factor G. Sci Adv. 2015;1(4):e1500169. doi:10.1126/sciadv.1500169
9. Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. The Mechanism for Activation of GTP Hydrolysis on the Ribosome. Science. 2010;330(6005):835–838. doi:10.1126/science.1194460
10. Rodnina MV, Peske F, Peng B-Z, Belardinelli R, Wintermeyer W. Converting GTP hydrolysis into motion: versatile translational elongation factor G. Biol Chem. 2019;401(1):131–142. doi:10.1515/hsz-2019-0313
11. Hesketh A, Oliver SG. High-energy guanine nucleotides as a signal capable of linking growth to cellular energy status via the control of gene transcription. Curr Genet. 2019;65(4):893–897. doi:10.1007/s00294-019-00963-1
12. Roostalu J, Thomas C, Cade NI, Kunzelmann S, Taylor IA, Surrey T. The speed of GTP hydrolysis determines GTP cap size and controls microtubule stability. eLife. 2020;9. doi:10.7554/elife.51992
13. Rodbell M, Schlegel W. The Role of GTP in the Coupling of Hormone Receptors and Adenylate Cyclase. In: Fuxe K, Hökfelt T, Luft R, editors. Central Regulation of the Endocrine System. New York: Plenum Press; 1979:71–83.
14. Takai Y, Sasaki T, Matozaki T. Small GTP-Binding Proteins. Physiol Rev. 2001;81(1):153–208. doi:10.1152/physrev.2001.81.1.153
15. Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980;284(5751):17–22. doi:10.1038/284017a0
16. Bergamini CM. GTP modulates calcium binding and cation-induced conformational changes in erythrocyte transglutaminase. FEBS Lett. 1988;239(2):255–258. doi:10.1016/0014-5793(88)80928-1
17. Naffouje R, Grover P, Yu H, et al. Anti-Tumor Potential of IMP Dehydrogenase Inhibitors: a Century-Long Story. Cancers. 2019;11:1346. doi:10.3390/cancers11091346
18. Hu M, Lin W. Effects of Exercise Training on Red Blood Cell Production: implications for Anemia. Acta Haematol. 2012;127(3):156–164. doi:10.1159/000335620
19. Ciekot-Sołtysiak M, Kusy K, Podgórski T, Zieliński J. Training-induced annual changes in red blood cell profile in highly-trained endurance and speed-power athletes. J Sports Med Phys Fitness. 2018;58(12):1859–1866. doi:10.23736/s0022-4707.17.07819-7
20. Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front in Physiol. 2013;4. doi:10.3389/fphys.2013.00332
21. Paraiso LF, Gonçalves-E-Oliveira AFM, Cunha LM, et al. Effects of acute and chronic exercise on the osmotic stability of erythrocyte membrane of competitive swimmers. PLoS One. 2017;12(2):e0171318. doi:10.1371/journal.pone.0171318
22. Lippi G, Sanchis-Gomar F. Epidemiological, biological and clinical update on exercise-induced hemolysis. Ann Transl Med. 2019;7(12):270. doi:10.21037/atm.2019.05.41
23. Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front in Physiol. 2014;5:84. doi:10.3389/fphys.2014.00084
24. Massaccesi L, Galliera E, Corsi Romanelli MM. Erythrocytes as markers of oxidative stress related pathologies. Mech Ageing Dev. 2020;191:111333. doi:10.1016/j.mad.2020.111333
25. Şentürk ÜK, Gündüz F, Kuru O, et al. Exercise-induced oxidative stress leads hemolysis in sedentary but not trained humans. J Appl Physiol. 2005;99(4):1434–1441. doi:10.1152/japplphysiol.01392.2004
26. Borden M, Nyhan WL, Bakay B. Increased Activity of Adenine Phosphoribosyltransferase in Erythrocytes of Normal Newborn Infants. Pediatr Res. 1974;8(1):31–36. doi:10.1203/00006450-197401000-00006
27. Bizjak DA, Tomschi F, Bales G, et al. Does endurance training improve red blood cell aging and hemorheology in moderate-trained healthy individuals? J Sport Health Sci. 2020;9(6):595–603. doi:10.1016/j.jshs.2019.02.002
28. Dudzinska W, Suska M, Lubkowska A, et al. Comparison of human erythrocyte purine nucleotide metabolism and blood purine and pyrimidine degradation product concentrations before and after acute exercise in trained and sedentary subjects. J Physiol Sci. 2018;68(3):293–305. doi:10.1007/s12576-017-0536-x
29. Pospieszna B, Kusy K, Słomińska EM, Dudzinska W, Ciekot-Sołtysiak M, Zieliński J. The Effect of Training on Erythrocyte Energy Status and Plasma Purine Metabolites in Athletes. Metabolites. 2019;10(1):5. doi:10.3390/metabo10010005
30. Sawada Y, Ichikawa H, Ebine N, et al. Effects of High-Intensity Anaerobic Exercise on the Scavenging Activity of Various Reactive Oxygen Species and Free Radicals in Athletes. Nutrients. 2023;15(1):222. doi:10.3390/nu15010222
31. Pospieszna B, Kusy K, Slominska EM, Zieliński J. Life-long sports engagement enhances adult erythrocyte adenylate energetics. Sci Rep. 2021;11:23759. doi:10.1038/s41598-021-03275-y
32. Zieliński J, Slominska EM, Król-Zielińska M, Krasiński Z, Kusy K. Purine metabolism in sprint- vs endurance-trained athletes aged 20‒90 years. Sci Rep. 2019;9:12075. doi:10.1038/s41598-019-48633-z
33. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi:10.1136/bjsports-2020-102955
34. Edvardsen E, Hem E, Anderssen SA. End Criteria for Reaching Maximal Oxygen Uptake Must Be Strict and Adjusted to Sex and Age: a Cross-Sectional Study. PLoS One. 2014;9(1):e85276. doi:10.1371/journal.pone.0085276
35. Slominska EM, Carrey EA, Foks H, et al. A Novel Nucleotide Found in Human Erythrocytes, 4-Pyridone-3-carboxamide-1-β-d-ribonucleoside Triphosphate. J Biol Chem. 2006;281(43):32057–32064. doi:10.1074/jbc.m607514200
36. Wu R, Delahunt E, Ditroilo M, Lowery M, De Vito G. Effects of age and sex on neuromuscular-mechanical determinants of muscle strength. AGE. 2016;38:57. doi:10.1007/s11357-016-9921-2
37. Kusy K, Zieliński J. Aerobic capacity in speed-power athletes aged 20-90 years vs endurance runners and untrained participants. Scand J Med Sci Sports. 2014;24(1):68–79. doi:10.1111/j.1600-0838.2012.01496.x
38. Kosenko EA, Tikhonova LA, Montoliu C, Barreto GE, Aliev G, Kaminsky YG. Metabolic Abnormalities of Erythrocytes as a Risk Factor for Alzheimer’s Disease. Front Neurosci. 2018;11:728. doi:10.3389/fnins.2017.00728
39. Calabria E, Mazza EMC, Dyar KA, et al. Aging: a portrait from gene expression profile in blood cells. Aging. 2016;8(8):1802–1821. doi:10.18632/aging.101016
40. Khecuriani R, Lomsadze G, Arabuli M, Sanikidze T. Deformability of red blood cells and human aging. Georgian Med News. 2010;182:42–46.
41. Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci. 2016;113(16):4252–4259. doi:10.1073/pnas.1603023113
42. Kosenko EA, Tikhonova LA, Pogosyan AS, Kaminsky YG. Erythrocyte antioxidants in aging and dementia. Biomed Chem. 2012;6(3):273–277. doi:10.1134/s1990750812030079
43. Huang Y-X, Z-J W, Mehrishi J, et al. Human red blood cell aging: correlative changes in surface charge and cell properties. J Cell Mol Med. 2011;15(12):2634–2642. doi:10.1111/j.1582-4934.2011.01310.x
44. Domingo-Ortí I, Lamas-Domingo R, Ciudin A, et al. Metabolic footprint of aging and obesity in red blood cells. Aging. 2021;13(4):4850–4880. doi:10.18632/aging.202693
45. Andriollo-Sanchez M, Hininger-Favier I, Meunier N, et al. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005;59(S2):S58–S62. doi:10.1038/sj.ejcn.1602300
46. Tavazzi B, Di Pierro D, Amorini AM, et al. Energy metabolism and lipid peroxidation of human erythrocytes as a function of increased oxidative stress. Eur J Biochem. 2000;267(3):684–689. doi:10.1046/j.1432-1327.2000.01042.x
47. Schranner D, Schönfelder M, Römisch‐Margl W, et al. Physiological extremes of the human blood metabolome: a metabolomics analysis of highly glycolytic, oxidative, and anabolic athletes. Physiol Rep. 2021;9(12):e14885. doi:10.14814/phy2.14885
48. Zarębska EA, Kusy K, Słomińska EM, Kruszyna Ł, Zieliński J. Plasma Nucleotide Dynamics during Exercise and Recovery in Highly Trained Athletes and Recreationally Active Individuals. BioMed Res Int. 2018;2018:4081802. doi:10.1155/2018/4081802
49. Zarębska EA, Kusy K, Słomińska EM, Kruszyna Ł, Zieliński J. Alterations in Exercise-Induced Plasma Adenosine Triphosphate Concentration in Highly Trained Athletes in a One-Year Training Cycle. Metabolites. 2019;9(10):230. doi:10.3390/metabo9100230
50. Zieliński J, Kusy K. Training-induced adaptation in purine metabolism in high-level sprinters vs. triathletes. J Appl Physiol. 2012;112(4):542–551. doi:10.1152/japplphysiol.01292.2011
51. Zieliński J, Krasińska B, Kusy K. Hypoxanthine as a Predictor of Performance in Highly Trained Athletes. Int J Sports Med. 2013;34(12):1079–1086. doi:10.1055/s-0033-1337947
52. Hoxhaj G, Hughes-Hallett J, Timson RC, et al. The mTORC1 Signaling Network Senses Changes in Cellular Purine Nucleotide Levels. Cell Rep. 2017;21(5):1331–1346. doi:10.1016/j.celrep.2017.10.029
53. Drey M, Sieber CC, Degens H, et al. Relation between muscle mass, motor units and type of training in master athletes. Clin Physiol Funct Imaging. 2016;36(1):70–76. doi:10.1111/cpf.12195
54. Kusy K, Zielinski J. Sprinters versus Long-distance Runners: how to Grow Old Healthy. ESSR. 2015;43(1):57–64. doi:10.1249/JES.0000000000000033
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



