Back to Journals » Infection and Drug Resistance » Volume 16
Spontaneous Prostatic Hemorrhage in a COVID-19 Patient: A Case Report
Authors Huang J , Ding H, Feng C, Mao D, Tai S
Received 5 March 2023
Accepted for publication 9 May 2023
Published 16 May 2023 Volume 2023:16 Pages 3035—3040
DOI https://doi.org/10.2147/IDR.S410962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jiaguo Huang,1,* Hongxiang Ding,1,* Chao Feng,2 Dikai Mao,1 Shengcheng Tai1
1Department of Urology, Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, People’s Republic of China; 2School of Medicine, Hangzhou Normal University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shengcheng Tai, Department of Urology, Affiliated Xiaoshan Hospital, Hangzhou Normal University, No. 728 North Yucai Road, Xiaoshan District, Hangzhou, People’s Republic of China, Tel +8613516811191, Email [email protected]
Abstract: Hematuria occurring in patients with acute kidney injury caused by Corona Virus Disease 2019 (COVID-19) infection has been reported. However, cases of macroscopic hematuria in COVID-19 patients leading to a severe decrease in hemoglobin have not been reported heretofore. Herein, we describe the case of a 56-year-old male patient who suffered from spontaneous prostatic hemorrhage caused by thrombocytopenia and coagulation dysfunction associated with COVID-19 infection, which manifested as macroscopic hematuria, bladder blood clot tamponade and severe hemoglobin decline. Prostatic hemorrhage was diagnosed by endoscopy. There was no recurrence of macroscopic hematuria after undergoing transurethral prostate electrocoagulation for hemostasis, infusing plasma to supplement coagulation factors and taking finasteride. One month after the bleeding event, the patient’s blood routine reexamination revealed that the platelet count returned to the normal value and coagulation was normal.
Keywords: COVID-19, hematuria, prostatic hemorrhage, thrombocytopenia, coagulation dysfunction, bleeding event
Introduction
With the changes introduced in China’s prevention and control policy in December 2022, the infection rate of Corona Virus Disease 2019 (COVID-19) in China has increased sharply. The majority of patients present with fever and sore throat, and a considerable number of aged individuals and people with low immunity have developed pneumonia. Cases of hematuria in COVID-19 patients have been reported,1 mainly due to acute kidney injury caused by COVID-19 infection, which is manifested as microscopic hematuria.2,3 In severe cases, it can cause macroscopic hematuria.4 In a study involving 400 COVID-19 cases, the total bleeding rate was 4.8% (21 bleeding events in 19 patients), including 3.1% in non-critical patients and 7.6% in critical patients. The rate of massive hemorrhage was 2.3%, of which only two cases were of gastrointestinal bleeding without anticoagulant drugs.5 Disseminated intravascular coagulation, clinically relevant thrombocytopenia, and reduced fibrinogen were rare and were associated with significant bleeding manifestations. However, cases of macroscopic hematuria in COVID-19 patients leading to a severe decrease in hemoglobin have not been reported heretofore. Herein, we describe the case of a 56-year-old male patient who presented with spontaneous prostatic hemorrhage caused by thrombocytopenia and coagulation dysfunction associated with COVID-19 infection, which manifested as macroscopic hematuria, bladder blood clot tamponade and a severe decline in hemoglobin.
Case Report
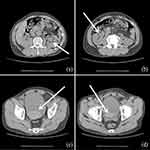
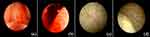
The above patient was a 56-year-old male patient with negative past medical and past surgical history. One week prior to his admission, the patient presented with fever and was diagnosed with COVID-19 infection according to the positive COVID-19 viral RNA test of throat swabs. After three days of antipyretic treatment, the febrile symptoms improved. One day before admission, the patient had obvious macroscopic hematuria. Small blood clots were seen in urine, which were followed by difficulty in urination and abdominal pain. His family sent him to the emergency department. A preliminary physical examination revealed that his vital signs were stable, and the lower abdomen was swollen and painful, especially in the bladder area. Blood routine test revealed that the hemoglobin level was 133 g/L (normal range: above 130 g/L), the platelet level was 73×109 /L (normal range: 125–350 × 109 /L), and platelet ocrit, that is, the product of mean platelet volume and platelet count was 0.09% (normal range: 0.1%–0.5%). The coagulation function test revealed that the thrombin time (TT) was 17.90 seconds (normal range: 10.30–16.60 seconds), the prothrombin time (PT) was 12.60 seconds (normal range: 9.40–12.50 seconds), the fibrinogen concentration was 1.70 g/L (normal range: 2.38–4.98 g/L). Finally, abdominal computer tomography (CT) revealed that a high-density mass in the bladder, with blood clots, urinary retention, and hydronephrosis (Figure 1). After the patient was admitted to hospital, a catheter was indwelt and retained, and the bladder was flushed. A large amount of blood clots, about 200 mL, were flushed out. Then the bladder was continuously flushed to keep the catheter unobstructed. However, the bladder irrigation fluid was bloody. On the second day of admission, we reexamined the blood routine test and found that hemoglobin dropped to 98 g/L, platelets dropped to 62×109 /L, platelet ocrit dropped to 0.07%, and bladder irrigation fluid was still bloody. An endoscopic examination was carried out, which revealed that the patient had dilated blood vessels in the prostate, congested prostate glands (Figure 2a), and obvious bleeding in the prostate (Figure 2b), with no bleeding point in the bladder mucosa (Figure 2c), and ureteric jets were for hematuria-free (Figure 2d). Therefore we performed transurethral prostate electrocoagulation hemostasis for the patient and infused 480 mL of plasma to supplement coagulation factors as well as administered finasteride 5 mg once a day. There was no recurrence of macroscopic hematuria after the above treatment. After one week, the catheter was removed, and the patient returned to normal spontaneous urination. One month after the bleeding event, the patient’s blood routine reexamination revealed that hemoglobin was 107 g/L, and platelet, platelet ocrit and coagulation function returned to normal.
Discussion
Prostatic hemorrhage is often secondary to an operation such as puncture biopsy or prostate resection. Spontaneous prostatic hemorrhage is an extremely rare emergency of the urinary system. It occurs in the elderly with severe prostatic hyperplasia, and the amount of bleeding can be life-threatening.6 In the course of disease development in COVID-19 patients, coagulation dysfunction and thrombocytopenia resulting in significant bleeding consequences beyond the thromboembolic risk cannot be overlooked.5 In the above case, it seems that prostatic hemorrhage was caused by thrombocytopenia and coagulation dysfunction associated with COVID-19 infection.
COVID-19 is a viral respiratory disease caused by severe acute respiratory syndrome corona virus 2 (SARS-CoV-2). Due to excessive inflammation, platelet activation, endothelial dysfunction, and stasis, patients are prone to thrombotic disease in venous and arterial circulation.7 The initial coagulation dysfunction of COVID-19 is characterized by a significant increase in D-dimer and fibrinogen degradation products, while PT, activated partial thromboplastin time (APTT), and platelet count abnormalities were relatively rare in the initial course of the disease.8 Clinically, some COVID-19 patients showed shortened PT, APTT, and increased fibrinogen,9 but a few patients also showed prolonged PT and APTT,10 indicating that the consumption of coagulation factors led to subsequent progression to low coagulation. A clinical characterization data of 1099 COVID-19 cases diagnosed from December 2019 to January 2020 in China showed that the vast majority of patients (83.2%) had lymphocytopenia, while some of the patients (36.2%) had thrombocytopenia.11 Thrombocytopenia aggravates the risk of bleeding in patients.
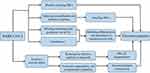
Thrombocytopenia is a common consequence of infectious diseases. The mechanisms of thrombocytopenia caused by SARS-CoV-2 have not yet been fully elucidated (Figure 3). SARS-CoV-2 may directly attack hematopoietic stem cells (HSCs). CoV particles may cause thrombocytopenia by inducing the production of autoantibodies and immune complexes and reducing the production of platelets. SARS-CoV-2 may bind to angiotensin-converting enzyme 2 (ACE2) protein and induce liver injury, thereby blocking the production of thrombopoietin and ultimately inhibiting the differentiation and maturation of megakaryocyte (MK) cells.12 An ultrastructural study demonstrated that interferon-α (IFN-α) inhibited MK cell maturation.13 Extensive alveolar injury tends to reduce the availability of effective capillary in the lung beds and affects MK cell fragmentation and the production of platelets for pulmonary microcirculation, leading to thrombocytopenia.14,15 Virus attack and replication can cause lung cell apoptosis, vascular leakage, and release of pro-inflammatory cytokines and chemokines.16 This damage to lung tissue and pulmonary endothelial cells leads to the activation, aggregation, and encapsulation of platelets in the lung and increases platelet consumption or thrombosis, leading to thrombocytopenia.16
A laboratory examination of the patient showed thrombocytopenia and coagulation dysfunction, and the abdominal CT showed blood clots in the bladder. Furthermore, an endoscopic examination showed prostatic hemorrhage. The size of the patient’s prostate was about 45×40 × 33 mm3 measured by B-mode ultrasonography, and it was not significantly larger than normal. According to previous studies, approximately 2.5% of men with benign prostatic hyperplasia (BPH) have macroscopic hematuria.17 While the precise etiology of bleeding in men with BPH is unclear, it may be attributable to increased microvessel density level18 and vascular endothelial growth factor (VEGF) over-expression.19 This is consistent with our endoscopic examination results, including dilated blood vessels in the prostate and congested prostate glands. Additionally, hypertension has been identified as a risk factor for hematuria in these patients.20 The patient had no difficulty in urination as well as no history of macroscopic hematuria and the use of anticoagulant drugs in the past. It was reported that the COVID-19 genome was successfully identified in the prostate specimen of a single patient, and SARS-CoV-2 particles can still be found in the prostate tissue four months after infection with COVID-19, but no symptoms of prostate bleeding have occurred.21,22 Therefore, we believe that there exists no significant correlation between prostatic hemorrhage and BPH.
There are many literature reports of hematuria caused by kidney damage caused by COVID-19 infection.1–3 There are also cases of hematuria after COVID-19 vaccine inoculation, in which a considerable part of the vaccinated population suffer from immunoglobulin A nephropathy. The main mechanism of hematuria is to aggravate the kidney injury.23–25 In clinical practice, macroscopic hematuria originating from the kidneys can be detected under endoscopy to have hematuria spurting from the ureter, while in the patient we reported, the endoscopic examination did not show hematuria spurting from the ureter. Therefore, we believe that it is related to thrombocytopenia and coagulation dysfunction caused by COVID-19 infection.
The patient underwent transurethral prostate electrocoagulation for hemostasis and infused plasma to supplement coagulation factors and was administered finasteride 5 mg once a day. Finasteride seems to be an effective drug to control macroscopic hematuria secondary to prostatic hemorrhage.26 It decreased the expression of VEGF, inhibited the angiogenesis of prostatic suburethral tissue, and significantly decreased the microvessel density.27 There was no recurrence of macroscopic hematuria after the above treatment. One month after the bleeding event, the patient’s laboratory reexamination showed that platelet, platelet ocrit, and coagulation function returned to normal. This case report is of clinical significance, reminding urologists and infectious disease physicians that hematuria caused by COVID-19 infection may originate from prostatic hemorrhage.
Conclusion
Although most patients with COVID-19 have a hypercoagulable state, the bleeding risk associated with COVID-19 infection cannot be ignored. There are many causes of hematuria, and it is vital to find the cause. If necessary, endoscopy can be performed to look for the cause of hematuria. For severe hematuria from spontaneous prostatic hemorrhage caused by COVID-19 infection, emergency intervention is necessary.
Abbreviations
ACE2, angiotensin converting enzyme 2; APTT, activated partial thromboplastin time; BPH, benign prostatic hyperplasia; COVID-19, Corona Virus Disease 2019; CT, Computer Tomography; HSCs, hematopoietic stem cells; IFN-α, Interferon-α; IgAN, immunoglobulin A nephropathy; MK, megakaryocyte; PT, prothrombin time; SARS-CoV-2, severe acute respiratory syndrome corona virus 2; TT, thrombin time; VEGF, vasoactive endothelial growth factor.
Data Sharing Statement
All data of this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Patient Consent
Study was approved by Ethical Committee of Affiliated Xiaoshan Hospital, Hangzhou Normal University (Approval NO.: 2023015). The patient in this manuscript has given written informed consent to publication of his case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Bozorgmehr R, Tajabadi Z. Hemoptysis and Hematuria as the Initial Symptoms of COVID-19: a Case Report. Tanaffos. 2021;20(1):75–78.
2. Chaudhri I, Moffitt R, Taub E, et al. Association of Proteinuria and Hematuria with Acute Kidney Injury and Mortality in Hospitalized Patients with COVID-19. Kidney Blood Press Res. 2020;45(6):1018–1032. doi:10.1159/000511946
3. Zheng X, Yang H, Li X, et al. Prevalence of Kidney Injury and Associations with Critical Illness and Death in Patients with COVID-19. Clin J Am Soc Nephrol. 2020;15(11):1549–1556. doi:10.2215/CJN.04780420
4. Almeida FJ, Olmos RD, Oliveira D, et al. Hematuria Associated With SARS-CoV-2 Infection in a Child. Pediatr Infect Dis J. 2020;39(7):e161. doi:10.1097/INF.0000000000002737
5. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi:10.1182/blood.2020006520
6. Wroclawski ML, Carneiro A, Tristão RA, et al. Giant prostatic hyperplasia: report of a previously asymptomatic man presenting with gross hematuria and hypovolemic shock. Einstein. 2015;13(3):420–422. doi:10.1590/S1679-45082015RC2905
7. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi:10.1016/j.jacc.2020.04.031
8. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi:10.1182/blood.2020006000
9. Arachchillage D, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(5):1233–1234. doi:10.1111/jth.14820
10. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
11. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
12. Zhang Y, Zeng X, Jiao Y, et al. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb Res. 2020;193:110–115. doi:10.1016/j.thromres.2020.06.008
13. Yamane A, Nakamura T, Suzuki H, et al. Interferon-alpha 2b-induced thrombocytopenia is caused by inhibition of platelet production but not proliferation and endomitosis in human megakaryocytes. Blood. 2008;112(3):542–550. doi:10.1182/blood-2007-12-125906
14. Niinikoski J, Goldstein R, Linsey M, Hunt TK. Effect of oxygen-induced lung damage on tissue oxygen supply. Acta Chir Scand. 1973;139(7):591–595.
15. Martin JF, Slater DN, Trowbridge EA. Abnormal intrapulmonary platelet production: a possible cause of vascular and lung disease. Lancet. 1983;1(8328):793–796. doi:10.1016/S0140-6736(83)91851-2
16. Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: from Mechanisms to Potential Therapeutic Tools. Virol Sin. 2020;35(3):266–271. doi:10.1007/s12250-020-00207-4
17. Hunter DJ, Berra-Unamuno A, Martin-Gordo A. Prevalence of urinary symptoms and other urological conditions in Spanish men 50 years old or older. J Urol. 1996;155(6):1965–1970. doi:10.1016/S0022-5347(01)66063-4
18. Foley SJ, Bailey DM. Microvessel density in prostatic hyperplasia. BJU Int. 2000;85(1):70–73. doi:10.1046/j.1464-410x.2000.00322.x
19. Häggström S, Tørring N, Møller K, et al. Effects of finasteride on vascular endothelial growth factor. Scand J Urol Nephrol. 2002;36(3):182–187. doi:10.1080/003655902320131848
20. Guo LJ, Tang Y, Guo CM, Zhang XH. Impact of primary hypertension on hematuria of the patients with benign prostatic hyperplasia. Chin Med J. 2010;123(9):1154–1157.
21. Reddy R, Farber N, Kresch E, Seetharam D, Diaz P, Ramasamy R. SARS-CoV-2 in the Prostate: immunohistochemical and Ultrastructural Studies. World J Mens Health. 2022;40(2):340–343. doi:10.5534/wjmh.210174
22. Elsaqa M, Rao A, Liu L, et al. Molecular detection of the COVID-19 genome in prostatic tissue of patients with previous infection. Bayl Univ Med Cent. 2022;35(6):759–761. doi:10.1080/08998280.2022.2101178
23. Nihei Y, Kishi M, Suzuki H, et al. IgA Nephropathy with Gross Hematuria Following COVID-19 mRNA Vaccination. Intern Med. 2022;61(7):1033–1037. doi:10.2169/internalmedicine.8787-21
24. Ritter A, Helmchen B, Gaspert A, et al. Clinical spectrum of gross haematuria following SARS-CoV-2 vaccination with mRNA vaccines. Clin Kidney J. 2022;15(5):961–973. doi:10.1093/ckj/sfab284
25. Kudose S, Friedmann P, Albajrami O, D’Agati VD. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021;100(2):468–469. doi:10.1016/j.kint.2021.06.011
26. Sieber PR, Rommel FM, Huffnagle HW, Breslin JA, Agusta VE, Harpster LE. The treatment of gross hematuria secondary to prostatic bleeding with finasteride. J Urol. 1998;159(4):1232–1233. doi:10.1016/S0022-5347(01)63567-5
27. Pareek G, Shevchuk M, Armenakas NA, et al. The effect of finasteride on the expression of vascular endothelial growth factor and microvessel density: a possible mechanism for decreased prostatic bleeding in treated patients. J Urol. 2003;169(1):20–23. doi:10.1016/S0022-5347(05)64025-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.