Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Splenic Infarction with Myocardial Injury in a Diabetic Patient: A Case Report
Authors Wang C, Wen S , Zhou L
Received 25 June 2023
Accepted for publication 18 September 2023
Published 22 September 2023 Volume 2023:16 Pages 2929—2937
DOI https://doi.org/10.2147/DMSO.S427586
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Congcong Wang,1 Song Wen,1 Ligang Zhou1,2
1Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China; 2Shanghai Key Laboratory of Vascular Lesions Regulation and Remodeling, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, Shanghai, People’s Republic of China
Correspondence: Ligang Zhou, Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China, Tel +86 13611927616, Email [email protected]
Abstract: Splenic infarction (SI) is an uncommon complication of type 2 diabetes (T2D). Diabetes predisposes individuals to blood vessel abnormalities, such as atherosclerosis or thrombosis, increasing the risk of vessel occlusion and subsequent tissue infarction. If the diabetic patient has other serious diseases, such as a severe pneumonia infection and acute cardiac infarction, SI incidence may go unrecognized, making it challenging for physicians to identify. This case report discussed an 80-year-old hospitalized diabetic woman with a history of chronic bronchitis and 20 years of T2D who suffered an SI. The patient was at elevated risk for thrombosis of atrial fibrillation, manifested as an embolism of the spleen characterized by a high concentration of white blood cells. This patient also demonstrated a rapid increase in cardiac biomarkers troponin I, suggesting acute myocardial infarction (AMI) and increased amylase, which could not preclude the concern about the existence of acute pancreatitis. Abdominal CT displayed the calcification of only the splenic and other arteries, and low-density shadows were observed at the center portion of the spleen. This case demonstrated the significant occurrence of thrombotic complications in various blood vessels of multiple organs in T2D patients. Thus, clinicians should be aware of the possibility of simultaneous acute vascular infarction of several organs in diabetic patients with prior vascular constriction.
Keywords: systemic thrombosis, splenic artery embolism, spleen infarction, type 2 diabetes
Introduction
Splenic infarction (SI) is an uncommon complication in type 2 diabetic patients (T2D). There is a lack of consensus and guidelines on diagnosing and treating SI in T2D patients, as the relevant information is mainly reported in case reports. SI is a condition in which the blood supply to the spleen is blocked, resulting in the necrosis of spleen tissue. In patients with SI, the risk of thrombosis in other arteries and veins throughout the body must be monitored, particularly in myocardial infarction, pulmonary embolism, and cerebral embolism. A multicenter retrospective study identified cardiogenic embolism and identifiable hypercoagulability as the cause of most SIs.1 Infarcts of the spleen may be the initial sign of other potentially severe predisposition diseases (such as blood diseases, malignancies, or cardiac embolism) that cause death or morbidity. Therefore, we must be vigilant when encountering patients with spleen infarction to detect severe potential diseases. Multiple thromboembolic events co-occurring in a single patient lend support to this theory.2 Herein, we described the case of an 80-year-old female admitted to the hospital for a pulmonary infection but developed SI and significantly elevated cardiac biomarkers indicating myocardial injury during her hospitalization. This case study aimed to increase clinicians’ awareness of the risk of multiple organ infractions in T2D patients and improve the management of these patients.
Case Presentation
An 80-year-old female with recurrent fever for over a month was admitted to our hospital on January 23, 2023. The patient had a history of chronic obstructive pulmonary disease (COPD) for over a decade. She had primary hypertension for 25 years, diabetes for 20 years, coronary artery disease for eight years, and paroxysmal atrial fibrillation without treatment for five years. Moreover, she had severe osteoporosis (spinal bending over with a 90-degree back hunch) and was allergic to penicillin and levofloxacin. Her daily meds consisted of enalapril, one pill, Human Insulin Novo and Lantus 30R, aspirin, and inhaled salbutamol sulfate.
At admission, the patient had severe dyspnea, was afebrile (37 °C), had a respiratory rate of 22 breaths/min, a heart rate of 100 beats/min, a blood pressure of 107/56 mmHg, and a peripheral oxygen saturation of 96% without oxygen inhalation and 98.3% in arterial blood gas analysis after nasal catheter oxygen inhalation.
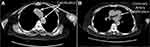
A chest computed tomography (CT)3 scan performed upon her admission indicated an expanded and disorganized broncho-vascular shadow, increased lung opacity, local bubble-like alterations, a few streak-like fuzzy shadows in the lungs and a central trachea with evident bronchial cavities. The mediastinum was not significantly enlarged, nor were the lymph nodes. Localized high-density calcium deposition was observed in the coronary and aortic arteries (Figure 1A and B).
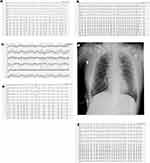
Following admission on January 23, 2023, she received antibiotics (azithromycin) for the evidence of mixed bacterial infection, steroids (betamethasone sodium phosphate), expectorants (bromhexine), cough suppressants and bronchodilators (theophylline and compound methoxyphenamine hydrochloride), antidiabetic agents (Degludec plus Insulin Aspart), diuretics and heart load-reducing agents (bumetanide and loop diuretics), and preventive dose of sodium heparin for coagulation. The electrocardiogram (ECG) profile of the patient upon admission is shown in Figure 2a.
On January 30, 2023, the patient complained of dizziness, shortness of breath, nausea, and vomiting twice without hematemesis and had a fever. The ECG (Figure 2b) showed sinus rhythm (HR 132 bpm), partial short-term ventricular tachycardia, and irregular intra-chamber conduction. The repeated arterial blood gas analysis revealed an oxygen saturation of 97.8%.
After having difficulties defecating in the afternoon of the same day, the patient presented with significant chest tightness and shortness of breath was unable to rest correctly and was in a forced position. Intravenous amiodarone was provided to maintain sinus rhythm after emergency consultation with a cardiovascular specialist, and we detected fast atrial fibrillation (HR 200 bpm) on the ECG (Figure 2c). A chest X-ray performed at the patient’s bedside revealed a symmetrical thorax, decreased lung transparency on the left side, and increased lung texture. Diaphragm and costophrenic angles were standard, as were the size and shape of the cardiac shadow (Figure 2d).
A re-examination of the patient’s ECG on January 30 evening (Figure 2e) revealed rapid atrial fibrillation (heart rate of 177 bpm), ST-T elevation, ST (II, III, V4, V5, V6 leads) with a horizontal or sloping depression greater than 0.05 mV, and low T waves (I teach). Laboratory tests indicated critical levels of cardiac biomarkers such as creatine kinase, troponin, and myoglobin, indicating an acute myocardial infarction (AMI) (data is given in Table 1).
 |
Table 1 Dynamic Evolutions in This Patient’s Laboratory Tests Following Hospitalization |
The cardiologist recommended antiplatelet aggregation, anticoagulation, plaque stabilization medications, a dynamic ECG recheck, and a laboratory test. The patient was prescribed enoxaparin sodium (40 mg/day), enteric-coated aspirin (100 mg/day), clopidogrel sulfate (75 mg/day), and atorvastatin calcium tablets (20 mg/day) for stabilizing the plaque of the arterial wall and improving dyslipidemia.
At 10 p.m. on January 30, the patient experienced abdominal pain and diarrhea five times in one night but had no fever. The physical examination indicated widespread abdominal pain without rebound tenderness or guarding. Laboratory tests (Table 1) revealed a moderately increased amylase level, suggesting an acute inflammatory response.
The following day, the patient suffered a brief fever with a maximum temperature of 38.2 °C (100.8 °F), alleviated abdominal pain, and no diarrhea. Re-examination of laboratory test results (Table 1 as above) exposed a significant increase in white blood cells, C-reactive protein (CRP), calcitonin, creatine kinase, myoglobin, and D-dimer but a slight decline in serum amylase compared to the previous day’s results. Multiple ECGs (Figure 2f) were performed from January 31 to February 1, and amiodarone was discontinued once sinus rhythm was recovered. The dynamic of laboratory tests in this patient are shown in Figure 3.
Abdominal enhanced CT on February 1 exhibited no abnormalities in liver parenchymal density, no apparent abnormal enhancing foci in the liver after enhancement, and no dilatation of bile ducts; no spleen enlargement; however, several high-density calcifications in the splenic arteries and a wedge-shaped region of low density within the spleen was observed, indicating an acute SI (Figure 4).
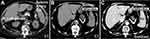 |
Figure 4 Several high-density calcifications in the splenic arteries (A–C), and a wedge-shaped region of low density within the spleen (B and C) were observed on an abdominal CT. |
As purpura emerged at the injection site after enoxaparin treatment, enoxaparin and aspirin were discontinued on February 5, 2023, and clopidogrel and atorvastatin were continued orally. We sustained monitoring the coagulation parameters and complete blood count. The patient’s creatine kinase, amylase, total blood count, CRP, calcium, and D-dimer levels returned to normal on February 8. The cardiologist considered the patient at a higher risk of pulmonary embolism and alerted us to be vigilant about this potential problem due to the manifestation of chest tightness and shortness of breath, accompanied by elevated D-dimers. Due to severe spinal bending, the patient could only lie on one side; therefore, she could not cooperate with the coronary computed tomography angiography (CTA) and pulmonary CT angiography examinations and refused coronary angiography. Consequently, we could not determine the precise blood supply situation of the coronary and pulmonary arteries.
Discussion
SI is an uncommon and easily overlooked thrombotic disorder, estimated at 0.004–0.01% in hospitalized patients.4 By analyzing 466 cases of spleen infarction, Professor Hakoshima et al classified the causes into five categories: infection, malignant tumor, aberrant red blood cells and hemoglobin, coagulation abnormality, vasculitis, and cardiovascular disease.5 A rare case describing a patient with spontaneous splenic infarction secondary to diabetes-induced, small-vessel atherosclerotic disease was reported.6 Thrombi development and the inflammatory response are tightly interconnected and mutually reinforcing.7 The production of thrombi in the microvasculature contributes to tissue ischemia and organ dysfunction.8
The patient was hospitalized due to a pneumonia infection. On the seventh day of hospitalization (January 30, 2023), the patient experienced a sudden onset of atrial fibrillation, resulting in a series of alterations in the patient’s condition. The imaging reports (February 1, 2023, abdomen contrast-enhanced CT) revealed SI. However, the specific time of onset was unknown. Several laboratory indicators, including white blood cells, D-dimer, procalcitonin (PCT), cardiac troponin I (cTnI), creatine kinase isoenzyme, and serum amylase, increased significantly following the onset of atrial fibrillation, as determined by an analysis of various laboratory indicators during the hospitalization of the patient. As the biggest lymphoid organ in the human body, it is commonly acknowledged that the spleen can contribute to immunological responses. The patient’s dramatic increase in white blood cells might have resulted from a leukemoid reaction followed by an SI. Simultaneously, the patient’s PCT and CRP levels were raised, indicating greater chances of a concurrent abscess or infection in the lung following SI. In this case, the symptoms of SI were minor, including brief diarrhea, stomach pain, and a one-day fever. However, increased levels of D-dimer were consistent with the hypercoagulable state associated with SI. During hospitalization, the patient was diagnosed with paroxysmal atrial fibrillation, a cardiovascular condition that increases the risk of vascular embolic events. Furthermore, the patient had apparent systemic atherosclerosis and coronary artery sclerosis; it was impossible to rule out the contribution of splenic artery sclerosis, all leading to SI.
Enhanced CT3 imaging is the gold standard for diagnosing SI.9 CT imaging can reveal the characteristic features of spleen infarction and its potential pathogenesis.10 Symptomatic arterial thrombosis is diagnosed clinically combined with imaging of CTA, which is preferable, while asymptomatic and atypical splenic infarction was discovered incidentally on CTA.11
The causes of SI include atherosclerotic embolus, hypercoagulation-related diseases such as cancer, arterial damage, abnormal clotting factors, and inflammation. In a retrospective study of patients with SI, Wand et al suggested that SI could be the initial presentation of previously unknown medical conditions in 38% of patients. The main underlying mechanisms were cardioembolic (54.4%), vascular (20%), hematologic disorders (15.6%), and multiple causes (21.1%). Atrial fibrillation and atherosclerosis were common in aged patients (age > 70), while antiphospholipid syndrome occurred exclusively in younger individuals.12 Additionally, pancreatic disorders are also an essential cause of SI, presumably due to the proximity of the pancreas to the splenic vessels.13
Co-occurrence renal infarction (RI) and SI are relatively high in patients with visceral infarction. Li et al reported a patient who experienced acute RI and SI secondary to atrial fibrillation.14 Weber et al compared acute RI with SI. In their study, researchers found that the primary cause of RI was attributed to the cardioembolic source, mainly atrial fibrillation, and that of SI was traced to the patient’s hypercoagulable states.15,16
Atherosclerosis is a widespread pathological condition in which lipid deposition, plaque formation, and even loss in the artery wall eventually result in lumen stenosis and occlusion. The occurrence and progression of atherosclerotic lesions are complicated.17 Compared with non-diabetic patients, patients with diabetes possess an increased risk of atherosclerosis and accelerated progression. Both type 1 and T2D are independent risk factors for rapid atherosclerosis development.18 Various pathological mechanisms, including dyslipidemia, hyperglycemia with AGE production, increased oxidative stress, and inflammation, were supposed to be associated with atherosclerosis.
A diabetic patient with severe atherosclerosis of arteries may experience cardiovascular disease or SI.19 Moreover, cTnI is the biomarker of AMI20 for its high specificity for myocardial infarction. During the few days following SI in this patient, the cardiac troponin level increased sharply despite the absence of ECG imaging characteristics of AMI. In addition, ischemia-related abnormalities were found on ECG, and the patient displayed the classic symptoms of AMI, such as chest tightness, shortness of breath, and transitory stomach pain. Therefore, AMI could be considered in this case.
Other than myocardial infarction, there could be other causes for elevated cardiac troponin levels during atrial fibrillation. Dr. Van den Bos suggested five common mechanisms: first, the fast ventricular response can cause demand ischemia in normal coronary arteries; second, coronary blood flow is reduced during atrial fibrillation; third, the co-existence of coronary artery stenosis can reduce blood perfusion, especially at tachycardia; fourth, left ventricular wall strain is increased during atrial fibrillation, exaggerated due to pre-existing heart failure; and finally, an acute thrombotic coronary event with pre-existing atrial fibrillation or atrial fibrillation caused by acute ischemia should also be considered.21 In this case, there may also be cTnI release caused by endothelial damage or subsequent neurohumoral or inflammatory responses associated with visceral infarction.22 Elevated cTnI in patients with atrial fibrillation is associated with adverse prognosis and increased thrombotic risk.23 There was a limitation to this study; the patient could not cooperate with the coronary CTA examination due to the spinal disease. Unfortunately, the patient’s coronary artery condition could not be shown.
T2D patients are at high risk of several cardiovascular diseases. Venous thromboembolism (VTE) occurs more than twice as often in patients with DM than in DM-free individuals.24 Glycosylated hemoglobin A1c (HbA1C) is often used as an indicator of blood sugar control. The significant association between the level of glycemic control and the risk of VTE is noticed. Studies regarding DM as an independent risk factor for VTE are inconsistent. In a relevant research, the authors concluded the possibility that female T2DM patients with HbA1c levels > 7% may have a slightly higher risk for unprovoked VTE compared to women with HbA1c levels > 6.5–7.0%.25 Our patient had an HbA1C level of 7.2%, increasing the risk of embolic events.
Interestingly, the patient’s serum amylase increased more than ten-fold concurrently with SI. Does this patient have simultaneously acute pancreatitis and SI? The connection between pancreatitis and SI has been confirmed based on anatomical evidence. Anatomically, the spleen is supplied by the splenic artery, the most significant branch of the abdominal aorta. It proceeds along the upper margin of the pancreas to the splenic hilum, dividing into numerous components, including the pancreatic branches, the left gastroepiploic artery, the short gastric arteries, the left gastro-omental artery, and the splenic branches. Thus, the splenic artery delivers blood to the stomach, pancreas, and spleen. In this patient, the calcified splenic artery was susceptible to thrombosis due to the absence of appropriate collateral circulation between the blood arteries and succeeded by a rapid and significant increase in serum amylase levels, which restored to normal levels within three days. This increase in serum amylase levels was inconsistent with the typical amylase change curve for patients with acute pancreatitis. The CT scan revealed no morphological changes in the pancreas or shadows of aberrant density in the pancreas’ periphery, which did not support the diagnosis of acute pancreatitis. Diagnosing acute pancreatitis requires two of the following: upper abdominal pain consistent with pancreatitis and amylase/lipase levels greater than three times the upper normal range and/or cross-sectional imaging abnormalities.26 At 10 p.m. on January 30, the patient experienced widespread abdominal pain and diarrhea and had an elevated serum amylase value of nearly 10 times the reference level. After one day, serum amylase dropped to only 1.7 times the upper limit of the normal range. Abdominal enhanced CT on February 1 showed no radiographic findings of peripancreatic exudation. The patient’s abdominal pain symptoms were significantly relieved on January 31. Based on the above analysis, the diagnosis of acute pancreatitis was not considered at present.
Our limitation was that dynamic detection of serum lipase, urinary trypsinogen-2 and urine amylase, indicators of acute pancreatitis, was not performed. Therefore, SI was suspected to result in the transient increase of serum amylase level. Another shortcoming of our study was that we could not perform an emergent coronary angiography on this patient. To detect the severity of myocardial infarction and the condition of the coronary artery due to the lower degree of collaboration.
Conclusion
A physician must look for thromboembolic events to diagnose an infected T2D patient correctly. A SI typically manifests atypical clinical signs. To evaluate the condition of SUCH patients, an abdominal CT scan and laboratory tests for AMI, stroke, and venous organ infarction are necessary. To prevent serious organ injury and fatal infarction due to thromboembolic events, the strategy should focus on early discerning the severity of cardiovascular atherosclerosis, especially visceral vessels, with a precaution on the increased risk of thrombosis in such group patients to obtain effective measures to protect and against the subsequent expansion of organ infarctions.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The study, including surveys, sampling, examinations, and access or utilization of the raw data for this study, has obtained ethics approvals and permissions from the Ethics Committee of Shanghai Pudong Hospital (NO. WZ-010). The guidelines outlined and procedures were under the Declaration of Helsinki. All data used in this study were anonymized before its use. All methods were conducted by relevant guidelines and regulations of the Pudong area Health Committee and Shanghai Pudong Hospital.
Consent for Publication
Informed consent for the publication was obtained from the patient.
Acknowledgments
We would like to thank the staff of Shanghai Pudong Hospital whom provide numerous invaluable contributions to this study.
Author Contributions
All authors contributed significantly to the work reported, whether in the conception, study design, execution, data acquisition, analysis, and interpretation, or all of these areas; participated in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the report was submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the Project of Key Medical Discipline of Pudong Hospital of Fudan University (Zdxk2020-11), Project of Key Medical Specialty and Treatment Center of Pudong Hospital of Fudan University (Zdzk2020-24), Integrative Medicine special fund of Shanghai Municipal Health Planning Committee (ZHYY- ZXYJHZX-2-201712), Special Department Fund of the Pudong New Area Health Planning Commission (PWZzk2017-03), Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (PWR12014-06), Pudong New Area Clinical Plateau Discipline Project (PWYgy-2021-03), the Natural Science Foundation of China (21675034), National Natural Science Foundation of China (81370932), Shanghai Natural Science Foundation (19ZR1447500), Fudan Zhangjiang Clinical Medicine Innovation Fund Project (KP0202118), Pudong New Area Clinical Characteristic Discipline Project (PWYts2021-11), Pudong New Area Clinical Characteristic Discipline Project (PWYts2021-01), Wenzhou Medical University Education Grant (JG2021197).
Disclosure
The authors declare no competing interests related to this study.
References
1. Yen CC, Wang CK, Chen SY, et al. Risk assessment and prognostic analysis of patients with splenic infarction in emergency department: a multicenter retrospective study. Sci Rep. 2021;11(1):21423. doi:10.1038/s41598-021-00897-0
2. Tanaka Y, Matsumoto M, Yahata T, Mineki T, Oiwa K. Two cases of multiple thromboembolism with asymptomatic atrial fibrillation. Cureus. 2022;14(1):e21645. doi:10.7759/cureus.21645
3. Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013;216(1):T1–T15. doi:10.1530/JOE-12-0498
4. Weber E, Grangeon F, Reynaud Q, et al. Acute renal and splenic infarctions: a review. QJM. 2020;113(3):186–193. doi:10.1093/qjmed/hcz252
5. Hakoshima M, Kitakaze K, Adachi H, Katsuyama H, Yanai H. Clinical, hematological, biochemical and radiological characteristics for patients with splenic infarction: case series with literature review. J Clin Med Res. 2023;15(1):38–50. doi:10.14740/jocmr4836
6. Tóth PP, Reuter RK, MacDonald J. Spontaneous splenic infarction secondary to diabetes-induced microvascular disease. Arch Fam Med. 2000;9(2):195–197. doi:10.1001/archfami.9.2.195
7. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–241. doi:10.1111/jth.13911
8. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709–725. doi:10.1161/ATVBAHA.117.309846
9. Cox M, Li Z, Desai V, et al. Acute nontraumatic splenic infarctions at a tertiary-care center: causes and predisposing factors in 123 patients. Emerg Radiol. 2016;23(2):155–160. doi:10.1007/s10140-016-1376-3
10. Antopolsky M, Hiller N, Salameh S, Goldshtein B, Stalnikowicz R. Splenic infarction: 10 years of experience. Am J Emerg Med. 2009;27(3):262–265. doi:10.1016/j.ajem.2008.02.014
11. Childers J, Do TVC, Smith F, Vangara A, Ganti SS, Akella R. Incidental and asymptomatic splenic infarction and infrarenal thrombus in a COVID-19 patient. Cureus. 2022;14(7):e26555. doi:10.7759/cureus.26555
12. Wand O, Tayer-Shifman OE, Khoury S, Hershko AY. A practical approach to infarction of the spleen as a rare manifestation of multiple common diseases. Ann Med. 2018;50(6):494–500. doi:10.1080/07853890.2018.1492148
13. Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER cardiac plug. EuroIntervention. 2016;11(10):1170–1179. doi:10.4244/EIJY15M01_06
14. Yihan L, Guanqi F, Tong H, Junye G, Zhong J, Chen T. Case report: acute renal and splenic infarctions secondary to atrial fibrillation. Front Cardiovasc Med. 2022;9:879322. doi:10.3389/fcvm.2022.879322
15. Antopolsky M, Simanovsky N, Stalnikowicz R, Salameh S, Hiller N. Renal infarction in the ED: 10-year experience and review of the literature. Am J Emerg Med. 2012;30(7):1055–1060. doi:10.1016/j.ajem.2011.06.041
16. Schattner A, Adi M, Kitroser E, Klepfish A. Acute splenic infarction at an academic general hospital over 10 years: presentation, etiology, and outcome. Medicine. 2015;94(36):e1363. doi:10.1097/MD.0000000000001363
17. Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27(4):705–713. doi:10.1161/01.ATV.0000261709.34878.20
18. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835. doi:10.3390/ijms21051835
19. Kitagawa I, Ono R, Tobe S, Nagatsuka M. Cardiobacterium hominis endocarditis associated with cerebral, renal, and splenic infarctions: a case report and review of the literature. IDCases. 2023;31:e01655. doi:10.1016/j.idcr.2022.e01655
20. Kaier TE, Alaour B, Marber M. Cardiac troponin and defining myocardial infarction. Cardiovasc Res. 2021;117(10):2203–2215. doi:10.1093/cvr/cvaa331
21. van den Bos EJ, Constantinescu AA, van Domburg RT, Akin S, Jordaens LJ, Kofflard MJ. Minor elevations in troponin I are associated with mortality and adverse cardiac events in patients with atrial fibrillation. Eur Heart J. 2011;32(5):611–617. doi:10.1093/eurheartj/ehq491
22. Huang Y, Liu Y, Ma Y, et al. Associations of visceral adipose tissue, circulating protein biomarkers, and risk of cardiovascular diseases: a Mendelian randomization analysis. Front Cell Dev Biol. 2022;10:840866. doi:10.3389/fcell.2022.840866
23. Providência R, Paiva L, Barra S, Faustino A. Troponin rise in patients with atrial fibrillation: a marker of adverse prognosis and increased thromboembolic risk. Int J Cardiol. 2013;168(5):4889. doi:10.1016/j.ijcard.2013.07.026
24. Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;48(5):1017–1021. doi:10.1007/s00125-005-1715-5
25. Shao S, Zhang X, Xu Q, Pan R, Chen Y. Emerging roles of Glucagon like peptide-1 in the management of autoimmune diseases and diabetes-associated comorbidities. Pharmacol Ther. 2022;239:108270. doi:10.1016/j.pharmthera.2022.108270
26. Szatmary P, Grammatikopoulos T, Cai W, et al. Acute pancreatitis: diagnosis and treatment. Drugs. 2022;82(12):1251–1276. doi:10.1007/s40265-022-01766-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.