Back to Journals » International Journal of General Medicine » Volume 14
Sonoclot Signature Analysis: A New Point-of-Care Testing Method for Defining Heat Stroke-Induced Coagulopathy
Authors Min J, Wan P, Liu G, Yu M, Su L
Received 3 June 2021
Accepted for publication 28 September 2021
Published 20 October 2021 Volume 2021:14 Pages 6925—6933
DOI https://doi.org/10.2147/IJGM.S321982
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jinyi Min,1,* Peng Wan,2,* Guiwei Liu,1 Min Yu,2 Lei Su3
1Department of Critical Care Medicine, The People’s Hospital, Dangyang City, Hubei, 444100, People’s Republic of China; 2Department of Critical Care Medicine, The People’s Hospital of China Three Gorges University, Yichang City, Hubei, 443000, People’s Republic of China; 3Department of Critical Care Medicine, Guangzhou General Hospital of Guangzhou Military Command, The Military Key Laboratory of Trauma Care in Hot Zone and Tissue Repair in PLA, Guangzhou, 510000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peng Wan
Department of Critical Care Medicine, The People’s Hospital of China Three Gorges University, 4th Street Hudi, Xiling District, Yichang City, Hubei, 443000, People’s Republic of China
Tel + 86717-6287551
Fax + 86717-6221636
Email [email protected]
Purpose: Data regarding the incidence of a coagulable state following heat stroke as assessed by Sonoclot signature analysis are limited. Our purpose was to appraise coagulopathy using a dynamic test capable of analyzing the entire coagulation cascade and to characterize coagulation in patients with heat stroke prior to transfusion.
Materials and Methods: The data of 106 patients were collected prospectively from the Critical Care Center of the General Hospital of Guangzhou Military Command. Coagulable state was defined as normal. Both hyper- and hypo-coagulable states were defined as coagulation defects. Hypercoagulability was defined as an activated clotting time (ACT) ≦195s and a clot rate (CR) > 23, and hypocoagulability was defined as an ACT ≧119s and a CR < 7. The Sonoclot signature t examination was performed at the time of admission. Conventional tests, such as the prothrombin time (PT) and activated partial thromboplastin time (aPTT), were compared with Sonoclot monitoring to identify coagulation defects.
Results: The average age of the 106 patients was 23.2± 2.5 years. There were 102 males (96.3%) and 4 females (3.7%). Thirty-four patients (32.1%) were hypercoagulable and 44 patients (41.5%) were hypocoagulable at the time of admission; 28 patients (26.4%) had no evidence of a coagulopathy. Patients with hypocoagulability, unlike patients with hypercoagulability, had a higher sequential Organ Failure Assessment score, indicating a more severe multiple organ dysfunction score. Mortality was 5.9% in patients with hypercoagulability compared with 3.5% in patients with normal coagulation, and 18.1% in patients with a hypocoagulable state (P < 0.05). ACT was a predictor of mortality, while the CR and platelet function did not show statistical significance.
Conclusion: This study determined the clinical outcomes and prognostic value of coagulability in patients with heat stroke, as defined by Sonoclot signature analysis at the time of admission.
Keywords: heat stroke, coagulopathy, prognosis
Introduction
Heat stroke (HS) resulting from exposure to high ambient temperatures is characterized by a rapid upsurge in core temperature above 40°C and is accompanied by central nervous system (CNS) changes, such as convulsions, delirium, or coma.1 Bleeding and coagulopathy are common in patients with HS injuries, with 28% of patients presenting with significant disseminated intravascular coagulation (DIC) in one case series.2,3 DIC has been shown to be an important prognostic factor for mortality in patients with HS.4 The recent literature suggests that HS-induced coagulopathy is a major process that is the result of inflammation and is thought to be an important part of the host defense against hyperthermia and vascular endothelial changes.5,6
Diagnosing HS-induced coagulopathy is not straightforward using the currently available conventional laboratory tests. Indeed, the activated partial thromboplastin time (aPTT), prothrombin time (PT), fibrinogen level (FL), platelet count, and specific factor tests only reflect isolated parts of the coagulation cascade.7 Specifically, the plasma levels of thrombin generation tests or natural anticoagulants, such as protein C, are not readily measurable for clinical use and not authenticated to detect hypercoagulable states. The International Society on Thrombosis and Hemostasis (ISTH) scoring system is an effective method for determining the DIC score in severe HS, but the DIC score is not necessarily good for application due to different situations.8,9
The Sonoclot analyzer (SIENCO, INC 7985 Vance Dr, Arvada, CO 80003 USA) is a viscoelastometric point-of-care testing device that delivers evidence on the complete hemostasis process in a qualitative signature (Sonoclot signature) and in quantitative grades, such as the activated clotting time (ACT), clot rate (CR) and platelet function (PF). The ACT is the time from sample activation to the appearance of fibrin, the presence of the coagulation cascade, and the fibrin-platelet interaction progressively used in trauma to measure for irregularities within the coagulation cascade.10,11 The Sonoclot signature demonstrates a real-time viscoelastic property of clot progression. Sonoclot signature analysis is based on the detection of viscoelastic variations of the plasma or the whole blood sample. As a dynamic and comprehensive test, Sonoclot signature analysis has proven to be clinically valuable in the appraisal of the hemostatic progression during hepatic transplantation, cardiac-surgical procedures.12–14 Previous studies that have investigated the mechanisms underlying HS-induced coagulopathy used standard laboratory testing to measure coagulation status, such as degradation products of thrombin and fibrin production or serum factor levels.15,16 Sonoclot profiling has been used to create and implement individualized peri-operational anticoagulation and antiplatelet remedies in patients with acute myocardial infarction undergoing emergency percutaneous coronary intervention.17 Sonoclot signature analysis has greater sensitivity in monitoring coagulation factors and platelet function in patients with coronavirus disease (COVID-19),18 severe alcohol-associated hepatitis,19 and ICU point-of-care coagulation testing.20 Thromboelastography is a dependable scheme for evaluating coagulability during sepsis and trauma;21 however, Sonoclot characterization is superior to thromboelastography with respect to hypercoagulable state sensitivity,22 practical cost, and usability.10
The purpose of the current study was to evaluate coagulopathies with a dynamic test capable of examining the entire coagulation cascade, and to characterize coagulation prior to transfusion in patients with HS injuries.
Materials and Methods
Study Design and Patients
A prospective observational study was conducted in the Critical Care Center of the General Hospital of Guangzhou Military Command from June 2010 to June 2015. All adult patients who exhibited all three Bouchama criteria,23 as follows, were eligible for the study: mental status alteration (delirium, disorientation, seizures, and coma); body core temperature >40.6°C or recognized evidence of cooling prior to the first confirmed temperature measurement; and a dependable history of well-matched environmental exposure (hot, dry, or flushed skin). The study exclusion criteria were as follows: <18 years of age; arrival time to ICU >1 h; hematopoietic malignancy; undergoing chemotherapy or radiation treatment; known related disorders or severe liver disease; anti-coagulation or anti-platelet therapy; and declined consent to use of data. One hundred seventy-two patients with excessive sweating, thirst, dizziness, headaches, and high fevers sought evaluation in the Critical Care Center and were diagnosed with HS injuries. The patients had the following characteristics: <18 years of age (n = 34); mortality (n = 2); death or discharge within 1 h (n=29); and declined to participate in the study (n = 1). Finally, 106 patients were analyzed (Figure 1).
 |
Figure 1 Flow chart of patient enrollment. |
The research project was approved by the Ethics Committee of Guangzhou General Hospital of Guangzhou Military Command and the work was undertaken conforming to the Declaration of Helsinki. All the subjects gave informed consent and patient anonymity was guaranteed.
Data Collection
Data on patient demographics and time of arrival to the ICU were collected prospectively. Standard clinical features and biological data were assessed on admission. A Sonoclot signature examination was performed on admission. A coagulable state was defined as normal. Hyper- and hypo-coagulable states were defined as coagulation defects. ACT and CR are useful for assessing coagulation in HS injuries because Sonoclot profiling is already available for point-of-care applications beyond DIC diagnosis.24 According to the work principle (see “Supplemental File-Application of Sonoclot”), and previous experiences of Sonoclot application, we identified and estimated the following criteria: Hypercoagulability was defined as an ACT ≦195s and a CR > 23; and hypocoagulability was defined as an ACT ≧119s and a CR < 7. Thirty-four cases (32.1%) were hypercoagulable and 44 cases (41.5%) were hypocoagulable at the time of admission, while 28 cases (26.4%) had no evidence of a coagulopathy.
Neurologic dysfunction was defined by the Glasgow coma score. DIC was defined according to the scoring algorithm criteria established by the ISTH.8 Acute Physiology and Chronic Health Evaluation (APACHE) II score and Sequential Organ Failure Assessment (SOFA) were recorded during the first 24 h after admission, and the ICU and hospital lengths of stay were also calculated. The primary outcome measure was the occurrence of multiple organ dysfunction score (MODS), as assessed by the SOFA score, which reliably assessed organ failure in patients with HS injuries and febrile strokes. The secondary outcome was mortality at day 28.
Blood Sampling
Peripheral venous blood samples were collected immediately upon admission to the ICU. Platelet counts were performed using a hematology analyzer (Sysmex XT1800i; Kobe, Japan) using EDTA anticoagulated blood samples. PT and FL were measured using CA7000 (Blood Coagulation Analyzer, Sysmex, Japan). Plasma quantitative D-dimer assays were performed on a mini-VIDAS analyzer (BioMe´rieux, Marcy l´E´toile, France) using the VIDAS D-dimer exclusion kit (quantitative enzyme-linked fluorescence assay; BioMe´rieux). Sonoclot characterization was performed as follows: A 0.4 mL fresh non-heparinized sample was immersed in the containing cuvette to a fixed depth and vibrated vertically at a frequency of 200 Hz. When the sample coagulated, the free vibration was impeded and the electronic circuitry driving the probe sensed the increase in impedance, which was transformed into an output signal on a paper chart recorder signaling the viscoelastic properties of the clot. A celite-activated cuvette was used (Sienco, Inc., Boulder, CO, USA) because it exhibited minimal differences from fresh native whole blood (data not shown). Records were obtained from the Sonoclot Signature viewer (Sienco, Inc.).
Conventional tests, such as the PT and aPTT, were compared with Sonoclot monitoring to identify coagulation defects.
General Treatment
Upon arrival at the ICU, all patients with HS injuries were immediately treated with a series of cooling treatments, such as continuous manual spraying with a thin stream of water, external cooling with inguinal and axillary ice packs, and intravenous infusions of cold fluids (<4°C).25 All patients were aggressively resuscitated to optimize fluid status and blood pressure maintenance. DIC treatment included the combination of anticoagulants, coagulation inhibitor concentrate, and plasma and platelet substitution therapy according to ISTH guidelines.26
Statistical Analysis
Sonoclot Signature data were analysed using SPSS 20.0 for Windows (IBM, Armonk, NY, USA). Data are reported as the mean ± standard deviation if not otherwise specified. The variables that were not normally distributed are shown as the median and interquartile range (IQR), and categorical variables are shown as n (%). Descriptive statistics were used to describe the study population. Continuous variables were analyzed using Fisher’s exact test, one-way ANOVA, and Bonferroni test. In addition, to judge independent factors affecting mortality and prognosis in DIC, Cox proportional hazards and multivariate regression models were used for analysis. The variable screening method was the Forward:LR method. P values <0.05 were deemed statistically significant. A death-censor strategy was used in this study. If there were missing or abnormal data, the mean replacement method was used, ie, replacing the missing data with the average of the observed value of the variable.27,28
Results
The average age of the 106 patients was 23.2±2.5 years. One hundred six patients were enrolled (males [96.3%] and 4 females [3.7%]). The majority of the patients were males undergoing military training. The average transport time from the field to the ICU was 26.1±10.3 min. The mean body temperature was 41.8±2.2°C. The systolic blood pressure (SBP) and GCS on admission were 101.4±31.6 mmHg and 10, respectively. The mean APACHE II, SOFA, and ISTH scores on admission were 22.4±6.6, 9, and 4, respectively. The mean lengths of stay in the ICU and hospital were 6.8±5.6 and 13.2 ± 16.5 days, respectively. The mortality rate was 10.4%. The patient characteristics are shown in Table 1.
 |
Table 1 Characteristics of Patients with Hyper-, Hypo- and Normo-Coagulable Sonoclot Signatures at the Time of Admission |
After stratification according to blood coagulation on the basis of the Sonoclot signature, no differences in age, gender, body temperature, SBP, GCS, or transport time to the ICU were detected. Patients with hypocoagulation had a higher incidence of severe HS, as evidenced by increased APACHE II, SOFA, and ISTH scores on admission (P < 0.05). Patients with hypocoagulation had longer ICU and overall lengths of stay (P < 0.05; Table 1).
No statistical differences were observed in the international normalized ratio (INR) and D-dimer when the coagulation parameters were compared between the three groups. Furthermore, patients with hypercoagulability on admission had shorter ACT (124.5±22.1 seconds vs 285.2±32.1 seconds, P < 0.05), longer CR (35.1±6.3 sig/min vs 3.5±1.1 sig/min, P < 0.05), and higher PF values (1.4±0.8 vs 0.8±0.3, P < 0.05) compared to hypocoagulable patients. Hypocoagulable patients had a longer aPTT (74.2±35.1 seconds vs 32.8±15.2 seconds, P < 0.05), PT (20.8±8.4 seconds vs 15.2±6.1 seconds, P < 0.05), and lower platelet counts (50.4±30.2×109/L vs 75.2±28.4 ×109/L, P < 0.05) compared to hypercoagulable patients (Table 2).
 |
Table 2 Comparison of Conventional Coagulation Test and Sonoclot Signatures |
MODS developed in 41% of patients with HS. Of patients with hypercoagulation or normal coagulation on admission 5.6% (6/106) developed MODS compared with 35.8% (38/106) of patients with hypocoagulation. APACHE II, ISTH, and SOFA scores were higher in patients with hypercoagulable or normocoagulable MODS compared with patients with hypocoagulable MODS (26.8±6.0 vs 18.5±5.5, 6 vs 3, and 12 vs 6, respectively; P < 0.001).
In addition, the admission Sonoclot signature in patients who developed MODS showed hypocoagulation compared to patients who did not develop MODS. Conventional coagulation screening has also shown that patients who develop MODS tend to have a hypocoagulable state. The coagulation parameters, such as aPTT, PT, ACT, and INR, were longer in the MODS group than the non-MODS group (aPTT: 72.6±43.2s v.s 41.7±25.1s, P < 0.001; PT: 20.4±6.5s vs 16.1±3.2s, P < 0.001; ACT: 389.6±112.8s vs 150.5±89.6s, P < 0.001; INR: 2.1±1.1 vs 1.5±0.6, P < 0.001). The FL, platelet count, and PF were lower in the MODS group than the non-MODS group (fibrinogen: 2.6±2.0 g/L vs 5.2±2.1g/L, P < 0.001; platelet count: 51.3±28.2×109/L vs 134.1±80.4×109/L, P < 0.001; PF: 1.1±0.7 vs 1.5±1.2, P < 0.05; Table 3, Figures 2–5).
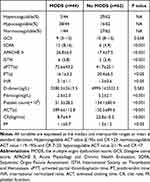 |
Table 3 Sonoclot Signatures on Admission for Patients Who Did and Did Not Develop MODS |
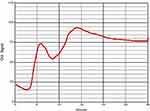 |
Figure 2 Example of normocoagulopathy (ACT192, CR20, PF3.6). Clot signals changed relatively smoothly during the testing time of 30 min. |
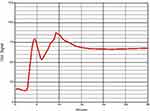 |
Figure 3 Example of hypercoagulopathy (ACT161, CR44, PF4.8). Clot signals grew and had peaks during the testing time of 30 min. |
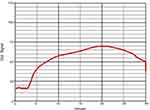 |
Figure 4 Example of hypocoagulopathy (ACT318, CR5.2, PF0.4). Clot signals climbed slowly and kept low during the testing time of 30 min. |
 |
Figure 5 Example of hypofibrinolysis (ACT 119, CR 1.0, PF 3.7). |
In addition, 28-day mortality was lower in patients with hypercoagulation at the time of admission compared with patients with hypocoagulation (5.9% vs 18.1%, respectively, P < 0.05) and was not statistically different compared to the normocoagulation group.
The demographic variables (age and gender), scores, and the coagulation variables were analyzed by Cox regression. We showed that increased APACHE II (odds ratio [OR]: 1.038, 95% CI: 1.003–1.064; P < 0.05), SOFA (OR: 1.125, 95% CI: 1.081–1.324; P < 0.05), and ISTH scores (OR: 1.312, 95% CI: 1.041 −1.811; P < 0.05), delayed ACT (OR: 1.003, 95% CI, 1.002 −1.004; P < 0.01), shortened CR (OR: 0.811, 95% CI: 0.697 −1.032; P <0.01), and lower PF (OR: 0.743; 95% CI: 0.614–0.928; P < 0.05) were independent variables affecting survival in patients with HS injuries (Table 4).
 |
Table 4 Cox Regression Model Analyzing the Prognosis of Patients with Heat Stroke |
Discussion
Coagulopathies occur in patients with HS injuries, and the mechanism underlying thermal injury to the vascular endothelium has recently been reviewed.29,30 Patients undergoing HS are also at high risk for hemorrhage complications, which remains a leading cause of mortality. The standard coagulation test represents the initial creation of thrombin in plasma and is not disturbed by any of the corpuscular elements in the blood.31 Our study indicated that patients with hypocoagulation had a higher incidence of severe HS, longer ICU and overall lengths of stay, and a higher rate of MODS than normal or hypercoagulation patients. Our findings suggest that the commonly used coagulation function determined by standard coagulation testing may be inadequate. Therefore, how to effectively discriminate coagulability in HS patients is important for reduction in thrombotic or hemorrhagic risk.
Compared to conventional laboratory tests, the DIC score, and thromboelastography, Sonoclot has advantages such as real-time dynamic viscoelastic property of clot progression, and higher sensitivity, as stated in the Introduction section.7–11,21,22 Furthermore, Sonoclot profiling does not require a special solid platform to absorb shocks. In the current study, we demonstrated a hypercoagulable state identified by Sonoclot signature analysis occurring in 34 patients (32%) at the time of admission prior to blood product transfusion. This indicates that early HS-induced coagulopathy is initially described by hypercoagulability because of increased thrombin and fibrin generation, as reported previously in another study.32 Hypofibrinolysis was also found in our series because 10 (9.4%) patients had normal ACT values and CRs <7 based on Sonoclot signature. This finding supports previous evidence that thrombin-dependent mechanisms may contribute to hypofibrinolysis during HS.33,34
Previous studies have shown that HS is accompanied by intense activation of inflammation and coagulation, which both contribute to organ dysfunction.29,35,36 Coagulopathy with diagnostic features can be observed at early stage after heat exposure and before MODS through accessible biomarkers. However, the clinical manifestations and consequences of coagulability after HS are not entirely clear. Our data indicate that patients with hypocoagulability on admission developed more severe organ dysfunction and had increased mortality in the future. Whether the occurrence of hypercoagulability was associated with MODS requires further study. Our data also showed that hypocoagulability predicted 28-day survival in patients with HS. Our finding that prolonged ACT was predictive of the mortality in HS suggests that the Sonoclot signature can be used to identify patients for whom hemostasis treatment may be promising. The reasons that CR and PF were not statistically significant might be due to the small sample size and single center study, thus further studies are warranted. What is more, hypocoagulable patients had a longer aPTT, and aPPT was longer in the MODS group than non-MODS group, which demonstrated aPTT might be a risk factor for severe HS as previous report stated.37
It is concerning that in many cases, anticoagulation may have been inadequate, highlighting the need for alternative or additional therapies. Many ongoing studies investigating the pathophysiology underlying COVID-19-associated coagulation may provide mechanistic insights that can guide appropriate interventional strategies.38 For example, based on the Sonoclot signature, which has the advantage of a comprehensive assessment of whole blood coagulation capacity, the role of various factors, such as platelets and clotting factors, on thrombosis can be assessed and targeted with effective anticoagulation prophylaxis to reduce the risk of thrombotic complications. Individuals with COVID-19 may have a number of complex and varied coagulation abnormalities that create a hypercoagulable state, presenting questions about proper evaluations and interventions to prevent or treat thrombosis. Normal or mildly prolonged aPTT and PT, slight thrombocytopenia or thrombocytosis, or a normal platelet count or elevated FL and D-dimer are typically noticed.32–37 The clinical significance of these aforementioned findings were also discovered to relate to the 28-day mortality rate based on multivariate regression testing of 447 patients in a retrospective investigation;39 however, the mechanisms underlying hypercoagulable state caused by HS and COVID-19 may be similar or completely different, and although both manifest as a hypercoagulable state, further evidence is needed.
Our study had some limitations: 1. The occurrence of venous thromboembolism was not systematically studied and Sonoclot signature analysis was performed in each patient at only one time point, ie, immediately after admission. 2. There was no thromboelastometry group to compare and no thromboelastography was shown. 3. It was not possible to assess the effect of late hypercoagulability on the development of organ dysfunction. 4. Power calculation was not proceeded and might have led to some deviation in statistical analysis.
Conclusions
Sonoclot signature analysis is a relatively reliable method for assessing coagulability. The presence of early hypocoagulability is associated with the development of MODS. In addition, Sonoclot characteristics indicating a hypocoagulable state on admission may be predictive of the development of death. This study determined the prognostic value of the clinical outcome and coagulation of HS as defined by Sonoclot signature analysis at the time of admission. Further large studies with large samples should aim at developing transfusion protocols based on Sonoclot signature and using anticoagulants or even prophylactic medication for patients with HS.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The research project was approved by the Ethics Committee of Guangzhou General Hospital of Guangzhou Military Command and the work was undertaken conforming to the Declaration of Helsinki. All the subjects gave informed consent and patient anonymity was guaranteed.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81101406 and 81071529) and the Project of Medical Research of PLA (BWS12J108).
Disclosure
The authors declare that there are no competing interests.
References
1. Beltran G. Emergency Medical Services: Clinical Practice and Systems Oversight.
2. Levi M. Burning issues surrounding inflammation and coagulation in heatstroke. Crit Care Med. 2008;36:2455–2456. doi:10.1097/CCM.0b013e31818114f6
3. Huisse MG, Pease S, Hurtado-Nedelec M, et al. Leukocyte activation: the link between inflammation and coagulation during heatstroke. A study of patients during the 2003 heat wave in Paris. Crit Care Med. 2008;36:2288–2295. doi:10.1097/CCM.0b013e318180dd43
4. Hifumi T, Kondo Y, Shimazaki J, et al. Prognostic significance of disseminated intravascular coagulation in patients with heat stroke in a nationwide registry. J Crit Care. 2018;44:306–311. doi:10.1016/j.jcrc.2017.12.003
5. Jilma B, Derhaschnig U. Disseminated intravascular coagulation in heat stroke: a hot topic. Crit Care Med. 2012;40:1370–1372. doi:10.1097/CCM.0b013e31823d785d
6. Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5:611–647.
7. Levi M, Meijers JC. DIC: which laboratory tests are most useful. Blood Rev. 2011;25:33–37. doi:10.1016/j.blre.2010.09.002
8. Taylor FB, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. doi:10.1055/s-0037-1616068
9. Gao TY, Yang WC, Zhou FH, Song Q. Analysis of D-dimer cut-off values for overt DIC diagnosis in exertional heat illness. Medicine. 2020;99(52):e23831. doi:10.1097/MD.0000000000023831
10. Hett DA, Walker D, Pilkington SN, et al. Sonoclot analysis. Br J Anaesth. 1995;75:771–776. doi:10.1093/bja/75.6.771
11. Saleem A, Blifeld C, Saleh SA, et al. Viscoelastic measurement of clot formation: a new test of platelet function. Ann Clin Lab Sci. 1983;13:115–124.
12. Saxena P, Bihari C, Rastogi A, et al. Sonoclot signature analysis in patients with liver disease and its correlation with conventional coagulation studies. Adv Hematol. 2013;2013:237351.
13. Lee B, Al-Waili N, Butler G, et al. Assessment of heparin anticoagulation by Sonoclot Analyzer in arterial reconstruction surgery. Technol Health Care. 2011;19:109–114. doi:10.3233/THC-2011-0612
14. Bischof DB, Ganter MT, Shore-Lesserson L, et al. Viscoelastic blood coagulation measurement with Sonoclot predicts postoperative bleeding in cardiac surgery after heparin reversal. J Cardiothorac Vasc Anesth. 2015;29:715–722. doi:10.1053/j.jvca.2015.01.015
15. Bouchama A, Bridey F, Hammami MM, et al. Activation of coagulation and fibrinolysis in heatstroke. Thromb Haemost. 1996;76:909–915. doi:10.1055/s-0038-1650685
16. Kawasaki T, Okamoto K, Kawasaki C, et al. Thrombomodulin improved liver injury, coagulopathy, and mortality in an experimental heatstroke model in mice. Anesth Analg. 2014;118:956–963. doi:10.1213/ANE.0000000000000170
17. Yang WX, Lai CL, Chen FH, et al. The value of Sonoclot detection technology to guide the clinical medication of the perioperative anticoagulation and antiplatelet therapy in patients with acute myocardial infarction undergoing emergent PCI. Exp Ther Med. 2017;13:2917–2921. doi:10.3892/etm.2017.4336
18. Song JC, Wang G, Zhang W, et al. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Military Med Res. 2020;7:19. doi:10.1186/s40779-020-00247-7
19. Premkumar M, Bihari C, Saxena P, et al. Heparin-like effect associated with risk of bleeding, sepsis, and death in patients with severe alcohol-associated hepatitis. Clin Gastroenterol Hepatol. 2020;18:486–495. doi:10.1016/j.cgh.2019.04.057
20. Govil D, Pal D. Point-of-care testing of coagulation in intensive care unit: role of thromboelastography. Indian J Crit Care Med. 2019;23(Suppl 3):S202–S206.
21. Mallett SV, Cox DJ. Thrombelastography. Br J Anaesth. 1992;69:307–313. doi:10.1093/bja/69.3.307
22. Francis JL, Francis DA, Gunathilagan GJ. Assessment of hypercoagulability in patients with cancer using the Sonoclot Analyzer and thromboelastography. Thromb Res. 1994;74:335–346. doi:10.1016/0049-3848(94)90149-X
23. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi:10.1056/NEJMra011089
24. Wan P, Tong HS, Zhang XQ, et al. Diagnosis of overt disseminated intravascular coagulation in critically ill adults by Sonoclot coagulation analysis. Int J Hematol. 2014;100:125–131. doi:10.1007/s12185-014-1601-3
25. Mutha B. CN management of heat stroke and heat exhaustion. Inter J Curr Trends Sci Tech. 2010;1:1–7.
26. Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi:10.1111/j.1365-2141.2009.07600.x
27. Kenward MG, Molenberghs G. Parametric models for incomplete continuous and categorical longitudinal data. Stat Methods Med Res. 1999;8:51–83. doi:10.1177/096228029900800105
28. Bello A. Imputation techniques in regression analysis: looking closely at their implementation. Comput Stat Data Anal. 1995;20:45–57. doi:10.1016/0167-9473(94)00024-D
29. Roberts GT, Ghebeh H, Chishti MA, et al. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol. 2008;28:1130–1136. doi:10.1161/ATVBAHA.107.158709
30. Bouchama A, Al-Mohanna F, Assad L, et al. Tissue factor/factor VIIa pathway mediates coagulation activation in induced-heat stroke in the baboon. Crit Care Med. 2012;40:1229–1236. doi:10.1097/CCM.0b013e3182387bef
31. Meybohm P, Zacharowski K, Weber CF. Point-of-care coagulation management in intensive care medicine. Crit Care. 2013;17:218. doi:10.1186/cc12527
32. Matsumoto H, Takeba J, Umakoshi K, et al. Successful treatment for disseminated intravascular coagulation (DIC) corresponding to phenotype changes in a heat stroke patient. J Intensive Care. 2019;7:2. doi:10.1186/s40560-019-0359-3
33. Aoki K, Yoshino A, Ueda Y, et al. Severe heat stroke associated with high plasma levels of plasminogen activator inhibitor 1. Burns. 1998;24:74–77. doi:10.1016/S0305-4179(97)00079-X
34. Watanabe R, Wada H, Watanabe Y, et al. Activity and antigen levels of thrombin-activatable fibrinolysis inhibitor in plasma of patients with disseminated intravascular coagulation. Thromb Res. 2001;104:1–6. doi:10.1016/S0049-3848(01)00331-0
35. Pease S, Bouadma L, Kermarrec N, et al. Early organ dysfunction course, cooling time and outcome in classic heatstroke. Intens Care Med. 2009;35:1454. doi:10.1007/s00134-009-1500-x
36. Proctor EA, Dineen SM, Van Nostrand SC, et al. Coagulopathy signature precedes and predicts severity of end-organ heat stroke pathology in a mouse model. J Thromb Haemost. 2020;18:1900–1910. doi:10.1111/jth.14875
37. Xing L, Liu SY, Mao HD, et al. The prognostic value of routine coagulation tests for patients with heat stroke. Am J Emerg Med. 2021;44:366–372. doi:10.1016/j.ajem.2020.04.062
38. Abou-Ismail MY, Diamond A, Kapoor S, et al. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi:10.1016/j.thromres.2020.06.029
39. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi:10.1111/jth.14817
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
