Back to Journals » Infection and Drug Resistance » Volume 15
Some Manifestations of Tuberculosis in Otorhinolaryngology – Case Series and a Short Review of Related Data from South-Eastern Europe
Authors Mocanu AI, Mocanu H , Moldovan C , Soare I , Niculet E , Tatu AL , Vasile CI , Diculencu D, Postolache PA, Nechifor A
Received 25 March 2022
Accepted for publication 12 May 2022
Published 31 May 2022 Volume 2022:15 Pages 2753—2762
DOI https://doi.org/10.2147/IDR.S367885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Adela-Ioana Mocanu,1,* Horia Mocanu,2 Cosmin Moldovan,3,4,* Ioana Soare,3,* Elena Niculet,5,6 Alin Laurentiu Tatu,6,7,* Claudiu Ionut Vasile,7,* Daniela Diculencu,8 Paraschiva A Postolache,9,* Alexandru Nechifor7
1Department of ENT & HNS, Polimed Medical Center, Bucharest, Romania; 2Department of ENT & HNS, Faculty of Medicine, “Titu Maiorescu” University, Bucharest, Romania; 3Faculty of Medicine, “Titu Maiorescu” University of Bucharest, Bucharest, Romania; 4General Surgery Ward, ‘Witting’ Clinical Hospital, Bucharest, Romania; 5Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galati, Romania; 6Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID), “Dunărea de Jos” University, Galați, Romania; 7Clinical Medical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galati, Romania; 8Medical Analysis Laboratory, Clinical Pneumoftisiology Hospital, Iasi, Romania; 9Clinical Medical Department, Faculty of Medicine, “Grigore T Popa” University of Medicine and Pharmacy, Iasi, Romania
*These authors contributed equally to this work
Correspondence: Adela-Ioana Mocanu, Department of ENT&HNS, Polimed Medical Center, Bucharest, Romania, Tel +40 751 209 060, Email [email protected] Elena Niculet, Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania, Tel +40 741 398 895, Email [email protected]
Abstract: Tuberculosis is a disease of global outreach that may affect the entire human body but is most commonly located in the lungs. Otorhinolaryngological manifestations of tuberculosis are rare, mostly occurring secondary to pulmonary disease but nevertheless represent significant diagnostic challenges. Nasopharyngeal tuberculosis is rare, representing around 1% of all upper air-way localizations and the most common presentation is in the form of adenoids. Tuberculous glossitis (oral tuberculosis) is even scarcer and may present in various clinical forms, usually mimicking a malignant neoplasm, or, less often, trauma or other infectious lesions. Oropharynx tuberculosis is usually misdiagnosed as hypertrophic chronic tonsillitis. We present four rare cases of ENT tuberculosis, primary adenoiditis and tonsillitis in a 13-year-old girl, a curious case of tuberculous glossitis in a 65-year-old woman, clinically diagnosed as a lingual neoplasm and two cases of tuberculous lymphadenopathy uncommonly located in the submandibular and supraclavicular regions. A comprehensive review of literature follows the case presentations. Tuberculous manifestation in the ear, nose and throat area remains a difficult diagnosis to establish, particularly because of its rarity and non-specific clinical appearance, and should be included in the differential diagnosis of pharynx lesions. An early diagnosis is essential to avoid occurrence of complications.
Keywords: case report, tuberculosis, nasopharynx, oral tuberculosis, head and neck, extra nodal
Introduction
Tuberculosis (TB) represents one of the oldest diseases in humans and at the same time one of the most common chronic granulomatous infections, especially in developing countries. Mycobacterium tuberculosis has been discovered by Robert Koch over 100 years ago, and, despite the successful anti-tuberculous chemotherapy treatments, it remains a public health concern. According to the World Health Organization (WHO) report from 2021, after a large increase in TB cases between 2017 and 2019, in 2020 there was a noticeable decrease (18%) in the reported cases: from 7.1 million the previous year, to 5.8 million, back to the level of 2012. This can be attributed to the ongoing COVID-19 (CoronaVirus Disease of 2019) pandemic. Worldwide, 9.9 million people fell ill with tuberculosis in 2020 (127/100.000), which represents a slight decline as compared to 2019, but the WHO predicts a worsening trend in TB deaths for 2021 and beyond. Presently, TB is ranked 13 amongst death causes worldwide. The total number of deaths reported an increase from 1.2 million to 1.3 million.1 In the WHO - European region, Romania occupies the first place in number of cases (23.4%) and a notify rate (59.9/100.000 inhabitants), followed by Lithuania with 37.9/100.000. Amongst the 18 countries considered high priority in the WHO-European Region, Romania is ranked number 8 with an incidence of 57.1/100.000 inhabitants. The proportion of extrapulmonary tuberculosis (EPTB) remained relatively stable over the last four years.1 TB of the head and neck represents about 10% of all extra-pulmonary cases2 and most of them are located in the cervical lymph nodes.3 In the ears, nose and throat (ENT) region the most common presentation is in the cervical lymph nodes, the majority located in the posterior triangle. Extra nodal ENT presentation represents less than 1% of all TB sites.4–6 Excluding cervical lymphadenitis, the most frequent otorhinolaryngological localizations are laryngeal TB in about 8% of cases,7,8 nose in 2.9% and middle-ear in 1.96% of cases.8 Other regions include adenoids, tonsils, oropharynx, parotid and submandibular gland, palate, and tongue. The aim of our study is to increase awareness on the diversity of presentations in head and neck TB and on diagnostic difficulties.
Infection usually occurs via lympho-hematogenous spread from primary lung disease but may sometimes appear after pharynx inoculation or reactivation of dormant acid-fast bacilli in the lymphatic system. Clinical examination and imaging and tuberculin skin tests are not specific and usually misleading. The diagnosis is based on histopathological examination since smear and culture tests take up to two months to provide a result and are difficult to perform in EPTB due to the low number of bacilli in the specimen. Modern laboratory methods such as PCR (polymerase chain reaction) are proving more and more useful.6,9,10
The WHO guidelines are very specific regarding treatment of EPTB. Surgery has no curative role and is more of an early diagnosis method. Antituberculous chemotherapy should be administered following the same 4 drug regimen for 2 months, followed by 2 drug regimen for 4 months.11
Primary nasopharyngeal TB represents the isolated infection of the nasopharynx without pulmonary or systemic disease. The condition is very unusual and only a few cases have been reported in the literature. The patient may have chronic nasal obstruction, rhinorrhea and, especially in children, it can be easily mistaken for chronic adenoiditis, as was the case of our patient. Tonsillar TB is more of a primary type of infection, usually involving a sore throat and difficulty swallowing; it is present in younger patients, in conjunction to TB adenoiditis. Before the development of specific chemotherapy, 6.5% of all tonsils removed from asymptomatic patients proved to contain tubercles.12,13 The introduction of medical treatment and the pasteurization of cow’s milk led to a considerable reduction of that percentage.14
TB glossitis is also rarely reported in the literature, usually secondary to pulmonary infection15–17 and frequently misdiagnosed as a neoplasm. Primary tuberculous glossitis is exceedingly rare.18,19
TB lymphadenopathy represents the most common localization in the head and neck area and has been heavily reported in the literature, usually in the posterior triangle but seldom in the submandibular region or the supraclavicular region.
Materials and Methods
Case 1- Tuberculous Adenotonsilitis
A 13-year-old female patient presented with typical chronic adenotonsillitis symptoms – bilateral chronic nasal obstruction, headaches, chronic muco-purulent rhinorrhea, oral breathing, and sore throat. The clinical examination revealed grade III tonsillar hypertrophy and a large pinkish mass obstructing the choanae bilaterally (upon endoscopic examination) and a mild retraction of the tympanic membranes. Routine blood work and a chest X-ray yielded close to normal results. The diagnosis of chronic adenotonsillitis was set and the patient underwent an adenotonsillectomy under total anesthesia. The surgical specimen (Figure 1) consisted of both palatine tonsils and the pharyngeal tonsil, all with an unusually grey-yellowish, elastic, almost cartilaginous gross aspect, which led us to ask for histopathological examination. This showed lymphatic tissue with an intensely modified architecture due to the presence of tuberculous granulomas with central caseous necrosis with Langhans giant cells and epithelioid cells. The patient followed an uneventful post-operative course and was referred to the Infectious Disease Department for further diagnosis and treatment. The final diagnosis of primary tuberculous adenotonsillitis was established through special cultures and acid-fast bacteria (AFB) staining of the sputum, and the classic WHO Category-3 regimen was employed (Isoniazid, Rifampicin and Pyrazinamide for 3 times/week in the first 2 months followed by only Isoniazid and Rifampicin for the next 4 months – 2H3R3Z3/4H3R3). Follow-ups at 3, 6 and 12 months revealed no sign of recurrence or residual disease. The patient’s legal guardians/parents have given and signed the informed consent for publication.
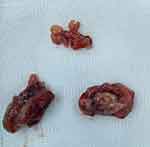 |
Figure 1 Gross examination of the palatine and pharyngeal tonsils, all with an unusually grey-yellowish, elastic, almost cartilaginous aspect. |
Case 2 – Tuberculous Glossitis
A 64-year-old female patient with a history of cervical cancer was admitted in our clinic for odynophagia, anorexia and weight loss, all due to a nodular, ulcerated, infiltrating mass on the left side margin of the tongue, extending inferiorly towards the ventral surface (Figure 2). The lesion was described as a tumor, and a biopsy was taken. Chest X-ray showed bilateral diffuse micronodular and nodular opacities, and an infectious substrate was suspected. Pneumology consult raised a suspicion of secondary pulmonary tuberculosis. A surgical consult for intense abdominal pain with muscular defense established a diagnosis of acute abdomen with generalized peritonitis. An emergency segmental enterectomy of the jejune was performed. Macroscopic inspection revealed a semi-circumferential and transverse ulcer. Histopathological examination of both lingual biopsy and jejune resection specimen showed numerous caseous and non-caseous epithelioid and giant cells granulomas (Figure 3). Acid-fast bacilli were identified using Ziehl–Neelsen stain (Figure 4), and the diagnosis of lingual and jejunal tuberculosis was established. Unfortunately, the patient’s postoperative evolution was severe, and she died within 10 days from being admitted in the intensive care unit, from acute circulatory and respiratory insufficiency.
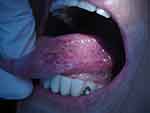 |
Figure 2 Clinical examination of the tongue revealing a nodular, ulcerated, infiltrating mass on the left side, extending inferiorly towards the ventral surface. |
 |
Figure 3 Microscopic examination revealing numerous caseous and non-caseous epithelioid and giant cell granulomas. (hematoxylin-eosin, 40x). |
 |
Figure 4 Ziehl-Neelsen special stain revealing acid-fast bacilli (pink rods on a blue background). |
Case 3 – Submandibular Tuberculous Lymphadenopathy
A 71-year-old female patient was referred to our clinic from a Psychiatric Hospital (where she was institutionalized for several years) for the diagnosis and treatment of a round, mobile, elastic, submandibular tumor of around 5 cm, which has been slowly growing in the last year (Figure 5). The fact that she had no close relatives and a scarce medical record, made it impossible to establish a history or opportunity of TB contact. However, the fact that she came from a psychiatric institution may be considered circumstantial evidence in this direction. The evaluation protocol included a complete ENT, chest X-ray and complete blood work, all of these showing minimal changes. The mass was excised under general anesthesia, and an additional smaller one (about 1 cm) was found intraoperatively, beneath it. Both masses had suppuration, being filled with a creamy yellow puss. The histopathological examination revealed lymph nodes with modified architecture, with frequent granulomas of various shapes and sizes, necrosis, giant multinuclear cells, areas of ulceration and granulation (Figure 6). The patient followed an uneventful post-operative course and was referred to the Infectious Disease Department for further diagnosis and treatment. The final diagnosis of primary tuberculous adenotonsilitis was set by special cultures and AFB staining of the sputum, and the classic WHO Category-3 regimen was employed. Follow-ups at 3, 6 and 12 months revealed no sign of recurrence or residual disease.
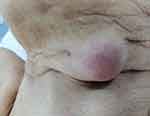 |
Figure 5 Clinical examination of a round, mobile, submandibular tumor. |
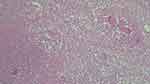 |
Figure 6 Microscopic examination revealing epithelioid granulomas of various shapes and sizes, necrosis, giant multinucleated cells (Langhans giant cells). |
Case 4 – Supraclavicular Tuberculous Lymphadenopathy
A 74-year-old male patient was referred to our clinic from the same Psychiatric Hospital as Case 3, which led us to the conclusion that a TB exposure is present in that institution. He presented with a 5 to 6 cm round, mobile, elastic, supraclavicular tumor on the left side that has been slowly growing for the last year. A complete medical history and TB exposure history was also impossible to obtain. The evaluation protocol included a complete ENT, chest X-ray and blood work showing minimal changes. The mass was excised under general anesthesia and sent for histopathological examination. It also had suppuration and was filled with creamy yellow puss. The histopathological examination revealed similar results as Case 3, with intensely modified lymph nodes by the presence of frequent granulomas of various shapes and sizes, necrosis, giant multinuclear cells, areas of ulceration and granulation. The post-operative course was in this case rather laborious due to the precarious healing of the wound (Figure 7) which had to be re-stitched several times until it completely healed. After complete recovery, the patient was referred to the Infectious Disease Department for further diagnosis and treatment. The final diagnosis of primary tuberculous adenotonsillitis was set by special cultures and AFB staining of the sputum and the classic WHO Category-3 regimen was employed. Follow-ups at 3, 6 and 12 months revealed no sign of recurrence or residual disease.
 |
Figure 7 Defective wound healing. |
Case 1, 3 and 4 did not receive the positive diagnosis of tuberculosis in our surgical clinic, but after they were sent to the infectious diseases clinic. Thus being said, the four cases of extra-pulmonary tuberculosis had minimal, non-specific changes in regards to the laboratory and imaging studies, having had modified erythrocyte sedimentation rates (elevated ranges of approximately 3 to 4 times the normal values) and elevated fibrinogen values (above the normal range), revealing the active inflammatory state of the patients. At the same time, the X-rays of patients 1, 3 and 4 had minimal changes, with accentuated interstitial pattern, while in case 2, the X-rays revealed a more complex reticular pattern with micronodules and a condensing area located peripherally, in the right medial lung lobe. In case 2, at first the patient refused the surgical intervention on her abdomen (due to intense, diffuse pain both spontaneous and when palpating, with muscle defence and right pneumoperitoneum), having accepted only the biopsy from the tongue pseudo-tumor. A diagnosis of perforated bowel (or perforated stomach) accompanied by acute peritonitis was established and due to worsening of symptoms, the patient consented to being operated, with the aforementioned final development.
Discussions
The human body is the only natural reservoir for Mycobacterium tuberculosis, an air-borne bacteria transmitted via Pflügge’s droplets during coughing, talking, or sneezing. The tuberculous bacilli are obligatory anaerobes, meaning they only affect oxygen-rich organs such as the lungs. However, the infection can spread and affect any organ of the human body causing what we call EPTB. This type of infection seems to have an increase in the number of cases, especially in developing countries. Of these, approximately 10% is tuberculosis of the head and neck region.20 The most frequently affected organs are cervical lymph nodes, larynx, deep neck spaces (abscesses) and middle-ear.8 Other less common locations are the nasal and paranasal region, nasopharynx (adenoids), oral cavity (tongue, palate), salivary glands etc.
The risk factors for TB infection include smoking, overcrowding, immunocompromised status (HIV), young age, malnutrition, indoor air pollution, diabetes, smoking and alcohol consumption.21 Our lymphadenitis patients came from a psychiatric institution where living conditions were poor and direct contact with other patients was probably common. This led us to receiving two very similar cases within a very short period. The only difference between the two cases was the location of the affected lymph nodes.
Diagnosis of head and neck tuberculosis is complex and requires the involvement of several departments. On top of a complete clinical examination, including endoscopy, chest X-ray and sometimes computed-tomography (CT) of the region may provide valuable information. Most of these lesions end up with a clinical diagnosis of neoplasm or other type of inflammatory lymphadenitis, leading to biopsy. The samples are then sent to the Histopathology Department where they are analyzed with the help of hematoxylin-eosin stains, revealing chronic granulomatous inflammation, necrosis/caseation with Langerhans giant cells, and also with the help of Ziehl–Neelsen stained slides; samples are also sent to the Microbiology Department for diagnosis (usually growth on the egg-based Lowenstein-Jensen on medium or the agar-based medium Middlebrook 7H10 or 7H11), after digestion and decontamination by modified Petroff’s method.9 This process can take up to six weeks, but results are usually obtained within 7–21 days. Drug susceptibility testing is also a reason to perform classical microbiology tests. More modern methods such as PCR detect deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) much faster, and can provide results in 12–24h.22 We should also underline the fact that such techniques are more expensive and at times not available in developing countries such as Romania.
The treatment for EPTB is standardized and follows the Guidelines of the WHO, usually involving an intensive 2 months, 4 drug treatment phase, followed by a continuous 4 months, 2 drugs treatment phase, as presented above. Sometimes multi-drug resistant TB can occur and that presents a complicated problem for the physician.
Cervical tuberculous lymphadenitis is by far the most common location, as reported by several studies in the literature, presenting ratios ranging from 72.6% to 97%.8,17,23–27 Although our study has no statistical data to report, the fact that two out of the four presented cases were of lymph node TB infection suggests that all previously reported results are accurate. This pathology usually manifests with multiple painless cervical lymph nodes, the majority of which are situated in the posterior triangle of the neck - 64.6% (according to Bokare et al), 78% (according to Sharma et al and Bayazit et al).17,28,29 Our two cases present unusual location in the supraclavicular and submandibular region. Sometimes, an abscess may be present30,31 as we have ourselves discovered in both cases.
Tuberculous glossitis was first described by Morgagni in 176132 and usually manifests with painful ulcerations. It was probably ignored for centuries or misdiagnosed and mistreated, since, unfortunately, medical services at that time were rudimentary and rather palliative, and were provided mainly in monastic hospitals, especially for poor people, with poor results due to the lack of sanitary conditions.33 Oral cavity TB is generally very rare: less than 0.2% of all cases of TB,34 and may be primary or secondary. Oral tuberculosis has been described as three clinical forms: acute miliary, chronic ulcerative and lupus vulgaris.35 Other types of lesions such as diffuse glossitis and tuberculomas, have also been reported in the specialty literature. The most common site is the dorsal part of the tongue, and a differential diagnosis usually includes neoplasms, syphilis, ulcers, granulomatous disease, and mycosis. Our case is unusual since the tumor showed infiltration of the left margin of the tongue, extending mostly towards the ventral surface; it had clear signs of a neoplasm and was also associated with what was later discovered as intestinal tuberculosis. The evolution of intestinal TB brought about the patient’s demise, which underlines the possible severe complications of EPTB. As far as Romania is concerned, another similar case was reported in 2015 by Nemes et al, which was also a lateral tongue lesion in a patient from a poor socio-economic environment, with a similar clinical presentation but lacking the life-threatening complication from our case.36 A larger study by Popescu et al presented 17 cases of oral TB selected over 23 years from an initial cohort of 774 patients with histologically established diagnosis of granulomatous inflammatory lesions of the oral cavity. This study emphasizes the rarity of oral tuberculosis; most of the lesions involved the tongue, the salivary glands, or an association of the two. The authors also reported on scarce findings in literature, where only 10 countries published over 10 cases of oral involvement in TB, starting from the year 2000, as follows: India (114 cases), China (43 cases), Turkey (30 cases), Brazil (23 cases), Spain (22 cases) and England (11 cases). This would bring the statistics at a mean of 3 cases for every 5 years.37
Primary tuberculous adenotonsillitis is described as the infection of the nasopharynx and tonsils without pulmonary or systemic disease. It is a rather unusual location with only a few cases described.38 It is easily mistaken for classical chronic adenotonsillitis (with the same clinical appearance and same symptoms), and ENT specialists usually treat it accordingly, by removing the affected organs (palatine and pharyngeal tonsils) as was the case from our study. A clear diagnosis can only be set by histopathology and microbiology. The aspect that caught our attention was the unusual gross aspect of the specimen, and we decided to investigate further. It is also worth mentioning the young age of the patient, in accordance with other reported cases,39 and the total lack of lung or lymph node involvement.
TB as a whole represents a very serious public health problem in Romania. Since 2002, the reported TB cases nationwide have declined, but the multidrug resistance of this disease has taken the spotlight. Although control measures have been taken (for example, national treatment programs) and have been successful, Romania’s TB burden is still severe. Multidrug-resistant TB (isoniazid and rifampicin-resistant) is a serious concern not only in Romania but also worldwide.40,41 Extensively drug resistant TB cases have also emerged, being resistant also to kanamycin, amikacin, capreomycin, and fluoroquinolones (at least one).42 In 2011, Romania was reported to have a high incidence of EPTB (14% of all TB cases reported that year), along with Estonia, Latvia, Bulgaria, Lithuania, Poland and Portugal. Even more worrisome is the TB-HIV infection association, in Eastern Europe TB representing the most frequent opportunistic infection in HIV patients (32% pulmonary TB, 12% EPTB), making it necessary to screen for TB at every HIV-positive patient’s visit; HIV is also associated with HPV (human Papilloma virus) infection, which determines the development of skin or mucosal lesions.43,44 Extensive measures must be taken in order to control TB better: appropriate financial support, electronic data collection and media support, drug susceptibility testing availability, proper protocols for TB and TB/HIV treatment.45
Conclusion
TB is still a prevalent health problem in developing countries since no country in the world, no matter how developed, has not yet reached complete control of this issue. The most common site is the lung but the fact that it can manifest in virtually any organ as extrapulmonary disease, brings about several diagnostic challenges. The mortality and morbidity of TB is extremely high, especially in overpopulated areas. The treatment is standardized by the WHO, but it also requires prompt and accurate diagnosis. Unrecognized, it can be lethal, as presented in this article.
In the head and neck region, it is a rather rare occurrence but can affect any organ, both as primary of secondary infection, and is frequently misdiagnosed as a malignancy due to its lack of characteristic symptoms. The most common location is in the lymph nodes, followed by larynx, middle-ear, nose, oral cavity, nasopharynx, salivary glands, etc. Since this type of disease is also paucibacillary and many times without lung involvement, any suspected case should be diagnosed with the histopathological examination of the specimens.
Adenotonsillar TB is exceedingly rare and is usually found in young patients where it is misdiagnosed as chronic adenotonsillar inflammation.
Tuberculous glossitis remains a difficult diagnosis to establish, particularly because of its rarity and non-specific clinical appearance, and should be included in the differential diagnosis of oral lesions. It is rarely a primary infection, and an early diagnosis is essential to avoid occurrence of complications.
Abbreviations
TB, tuberculosis; EPTB, extrapulmonary tuberculosis; ENT, ears, nose and throat; PCR, polymerase chain reaction; AFB, acid-fast bacilli; CT, computed tomography; DNA, deoxyribonucleic acid; RNA, ribonucleic acid.
Data Sharing Statement
The information will be granted access to under reasonable request.
Ethics Approval and Informed Consent
For this study, the agreement was obtained from the Research Ethics Committee of the Faculty of Medicine, “Titu Maiorescu” University.
Consent for Publication
All patients provided informed consent and approved the publication of data.
Acknowledgments
The authors wish to acknowledge that the present study was academically supported by the ‘Dunarea de Jos’ University of Galati, Romania, through the research center – Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID). The authors also wish to acknowledge Constantin Ghimus’s and Stefan Sandulache’s contribution in data collection.
Funding
The present manuscript’s article publishing charges were paid by the “Dunărea de Jos” University of Galați.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2021. Geneva: World Health Organization; 2021. License: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/item/9789240037021.
2. Menon K, Bem C, Gouldesbrough D, Strachan DR. A clinical review of 128 cases of head and neck tuberculosis presenting over a 10-year period in Bradford, UK. J Laryngol Otol. 2007;121(4):362–368. doi:10.1017/S0022215106002507
3. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72(9):1761–1768.
4. Bharatha A, Bartlett ES, Yu E. Pharyngeal and retropharyngeal tuberculosis with nodal disease. Radiology. 2010;254(2):629–632. doi:10.1148/radiol.2542081660
5. Moon W, Han MH, Chang KH, et al. CT and MR imaging of head and neck tuberculosis. Tuberc Radiograph. 1997;17(2):391–402. doi:10.1148/radiographics.17.2.9084080
6. El Ayoubi A, Benhammou A, El Ayoub F, et al. Primary extranodal ENT tuberculosis. Annales d’otolaryngologie et chirurgie cervico-faciale. 2009;165:1–8.
7. Harney M, Hone S, Timon C, Donnelly M. Laryngeal tuberculosis: an important diagnosis. J Laryngol Otol. 2000;114(11):878–880. doi:10.1258/0022215001904220
8. Khan KA, Khan NA, Maqbool M. Otorhinolaryngological manifestations of tuberculosis. JK Sci. 2005;4:3.
9. Michael RC, Michael JS. Tuberculosis in otorhinolaryngology: clinical presentation and diagnostic challenges. Int J Otolaryngol. 2011;2011:686894. doi:10.1155/2011/686894
10. Gassab E, Kedous S, Berkaoui A, et al. Extra-nodal head, and neck tuberculosis. J Tun Orl. 2010;24:26–30.
11. World Health Organization. Treatment of Tuberculosis: Guidelines.
12. Jadia S, Chauhan A, Hazari R, Maurya A, Biswas R. An unusual case of recurrent tonsillitis. BMJ Case Rep. 2010;2010(1):2561. doi:10.1136/bcr.12.2009.2561
13. Sriram R, Bhojwani KM. Manifestations of tuberculosis in otorhinolaryngology practice: a retrospective study conducted in a coastal city of South India. Indian. J Otolaryngol Head Neck Surg. 2017;69(2):210–215.
14. Kulkarni NS, Gopal GS, Ghaisas SG, Gupta NA. Epidemiological considerations and clinical features of ENT tuberculosis. J Laryngol Otol. 2001;115(7):555–558. doi:10.1258/0022215011908487
15. Soni NK, Chatterjee P. Lingual tuberculosis. Indian J Otolaryngol. 1979;31:92.
16. Komet H, Schaefer RF, Mahoney PL, Antonio S. Bilateral tuberculosis granulomas of the tongue. Arch Otolaryngol. 1965;82(6):649–651. doi:10.1001/archotol.1965.00760010651018
17. Bokare B, Mehta K. Otolaryngological manifestations of tuberculosis: a clinical study. Indian J Otolaryngol Head Neck Surg. 2020. doi:10.1007/s12070-020-01789-x
18. Gupta A, Shinde KJ, Bhardwaj I. Primary lingual tuberculosis: a case report. J Laryngol Otol. 1998;112(1):86–87. doi:10.1017/S0022215100139982
19. Iype EM, Ramdas K, Pandey M, et al. Primary tuberculosis of the tongue: report of three cases. Br J Oral Maxillofac Surg. 2001;39(5):402–403. doi:10.1054/bjom.2000.0663
20. Gupta KB, Yadav SPS, Manchanda M. Primary pharyngeal tuberculosis. Lung India. 2005;22:127–129.
21. Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013:828939. doi:10.1155/2013/828939
22. Honore-Bouakline S, Vincensini JP, Giacuzzo V, Lagrange PH, Herrmann JL. Rapid diagnosis of extrapulmonary tuberculosis by PCR: impact of sample preparation and DNA extraction. J Clin Microbiol. 2003;41(6):2323–2329. doi:10.1128/JCM.41.6.2323-2329.2003
23. Akkara AS, Singhania A, Akkara AG, Shah A, Adalja M, Chauhan N. A study of manifestations of extrapulmonary tuberculosis in the ENT region. Indian J Otolaryngol Head Neck Surg. 2014;66(1):46–50. doi:10.1007/s12070-013-0661-7
24. Das S, Das D, Bhuyan UT, Saikia N. Head and neck tuberculosis: scenario in a tertiary care hospital of Northeastern India. J Clin Diagn Res. 2016;10(1):MC04–MC07. doi:10.7860/JCDR/2016/17171.7076
25. Chiesa Estomba CM, Betanses Reionoso CA, Rivera Schmitz T, Ossa Echeverri CC, Gonzales Cortes MJ, Santidrian Hidalgo C. Head and neck tuberculosis: 6-year retrospective study. Acta Otorrinolaringol Esp. 2016;67(1):9–14. doi:10.1016/j.otorri.2014.11.003
26. Pandurang K, Shenoy VS, Bhojwani K, et al. Tuberculosis in the head and neck in India: down but not yet dead. J Mycobact Dis. 2014;4(2):148.
27. Hafeez M, Ullah I, Ahmad I, Ullah Z. Otorhinolaryngological manifestations of tuberculosis. Pak J Med Sci. 2011;27(4):855–857.
28. Sharma S, Rana AK. ENT manifestations of tuberculosis: an important aspect of ENT practice. Pan Afr Med J. 2020;36(295). doi:10.11604/pamj.2020.36.295.24823
29. Bayazit YA, Bayazit N, Namiduru M. Mycobacterial cervical lymphadenitis. ORL J Otorhinolaryngol Relat Spec. 2004;66(5):275–280. doi:10.1159/000081125
30. Chen YM, Lee PY, Su WJ, Perng RP. Lymph node tuberculosis: 7-year experience in Veterans General Hospital, Taipei, Taiwan. Tuber Lung Dis. 1992;73(6):368–371. doi:10.1016/0962-8479(92)90042-I
31. Fain O, Lortholary O, Djouab M, et al. Lymph node tuberculosis in the suburbs of Paris: 59 cases in adults not infected by the human immunodeficiency virus. Int J Tuberc Lung Dis. 1999;3(2):162–165.
32. Popescu MR, Călin G, Strâmbu I, et al. Lymph node tuberculosis – an attempt of clinico-morphological study and review of the literature. Rom J Morphol Embryol. 2014;55(2 Suppl):553–567.
33. Alecu I, Mocanu H, Călin IE. Intellectual mobility in higher education system. Rom J Mil Med. 2017;CXX(2):16–21.
34. Prada JL, Kindelan JM, Villanueva JL, Jurado R, Sanchez-Guijo P, Torre-Cisneros J. Tuberculosis of the tongue in two immunocompetent patients. Clin Infect Dis. 1994;19(1):200–202. doi:10.1093/clinids/19.1.200
35. Koksal D, Acican T, Kanat F, Durmaz G, Ataoglu O, Cobanli B. Tuberculous ulcer of the tongue secondary to pulmonary tuberculosis. Aust NZ J Med. 2000;30(4):518–519. doi:10.1111/j.1445-5994.2000.tb02068.x
36. Nemes RM, Ianosi ES, Pop CS, et al. Tuberculosis of the oral cavity. Rom J Morphol Embryol. 2015;56(2):521–525.
37. Popescu MR, Please IR, Olaru M, et al. Morphological aspects in tuberculosis of oral cavity – our experience and a review of the literature attempt. Rom J Morphol Embryol. 2015;56(3):967–987.
38. Prstacic R, Jurlina M, Zizic-Mitrecic M, Janjanin S. Primary nasopharyngeal tuberculosis mimicking exacerbation of chronic rhinosinusitis. J Laryngol &otol. 2011;125(7):747–749. doi:10.1017/S0022215110002835
39. Patil C, Kharat R, Deshmukh P, Biswas J, Bassin J. Primary tuberculosis of nasopharynx (adenoid)- A rare presentation. Asian Pac J Trop Med. 2013;6(3):246–248. doi:10.1016/S1995-7645(13)60033-4
40. Golli AL, Niţu MF, Turcu F, Popescu M, Ciobanu-Mitrache L, Olteanu M. Tuberculosis remains a public health problem in Romania. Int J Tuberc Lung Dis. 2019;23(2):226–231. doi:10.5588/ijtld.18.0270
41. Marica C, Didilescu C, Chiotan D, et al. Tuberculoza multidrog rezistentă în România în ultimii ani (2004–2007)–un fenomen social de maximă importanţă [Multidrug resistant tuberculosis in Romania in the last years (2004–2007)–an extremely important social phenomenon]. Pneumologia. 2008;57(4):195–200. Romanian.
42. Ruesen C, Riza AL, Florescu A, et al. Linking minimum inhibitory concentrations to whole genome sequence-predicted drug resistance in Mycobacterium tuberculosis strains from Romania. Sci Rep. 2018;8(1):1–8. doi:10.1038/s41598-018-27962-5
43. Nițu FM, Olteanu M, Streba CT, et al. Tuberculosis and its particularities in Romania and worldwide. Rom J Morphol Embryol. 2017;58(2):385–392.
44. Draganescu M, Baroiu L, Iancu A, et al. Perspectives on skin disorder diagnosis among people living with HIV in southeastern Romania. Exp Ther Med. 2021;21(1):1. doi:10.3892/etm.2020.9433
45. Ibraim E, Stoicescu IP, Homorodean D, et al. Tuberculosis in Romania. Problems and solutions. Pneumologia. 2010;59(1):6–12.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
