Back to Journals » Journal of Inflammation Research » Volume 16
Soluble TAM Receptor Tyrosine Kinases Correlate with Disease Severity and Predict the Early Responsiveness of Sublingual Immunotherapy in Allergic Rhinitis
Authors Zhou Y, Feng Z, Wen J, Yang C, Jing Q
Received 25 July 2023
Accepted for publication 17 October 2023
Published 25 October 2023 Volume 2023:16 Pages 4845—4855
DOI https://doi.org/10.2147/JIR.S432281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yandan Zhou,1 Zhili Feng,2,3 Jie Wen,2,3 Chi Yang,2,3 Qiancheng Jing2,3
1Changsha Aier Eye Hospital, Aier Eye Hospital Group, Changsha, Hunan, People’s Republic of China; 2Department of Otolaryngology Head and Neck Surgery, the Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, Hunan, People’s Republic of China; 3Institute of Otolaryngology Head and Neck Surgery, Hengyang Medical School, University of South China, Changsha, Hunan, People’s Republic of China
Correspondence: Qiancheng Jing, Department of Otolaryngology-Head and Neck Surgery, the Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, Hunan, People’s Republic of China, Email [email protected]
Background: Allergic rhinitis (AR) is a common allergic disease, and SLIT has shown effectiveness as a treatment method. This study focuses on the evaluation of serum TAM receptor tyrosine kinases (TYRO3, AXL, and MER) levels as potential indicators of disease severity and predictive markers for sublingual immunotherapy (SLIT) responsiveness in AR patients.
Methods: A total of 160 AR subjects, including 40 mild AR (MAR) and 120 moderate-severe AR (MSAR) patients, and 40 healthy controls (HC) were recruited. Serum concentrations of TYRO3, AXL, and MER were measured and their relationships with disease severity were examined. In the MSAR group, 102 patients underwent SLIT, and the early efficacy was evaluated. The correlations between the baseline serum concentrations of TYRO3, AXL, and MER and the early responsiveness of SLIT were analyzed.
Results: Serum concentrations of TYRO3, AXL, and MER were significantly reduced in AR patients, particularly in those MSAR subjects. Correlation analysis results indicated that serum TYRO3 and MER levels were negatively correlated with the visual analog scale (VAS) and the total nasal symptom score (TNSS). After one year of follow-up, 80 AR patients completed the treatment and were divided into effective and ineffective groups. Serum baseline levels of TYRO3 and MER were found to be lower in the effective group compared to the ineffective group. Additionally, there was a significant increase in serum TYRO3 and MER levels compared to baseline levels. Receiver operating characteristic (ROC) analysis revealed that circulating TYRO3 and MER had potential values for reflecting AR severity and predicting early SLIT responsiveness.
Conclusion: Serum TYRO3 and MER concentrations were decreased in AR patients and negatively associated with disease severity. Circulating TYRO3 and MER seem to be promising indicators for monitoring the efficacy of SLIT in AR patients.
Keywords: allergic rhinitis, sublingual immunotherapy, TAM receptor tyrosine kinases, TYRO3, AXL, MER
Introduction
Allergic rhinitis (AR) is characterized as an inflammatory disorder affecting the upper respiratory tract mediated by immunoglobulin E (IgE) upon exposure to allergens.1 The main pathological feature of AR is T helper 2 (Th2)-type inflammation with eosinophil infiltration, which was primarily mediated by dendritic cells (DC) and macrophages.2,3 Previous research has demonstrated that AR exhibits a high degree of clinical heterogeneity, with varying levels of disease severity observed among affected individuals, which poses significant challenges for clinical diagnosis, assessment, and treatment.4,5 Despite previous publications finding that several indicators or biomarkers correlated with disease severity, their sensitivities and specificities were limited. Therefore, it is important to explore clinically meaningful biomarkers to monitor the severity of AR, which may contribute to standardizing diagnosis and evaluation and achieving personalized treatment. Furthermore, AR is a prevalent allergic condition that affects approximately one-fifth of the population, causing significant negative impacts on patients’ quality of life and a substantial socioeconomic burden.6 Allergen-specific immunotherapy (AIT) is a unique treatment approach that targets the underlying cause of the disease, effectively modulating its natural progression and providing long-lasting clinical benefits. These benefits can persist for several years, even after the completion of treatment.7,8 AIT encompasses subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT), with many patients with AR opting for SLIT due to its proven efficacy and simplicity.9,10 However, the effectiveness of SLIT varies among individuals, ranging from 45.0% to 75.0%, and there is a lack of consistent definitions for assessing its efficacy.11 Consequently, there is a pressing need to identify objective indicators or methods that can predict the early response to SLIT, which would be of great clinical significance.
The TAM receptor tyrosine kinases, namely TYRO3, AXL, and MER, are a primary family of receptors that provide a pleiotropic anti-inflammatory response, along with their corresponding glycoprotein ligands, growth arrest-specific 6 (Gas6) and protein S (Pros1), play crucial roles in maintaining tissue homeostasis and regulating inflammatory processes.12 A prior study has shown that TAM receptor tyrosine kinases are expressed in various cell types, including macrophages, dendritic cells, T cells, endothelial cells, and epithelial cells. Their broad expression implies their participation in the initiation and advancement of numerous inflammatory diseases.13 Ekman et al14 reported that the circulating levels of Axl and Gas6 were increased in systemic lupus erythematosus patients and varied with disease activity, suggesting that AXL and Gas6 might serve as objective biomarkers for reflecting disease activity.14 Additionally, previous studies have suggested that decreased expression of TAM receptor tyrosine kinases may contribute to persistent airway inflammation, which has been strongly linked to the development of chronic lung disease in the future.15,16 However, the specific function of TAM receptor tyrosine kinases in AR is not clearly understood. Therefore, the current study aims to evaluate the potential of serum TAM receptor tyrosine kinases as a novel biomarker for assessing disease severity and predicting the clinical outcome of SLIT in AR patients.
Materials and Methods
Study Design and Participants
This study received approval from the Medical Ethics Committee of the Affiliated Changsha Central Hospital, Hengyang Medical School of the University of South China (protocol:2023005). Before their enrollment, all participants provided informed consent. One hundred and sixty house dust mite (HDM)-induced AR adults, including 40 mild AR (MAR) participants and 120 moderate-severe AR (MSAR) participants treated between October 2021 and March 2022 were included in this study. Forty healthy volunteers, matched for age and sex, were recruited as healthy controls (HC). All participants diagnosed with AR met the criteria outlined in the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines as previously described.6 The diagnostic criteria for MAR and MSAR were performed based on total nasal symptom score (TNSS) as our previous publication described, TNSS≤4 was defined as MAR, and TNSS >4 was regarded as MSAR.17 The exclusion criteria for this study were performed as our previous study:18 the presence of concomitant immune or inflammatory diseases, severe renal, hepatic, or cardiac dysfunction, age below 18 years, pregnancy or potential pregnancy, and history of immunotherapy or systemic steroid or anti-allergy drug use within 1 month before enrollment. HCs were excluded if they were currently undergoing immunotherapy or receiving systemic steroid or anti-allergy treatment and had severe cardiac or renal dysfunction. Clinical and demographic data, including age, gender, body mass index (BMI), asthma, duration of disease, polysensitization, visual analogue scale (VAS), and total nasal symptom score (TNSS), were collected from all participants.
Serum Collection and TAM Receptor Tyrosine Kinases Measurement
Serum samples were obtained using a 5-milliliter vacuum tube. For AR patients undergoing SLIT, serum samples were collected before the initiation of treatment and one year after undergoing SLIT. The collected blood specimens were allowed to clot at room temperature for 1 h to separate the blood cells. Subsequently, the specimens were centrifuged at 3000 rpm for 15 min at 4°C, and the resulting serum was stored at −80°C for further analysis. The serum levels of TYRO3, AXL, and MER were measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from R&D Systems (Minneapolis, MN, USA), following the manufacturer’s instructions. The operators responsible for conducting the assay were blinded to the detailed data of the patients.
Immunotherapy and Clinical Efficacy Evaluation
The standardized Der f drops, supplied by Wolwo Pharma Biotechnology Company (Zhejiang, China), were self-administered by the AR patients as previously described.18 The initial administration was supervised by a physician. Any adverse reactions that occurred during the therapy period were documented.19 The medication score was determined using a pre-existing method, and the combined symptom medication score (SMS) was calculated as the sum of the symptom score and medication score.20 The efficacy evaluation was conducted following a previously described method, where the term “effective” was defined as a patient achieving a minimum 30% reduction in SMS compared to their baseline level. Patients who did not meet the criteria were considered “ineffective” in terms of treatment efficacy.17,18
Statistical Analysis
Variables with normal distribution were shown as mean and standard deviation (SD), while non-normally distributed variables were reported as median and interquartile range. One-way analysis of variance (ANOVA) or the Mann–Whitney U-test was used to compare among three groups, followed by the student-Newman-Keuls method for subsequent pairwise comparisons. Student’s t-test or Kruskal–Wallis H-test was used for comparison between the two groups. Multivariate logistic regression analysis was performed to explore independent factors associated with the early effectiveness of SLIT. Receiver operating characteristic (ROC) curves were generated. A P-value < 0.05 was regarded as statistically significant. The statistical analyses were conducted on SPSS and GraphPad Prism.
Results
Characteristics of All Subjects
The clinical and demographic characteristics of all participants in this study are displayed in Table 1. The number of patients with asthma, TNSS scores and VAS scores were significantly higher in the MSAR group than in the HC group and MAR (P<0.05). No significant differences were found in gender, age, BMI, duration of disease, and polysensitization among the three groups (P>0.05).
 |
Table 1 Clinical Characteristics of Recruited Individuals Among the Three Groups |
Serum TAM Receptor Tyrosine Kinase Levels Were Decreased in AR Patients and Correlated with Disease Severity
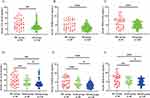
The baseline concentrations of serum TAM receptor tyrosine kinases tyrosine kinases (AXL, TYRO3, MER) were lower in the AR group compared to the HC group (P<0.05). Notably, the MSAR group showed significantly lower serum levels of TYRO3 and MER compared to both the HC and MAR groups (P<0.05) (Figure 1). The correlation analysis presented a negative association between serum levels of TYRO3 and MER with TNSS score and VAS score, while serum AXL levels did not show a significant correlation with TNSS score and VAS score in AR patients (Figure 2). To further explore the value of TAM receptors tyrosine kinases in predicting disease severity, ROC curves were applied. The results showed that serum TYRO3 and MER levels had more powerfully predictive values in assessing disease severity in AR patients in comparison with serum AXL (Figure 3).
Serum TAM Receptors Tyrosine Kinases Levels Correlated with Early Effectiveness of SLIT
After one-year of follow-up, a total of 80 patients completed the treatment and provided follow-up data. Among these patients, 49 were divided into the effective group, while 31 were categorized into the ineffective group. Table 2 presents the demographic and clinical data for both groups. The baseline levels of serum TYRO3 and MER were significantly lower in the effective group in comparison with the ineffective group (P<0.05), while serum AXL levels were not significantly different (P>0.05) (Figure 4A-C). Furthermore, the ROC analysis results suggested that serum TYRO3 and MER had the potential to predict the early efficacy of SLIT (Figure 4D-F). Interestingly, we also found a significant increase in serum TYRO3 and MER levels between baseline and 1-year post-SLIT (P<0.05), but serum AXL levels did not change significantly (P>0.05) (Figure 5A-C). Moreover, compared to AXL, serum TYRO3, and MER levels were substantially elevated between pre-SLIT and 1 year after SLIT in the effective group (P<0.05) (Figure 5D-F). However, in the ineffective group, no significant changes were observed in the levels of TAM receptor tyrosine kinases between the pre-and post-SLIT periods (P>0.05) (Figure 5G-I).
 |
Table 2 Comparisons of Baseline Clinical Characteristics Between the Two Groups |
Discussion
In the present study, TAM receptor tyrosine kinase levels were found to be decreased in the serum samples of AR patients, particularly in MRAR cases, and were negatively associated with symptom scores. Furthermore, our findings revealed a significant association between the early effectiveness of SLIT and the serum levels of TYRO3 and MER. Specifically, the effective group exhibited significantly lower levels of TYRO3 and MER compared to the ineffective group. Furthermore, we observed that the concentrations of TYRO3 and MER significantly increased 1 year after SLIT. ROC curves showed that TYRO3 and MER had good accuracy in reflecting disease severity and predicting the clinical effectiveness of SLIT in AR patients. Taken together, our findings suggested that the imbalanced levels of circulating TAM receptor tyrosine kinases were closely involved in the pathological mechanism of AR, and circulating TYRO3 and MER might serve as objective biomarkers in reflecting disease severity and monitoring the clinical responsiveness of SLIT in AR patients.
The TAM receptor tyrosine kinases, which include TYRO3, AXL, and MER, are a subclass of phosphatidylinositol 3-kinases (PI3K)-related kinases. Together with their ligands GAS6 and PROS1, they play a crucial role in the regulation of immune and inflammatory responses.13 Recent studies have highlighted the involvement of TAM receptor tyrosine kinases in the development of various diseases through their regulatory role in immune cell development and function.21 Prior evidence highlighted that TAM receptor tyrosine kinases were important negative regulators of the immune system, and their binding to ligands effectively reduces the secretion of downstream inflammatory cytokines by interfering with DC activation and macrophage polarization.22 Chan et al23 reported that TYRO3-deficient mice sensitized to HDM exhibited a stronger type 2 response, characterized by increased leukocytes and eosinophils in both bronchoalveolar lavage fluid and lungs. Previous studies suggested that DC and eosinophils played important roles in the pathogenesis and development of AR.3,24 An increase in the number and activity of DC has been observed in patients with AR, especially in those with moderate-to-severe symptoms, which suggests an overactive immune response.25 In addition, it has been demonstrated that DC can facilitate the differentiation of Th2 cells and stimulate the production of IgE, as well as facilitate the infiltration of eosinophils into nasal mucosal tissue.26 Furthermore, the elevated levels of eosinophils can further aggravate tissue inflammation by releasing various inflammatory mediators and factors that cause tissue damage.27 Furthermore, previous studies have indicated that high levels of TAM receptor tyrosine kinases can hinder the differentiation of M1-type macrophages, which may contribute to disease progression by producing pro-inflammatory mediators.28 Notably, a recent publication demonstrated that the number of M1-type macrophages was elevated in a mouse model of allergic asthma, which subsequently led to the production of multiple inflammatory mediators,29 These mediators have the potential to activate an inflammatory response in the airway mucosa, thereby exacerbating and prolonging the manifestation of symptoms.30,31 Moreover, M1-type macrophages could crosstalk with other immune cells and contribute to the inflammatory response, thereby intensifying the severity of allergic disorders.32,33 In the present study, we revealed that the concentrations of serum TYRO3, AXL, and MER were decreased in AR patients, and serum TYRO3 and MER levels were negatively associated with disease severity. Thus, we hypothesized that the reduction in TAM receptor tyrosine kinase levels in AR patients could weaken their regulatory functions, thereby promoting the activation of DC cells and polarization of M1-type macrophages, the infiltration of eosinophils, and the production of inflammatory mediators, ultimately worsening the allergic symptoms in AR patients. However, the underlying mechanisms are poorly discovered and further investigations are needed to better understand their roles in the pathogenesis of AR.
Mounting evidence suggests that SLIT can significantly alleviate allergic symptoms in AR patients and improve their quality of life.34,35 An increasing amount of real-world data indicates that a significant proportion of patients still do not benefit from SLIT despite receiving standard and long-term treatment.11,36 Hence, there is a particular significance in investigating objective biomarkers that can predict the effectiveness of SLIT. Here, we first observed that serum TYRO3 and MER baseline levels were lower in the effective group than in the ineffective group, and their concentrations progressively increased with the duration of SLIT treatment. Additionally, using ROC curve analysis, we observed that circulating TYRO3 and MER levels were reliable and precise biomarkers for predicting the early efficacy of SLIT. DCs, as primary antigen-presenting cells, have a crucial role in AR by promoting the differentiation of Th2 cells and type 2 responses. The tolerance of DCs is regarded as a vital indicator of the effectiveness of AIT.37,38 TAM receptor tyrosine kinases are expressed in DCs and play important roles in regulating immune responses. They exert their function by inhibiting the activation of DCs.13 In addition, TAM receptor tyrosine kinases were proven to regulate immune responses by promoting macrophage M2 polarization.28,39 Previous studies indicated that M2 macrophages were involved in the tissue immune microenvironment of AR, and the secretions of relevant cytokines by M2 macrophages participated in anti-inflammatory and tissue repair processes.40,41 Importantly, the induction of tolerance of DC and polarization of M2 macrophages are pivotal in the development of immune tolerance and exert significant impacts on the therapeutic effectiveness of AIT.42 TYRO3 and MER, as important anti-inflammatory cytokines, exhibit weaker inhibitory effects on DC activation and weaker promotion of M2 polarization when their concentrations are lower. Activated DCs and M1-polarized macrophages are more prevalent in this scenario.43,44 Conversely, effective SLIT promotes the reshaping of DCs and macrophages in AR patients, facilitating immune tolerance in DCs and M2 polarization in macrophages.45 Therefore, we have reason to believe that lower concentrations of TYRO3 and MER are associated with better efficacy of SLIT. Furthermore, we hypothesized that the upregulation of TYRO3 and MER during SLIT might have dual effects. On one hand, they can induce DC dysfunction, consequently impairing the initiation of the immune response and resulting in a decrease in the number of circulating allergen-specific Th2 cells. On the other hand, TAM receptors may promote the polarization of M2 macrophages, leading to the production of anti-inflammatory cytokines. This, in turn, reduces mucosal inflammation and attenuates the allergic response. Based on our findings, it can be suggested that soluble TYRO3 and MER exhibited the values to serve as reliable biomarkers to predict the efficacy of SLIT in AR patients and monitor therapeutic effects. However, further research is required to gain a better understanding of the underlying mechanisms by which TYRO3 and MER influence the response to SLIT in AR patients.
We acknowledge several limitations. Firstly, the sample size is relatively small, and there is a lack of validation cohort with a large sample size to strengthen the findings. Secondly, TNSS and VAS are subjective scoring methods and may not accurately reflect the disease severity. Additionally, due to the relatively small sample size included, this study concludes a relatively weak correlation between TAM receptor tyrosine kinases and disease severity. Thirdly, all patients were from a single medical center, and only HDM-induced AR patients were recruited, which might limit its generalization. Lastly, the recruited AR patients only followed up for one year, and the predictive values of serum TAM receptor tyrosine kinases for long-term efficacy were not evaluated. We will continue to follow up with these patients and collect serum samples to comparatively assess the values of TAM receptor tyrosine kinases in the long-term efficacy of SLIT. Moreover, further support and expansion of our current findings require additional multicenter studies with larger sample sizes and functional research.
Conclusion
We measured systematically for the first time and indicated that the serum TAM receptor tyrosine kinase concentrations were decreased in the AR patients and negatively correlated with disease severity. Additionally, our study demonstrated that serum TYRO3 and MER levels showed promise in differentiating patients who respond positively to SLIT. These findings indicate that serum TYRO3 and MER have the potential to be valuable and objective biomarkers for assessing the severity of AR and predicting the clinical effectiveness of SLIT.
Data Sharing Statement
Data will be available upon reasonable request.
Ethical Approval
This study was conducted following the recommendations of the Declaration of Helsinki. The Human Ethical Committee of the Affiliated Changsha Central Hospital, Hengyang Medical School of the University of South China approved this study (protocol:2023005).
Funding
This research was supported by the Natural Science Foundation of Hunan Province (2021JJ30754), Hunan Provincial Health Commission Scientific Research Project (202107010164), and Changsha Municipal Natural Science Foundation (kq2007050).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Abdullah B, Pawankar R, Latiff AHA, et al. Malaysian Society of Allergy and Immunology Consensus Statement on Sublingual Immunotherapy in Allergic Rhinitis. J Clin Med. 2023;12.
2. Zhang Y, Lan F, Zhang L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy. 2022;77(11):3309–3319. doi:10.1111/all.15454
3. Liu P, Kang C, Zhang J, et al. The role of dendritic cells in allergic diseases. Int Immunopharmacol. 2022;113:109449. doi:10.1016/j.intimp.2022.109449
4. Del Cuvillo A, Santos V, Montoro J, et al. Allergic rhinitis severity can be assessed using a visual analogue scale in mild, moderate, and severe. Rhinology. 2017;55:34–38. doi:10.4193/Rhin16.025
5. Feng LL, Sun FY, Chen Y, et al. Studying the Effects of Vitamin A on the Severity of Allergic Rhinitis and Asthma. Iran J Allergy Asthm. 2021;20:684–692.
6. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immun. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
7. Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi:10.1111/all.13317
8. Shamji MH, Sharif H, Layhadi JA, et al. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J Allergy Clin Immun. 2022;149:791–801. doi:10.1016/j.jaci.2022.01.016
9. Han DH, Cho S, Lockey R, et al. Early Responses of specific IgE Can Predict the Outcome of House Dust Mite Sublingual Immunotherapy. J Allergy Clin Immun. 2020;145:Ab63–Ab63. doi:10.1016/j.jaci.2019.12.721
10. Zhang Y, Lan F, Zhang L. Advances and highlights in allergic rhinitis. Allergy. 2021;76(11):3383–3389. doi:10.1111/all.15044
11. Li HB, Chen S, Cheng L, et al. Chinese guideline on sublingual immunotherapy for allergic rhinitis and asthma. J Thorac Dis. 2019;11(12):4936–4950. doi:10.21037/jtd.2019.12.37
12. Lee CH, Chun T. Anti-Inflammatory Role of TAM Family of Receptor Tyrosine Kinases Via Modulating Macrophage Function. Mol Cells. 2019;42(1):1–7. doi:10.14348/molcells.2018.0419
13. Rothlin CV, Carrera-Silva EA, Bosurgi L, et al. TAM Receptor Signaling in Immune Homeostasis. Annu Rev Immunol. 2015;33:355–391. doi:10.1146/annurev-immunol-032414-112103
14. Ekman C, Jonsen A, Sturfelt G, et al. Plasma concentrations of Gas6 and sAxl correlate with disease activity in systemic lupus erythematosus. Rheumatology. 2011;50(6):1064–1069. doi:10.1093/rheumatology/keq459
15. Grabiec AM, Denny N, Doherty JA, et al. Diminished airway macrophage expression of the Axl receptor tyrosine kinase is associated with defective efferocytosis in asthma. J Allergy Clin Immunol. 2017;140(4):1144–1146 e1144. doi:10.1016/j.jaci.2017.03.024
16. Xia J, Li J, Deng M, et al. Diosmetin alleviates acute lung injury caused by lipopolysaccharide by targeting barrier function. Inflammopharmacology. 2023;1–11.
17. Zhou YD, Xu M, Gong W, et al. Circulating MMP-12 as Potential Biomarker in Evaluating Disease Severity and Efficacy of Sublingual Immunotherapy in Allergic Rhinitis. Mediat Inflamm. 2022;2022:1–10. doi:10.1155/2022/3378035
18. Zhou Y, Xu M, Gong W, et al. Circulating MMP-12 as Potential Biomarker in Evaluating Disease Severity and Efficacy of Sublingual Immunotherapy in Allergic Rhinitis. Mediators Inflamm. 2022;2022:3378035.
19. Wang H, Lin X, Hao C, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy. 2006;61(2):191–197. doi:10.1111/j.1398-9995.2005.00913.x
20. Zhu K, Xia C, Chen J, et al. Serum Soluble ST2 Correlated with Symptom Severity and Clinical Response of Sublingual Immunotherapy for House Dust Mite-Induced Allergic Rhinitis Patients. Mediators Inflamm. 2021;2021:5576596. doi:10.1155/2021/5576596
21. Wu H, Zheng J, Xu S, et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation. 2021;18(1):2. doi:10.1186/s12974-020-02041-7
22. Carrera Silva EA, Chan PY, Joannas L, et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity. 2013;39(1):160–170. doi:10.1016/j.immuni.2013.06.010
23. Chan PY, Carrera Silva EA, De Kouchkovsky D, et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science. 2016;352(6281):99–103. doi:10.1126/science.aaf1358
24. Colas L, Magnan A, Brouard S. Immunoglobulin E response in health and disease beyond allergic disorders. Allergy. 2022;77(6):1700–1718. doi:10.1111/all.15230
25. KleinJan A, Willart M, van Rijt LS, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118(5):1117–1125. doi:10.1016/j.jaci.2006.05.030
26. Yin X, Chen S, Eisenbarth SC. Dendritic Cell Regulation of T Helper Cells. Annu Rev Immunol. 2021;39(1):759–790. doi:10.1146/annurev-immunol-101819-025146
27. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–751. doi:10.1038/s41577-021-00538-7
28. Ubil E, Caskey L, Holtzhausen A, et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J Clin Invest. 2018;128(6):2356–2369. doi:10.1172/JCI97354
29. Hong JY, Chung Y, Steenrod J, et al. Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma. Respir Res. 2014;15(1):63. doi:10.1186/1465-9921-15-63
30. Wang HR, Wei SZ, Song XY, et al. IL-1beta and Allergy: focusing on Its Role in Allergic Rhinitis. Mediators Inflamm. 2023;2023:1265449. doi:10.1155/2023/1265449
31. Kato A. Group 2 Innate Lymphoid Cells in Airway Diseases. Chest. 2019;156(1):141–149. doi:10.1016/j.chest.2019.04.101
32. Saradna A, Do DC, Kumar S, et al. Macrophage polarization and allergic asthma. Transl Res. 2018;191:1–14. doi:10.1016/j.trsl.2017.09.002
33. Lee JW, Chun W, Lee HJ, et al. The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells-Basel. 2021;10.
34. Drazdauskaitė G, Layhadi JA, Shamji MH. Mechanisms of Allergen Immunotherapy in Allergic Rhinitis. Curr Allergy Asthma Rep. 2020;21:2. doi:10.1007/s11882-020-00977-7
35. Lin Z, Liu Q, Li T, et al. The effects of house dust mite sublingual immunotherapy in patients with allergic rhinitis according to duration. Int Forum Allergy Rhinol. 2016;6(1):82–87. doi:10.1002/alr.21657
36. Tanaka Y, Fukumoto S, Sugawara S. Mechanisms underlying the induction of regulatory T cells by sublingual immunotherapy. J Oral Biosci. 2019;61(2):73–77. doi:10.1016/j.job.2019.02.001
37. Fargen KM, Singla A, Mocco J. The New England Journal of Medicine Stroke Trials: what Do They Really Mean? Neurosurgery. 2015;62(Suppl 1):137–140. doi:10.1227/NEU.0000000000000780
38. Yu S, Han B, Liu S, et al. Derp1-modified dendritic cells attenuate allergic inflammation by regulating the development of T helper type1 (Th1)/Th2 cells and regulatory T cells in a murine model of allergic rhinitis. Mol Immunol. 2017;90:172–181. doi:10.1016/j.molimm.2017.07.015
39. Loges S, Schmidt T, Tjwa M, et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115(11):2264–2273. doi:10.1182/blood-2009-06-228684
40. Lou H, Huang Y, Chu X, et al. M2 Macrophages Upregulated by Allergen Exposure in Seasonal Allergic Rhinitis. Int Arch Allergy Immunol. 2023;1–11.
41. Shiratori H, Feinweber C, Luckhardt S, et al. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol. 2017;88:58–68. doi:10.1016/j.molimm.2017.05.027
42. Zissler UM, Schmidt-Weber CB. Predicting Success of Allergen-Specific Immunotherapy. Front Immunol. 2020;11:1826. doi:10.3389/fimmu.2020.01826
43. Giroud P, Renaudineau S, Gudefin L, et al. Expression of TAM-R in Human Immune Cells and Unique Regulatory Function of MerTK in IL-10 Production by Tolerogenic DC. Front Immunol. 2020;11:564133. doi:10.3389/fimmu.2020.564133
44. Gadiyar V, Patel G, Chen J, et al. Targeted degradation of MERTK and other TAM receptor paralogs by heterobifunctional targeted protein degraders. Front Immunol. 2023;14:1135373. doi:10.3389/fimmu.2023.1135373
45. Xie S, Zhang H, Wang F, et al. Circulating MIF Associated With Disease Severity and Clinical Response of Sublingual Immunotherapy in House Dust Mite-Induced Allergic Rhinitis. Front Pharmacol. 2021;12:681724. doi:10.3389/fphar.2021.681724
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.