Back to Journals » Infection and Drug Resistance » Volume 15
Soluble Programmed Cell Death-1 is a Novel Predictor of HBsAg Loss in Chronic Hepatitis B Patients When Long-Term Nucleos(t)ide Analog Treatment is Discontinued
Authors Liao G, Liu Z, Xia M, Chen H, Wu H, Li B, Yu T, Cai S , Zhang X, Peng J
Received 5 February 2022
Accepted for publication 23 April 2022
Published 29 April 2022 Volume 2022:15 Pages 2347—2357
DOI https://doi.org/10.2147/IDR.S360202
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guichan Liao, Ziying Liu, Muye Xia, Hongjie Chen, Houji Wu, Bing Li, Tao Yu, Shaohang Cai, Xiaoyong Zhang, Jie Peng
State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Viral Hepatitis Research, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China
Correspondence: Jie Peng, State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Viral Hepatitis Research, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China, Tel +86 20 6278 7428, Fax +86 20 8771 9653, Email [email protected]
Purpose: The immunoinhibitory receptor, programmed death 1 (PD-1), plays a critical role in immune suppression during chronic viral infection. The significance of circulating soluble PD-1 (sPD-1) in patients with chronic hepatitis B who have discontinued long-term nucleos(t)ide analog (NA) treatment remains unknown.
Patients and Methods: A prospective cohort study was conducted using serial blood samples from chronic hepatitis B patients who discontinued long-term NA treatment. The current analysis included 115 non-cirrhotic patients with HBV DNA negative and HBsAg positive at the moment of NA discontinuation. Levels of sPD-1 were measured in all available samples using sandwich enzyme-linked immunoassay.
Results: Sixty-two patients experienced a clinical relapse and 14 occurred HBsAg loss, with 8-year cumulative rates of 56.6% and 23.4%, respectively. Time-dependent receiver operating characteristic curve analysis for sPD-1 derived 156 pg/mL, which is equivalent to the detectable threshold, as an optimal cut-off value for predicting 8-year clinical relapse. Patients with detectable sPD-1 at end of treatment (EOT) had a significant lower incidence of clinical relapse (48% vs 67%, hazard ratio [HR] 0.454, p = 0.006), but a remarkable higher probability of HBsAg loss (33.7% vs 2.4%, HR 9.17, p = 0.038), compared to those who with undetectable sPD-1, respectively.
Conclusion: EOT sPD-1 levels predicted clinical relapse and HBsAg loss after treatment discontinuation and may help to guide a finite NA treatment plan for patients with chronic hepatitis B virus infection.
Keywords: chronic hepatitis B, discontinuation, programmed cell death 1 protein, nucleos(t)ide analogs
Introduction
Nucleos(t)ide analogue (NA) treatment is one of the primary antiviral therapies for patients with chronic hepatitis B virus (HBV) infection.1–3 However, NA therapy does not impact intrahepatic covalently closed circular DNA (cccDNA) so cannot protect against rebound infection or even recurrence of active hepatitis after treatment cessation. The established treatment endpoint of hepatitis B surface antigen (HBsAg) loss is an infrequent event, leading to decades of NA therapy for most patients. Interestingly, HBsAg loss occurs in 0–25% of patients who have received finite NA treatment.4,5 Discontinuing treatment before HBsAg loss is being assessed as a new strategy to increase functional cure rates in HBV infected patients.
The factors that facilitate cure in off-therapy patients remain unknown. Suppression and control of HBV infection occur through a complex interaction between the virus and the host immune response. Circulating viral markers including HBsAg, hepatitis B core-related antigen (HBcrAg), and HBV RNA, are proposed as markers for possible treatment cessation because of their close association with active transcription and cccDNA replication.6,7 However, an understanding of the immunological factors that contribute to a sustained off-treatment response is limited.
Functional HBV-specific T cells play a major role in controlling HBV.8 However, HBV-specific T cells in patients with chronic HBV infection are hardly detectable and display dysfunction with high expression of an exhausted phenotype.9,10 Upregulation of programmed death-1 (PD-1) is demonstrated as one of the classic hallmarks of T cell exhaustion during chronic infections.11 Indeed, exhausted T cells can be functionally restored by blocking PD-1 and programmed death-ligand 1 (PD-L1) signaling.12,13 A study recently described soluble PD-1 (sPD-1) may serve as a deactivator of PD-1/PD-L1 signaling and subsequent T cell activation.14 Indeed, more studies are showing an association between sPD-1 and prognosis of tumor and inflammatory diseases, such as lung cancer, hepatocellular carcinoma, and rheumatoid arthritis.15–17 However, very little research has assessed the concentration and kinetics of sPD-1 during chronic HBV infection.18–22 Recently, Tan et al20 showed that sPD-1 expression is associated with functional cure during NA therapy. However, the potential value of sPD-1 in patients with NA discontinuation is not well understood. The aim of this study was to investigate the ability of sPD-1 to predict clinical outcomes in patients who discontinue NA treatment.
Materials and Methods
Patient Population
Patients with chronic HBV infection who discontinued NA according to prespecified cessation criteria were enrolled in an observational cohort study conducted at Nanfang Hospital of Southern Medical University in Guangzhou, China (clinical study no. ChiCTR-OOC-17013970). This study is part of an ongoing effort to prospectively investigate the discontinuation of NA therapy, and follow-up data through November 2012 to August 2021 were analyzed. The study was approved by the Institutional Ethics Committee of Nanfang Hospital (study identifier NFEC-201209-K3), and the study protocol was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent and agreed to follow the protocol, provide specimens, and have details from their medical records published anonymously.
The stopping rule was based on Asian Pacific Association for the Study of t he Liver (APASL) guidelines, as described previously.23,24 In brief, patients who were initially HBeAg-positive were required to administrate consolidation treatment for at least 12 months after HBeAg seroconversion and HBV DNA suppression. Patients who were initially HBeAg-negative were required to administrate consolidation treatment for at least 18 months after HBV DNA suppression. Patients with combined HBV-hepatitis C virus or hepatitis D virus infection, biopsy-proven cirrhosis, or liver stiffness > 9 kPa, as determined via FibroScan (Echosens, Paris, France), alcohol abuse, a liver transplantation history, or malignancy were excluded from the study.
Follow-Up and End Points
After NA discontinuation, patients were followed once a month for the initial 3 months, then every 3 months for 2 years, and every 6 months from then on. At each follow-up visit, comprehensive biochemical and virological tests were performed, and blood samples were stored at −80°C. Ultrasonography and liver stiffness measurements were performed annually after off-treatment follow-up.
A sustained response was defined as an HBV DNA level of <2000 IU/mL with or without normal alanine aminotransferase (ALT) levels. Clinical relapse was defined as an HBV DNA level >2000 IU/mL with an elevated ALT level greater than twice the upper limit of normal (ULN, 40 U/L). All patients with clinical relapse restarted NA treatment (0.5 mg Entecavir or 10 mg Tenofovir disoproxil fumarate per day) and were followed every 3–6 months for 5 years.
Biochemistry and Laboratory Methods
Serum HBV DNA levels were detected using the Cobas TaqMan polymerase chain reaction HBV assay (Roche Diagnostics, Basel, Switzerland) with a lower limit of detection (LLOD) of 20 IU/mL. HBsAg, HBeAg, and anti-hepatitis B e antibody levels were measured using ARCHITECT i1000SR (Abbott Laboratories, Chicago, IL, USA). The HBsAg test had a LLOD of 0.05 IU/mL. Local standardized automated techniques were used for biochemical testing.
Enzyme-Linked Immunosorbent Assay (ELISA)
Plasma levels of sPD-1 were measured at off-treatment at weeks 0, 4, 8, 12, 24, and 48 and at clinical relapse using a sandwich enzyme-linked immunoassay (DuoSet Human PD-1, R&D Systems, Minneapolis, MN, USA, DY1086) according to the manufacturer’s instructions. The absorbance was measured at 450 nm, while wavelength correction was set by 570 nm. Each sample was analyzed in duplicate using the average of the optical density (OD) values and a standard curve fitted with 4-parameter logistic regression. The minimum detection limit was 156 pg/mL and the upper limitation was 10,000 pg/mL, based on two standard deviations of the blanks. Sera containing > 10,000 pg/mL of sPD-1 were diluted 10-fold or 100-fold in sterile physiological saline solution and retested to obtain the exact concentration. Values below the detectable limit were assigned a value of 78 pg/mL.
Statistical Analysis
End of treatment (EOT) was defined as the time when NA therapy was discontinued. Follow-up time was calculated from the EOT to the last follow-up appointment or loss to follow-up, as appropriate. Continuous variables with a normal distribution were expressed as the mean ± standard deviation (SD), or medians and interquartile ranges (IQR), and assessed using the Student’s t-test and Mann–Whitney test, respectively. Groups were compared using the Fisher’s exact test. Paired t-tests were performed when two groups were compared. Time-dependent receiver operating characteristic (ROC) curves were generated via the “Kaplan-Meier” method in R 4.1.2 (Institute for Statistics and Mathematics, Vienna, Austria; http://www.r-project.org/). The discriminative of areas under the ROC curves (AUROCs) between two factors were compared by DeLong test. Kaplan–Meier analysis and the Log rank test were used to analyze longitudinal data. Cox proportional hazards regression analysis was used to analyze the association between variables and endpoints. In multivariate analysis, independent variables with a p-value > 0.1 in the regression coefficient test were eliminated, and the above process was repeated until no variables could be eliminated. Spearman correlation analysis was used to study the correlation between sPD-1 and other parameters.
Graphs were plotted using GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). All analyses were performed using IBM SPSS version 23.0 (IBM, Armonk, NY, USA). A two-tailed p-value < 0.05 was considered statistically significant.
Results
Study Population
A total of 115 patients who were HBsAg-positive and HBV DNA-negative at NA discontinuation were analyzed for this study. Seventy-nine (68.7%) patients were positive for HBeAg at the start of NA treatment, while 36 (31.3%) were negative. HBeAg-negative patients were older (p < 0.001) and had received consolidation for a longer period (p = 0.044) after treatment than HBeAg-positive patients. The two groups did not differ significantly in gender, treatment duration, antiviral regiment, HBsAg levels, Liver stiffness value, and sPD-1 levels at end of treatment (Table 1). All patients were followed for a median of 5.5 years (interquartile range [IQR]: 3.5–7.5 years). Eight patients were lost-to-follow-up (at weeks 8, 24, 36, 36, 48, 72, 72, and 96).
 |
Table 1 Clinical Characteristics of the Study Cohort |
Plasma sPD-1 and Clinical Relapse After Stopping NA Therapy
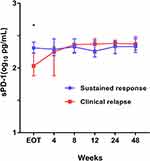
The median concentration of plasma sPD-1 was 2.25 (IQR, 2.08–2.45) log10 pg/mL at EOT, and detectable sPD-1 was found in 58.3% (n = 67) of patients. During the study, 62 patients developed a clinical relapse, resulting in an 8 year cumulative rate of 56.9% (HBeAg-positive vs HBeAg-negative: 50.6% vs 64.8%, Log rank test: p = 0.108, Supplementary Figure 1A). The plasma sPD-1 of patients was stratified according to whether clinical relapse was confirmed, as shown in Figure 1. At EOT, the median levels of sPD-1 were 2.04 (IQR, 1.89–2.42) and 2.37 (IQR, 2.20–2.50) log10 pg/mL, in patients with and without clinical relapse, respectively (p = 0.014). Patients with sustained responses had a stable level of sPD-1. However, increased sPD-1 concentrations were observed in patients with clinical relapse after treatment discontinuation, and growing with an estimated 1.05 log10 pg/mL at time of clinical relapse, compared to that at end of treatment (p < 0.001, Supplementary Figure 2).
Analysis of the Performance of Plasma sPD-1 as a Predictor of Clinical Relapse
The AUROCs of EOT sPD-1 for clinical relapse at 2, 4, 6 and 8 year after NAs discontinuation were 0.71 (95% CI, 0.61–0.82), 0.67 (95% CI, 0.56–0.78), 0.64 (95% CI, 0.53–0.75) and 0.65 (95% CI, 0.54–0.76), respectively (all p values < 0.05, Figure 2A). When the cut-off value was 78 pg/mL, the value of true positive rate (TPR) minus false positive rate (FPR) was maximal for the prediction of 8-year clinical relapse. However, 78 pg/mL is below the LLOD level of sPD-1, so that the detectable threshold value of 156 pg/mL replaced it as the optimal cut-off value in subsequent analysis. The detectable EOT sPD-1 gave 67.3% of TPR and 43.6% of FPR, respectively. Using the Kaplan–Meier curve, patients with detectable sPD-1 exhibited significantly lower cumulative clinical relapse than patients with undetectable sPD-1 (48% vs 67.7% at year 8, Log rank test: p = 0.002, Figure 2B).
The univariate analysis showed that detectable sPD-1 at EOT (hazard ratio [HR] = 0.479, 95% confidence interval [CI] 0.290–0.792, p = 0.004) and age (HR = 1.035, 95% CI 1.007–1.065, p = 0.015) were identified as independent predictors for clinical relapse. Three variables, including younger age (HR = 1.045, 95% CI 1.012–1.078, p = 0.007), lower levels of EOT HBsAg level (HR = 1.418, 95% CI 1.006–2.000, p = 0.046) and detectable EOT sPD-1 (HR = 0.498, 95% CI 0.296–0.840, p = 0.009) were found to be associated with significantly reduced risk of clinical relapse, after adjusting for treatment duration in multivariate analysis (Table 2).
 |
Table 2 Cox Proportional Hazard Model for Clinical Relapse (n=115) |
Plasma sPD-1 Predicted HBsAg Loss After NA Discontinuation
HBsAg loss was observed in 14 patients with an 8-year cumulative incidence of 23.4% (Supplementary Figure 1B). Of patients who lost HBsAg, 11 patients were treatment-free with a sustained response and three patients were re-treated after clinical relapse. The median time to HBsAg loss was 3.5 years (IQR, 1.0–6.5 years). For all 115 patients, Cox regression analysis revealed that HBsAg (HR = 0.233, 95% CI 0.121–0.450, p < 0.001) and detectable sPD-1 (HR = 9.179, 95% CI 1.131–74.506, p = 0.038) were independent predictors of HBsAg loss (Table 3). Previous studies have reported an EOT HBsAg level < 100 IU/mL is a strong predictor of HBsAg loss.25 In this study, the EOT HBsAg level < 100 IU/mL (n=23) and ≥ 100 IU/mL (n=92) contributed 66.2% and 15.1% 8-year cumulative rates of HBsAg loss, respectively (Log rank test: p < 0.001, Figure 3A). Furthermore, the 8-year cumulative incidence of HBsAg loss was significantly higher in patients with detectable sPD-1 than patients with undetectable sPD-1 (33.7% vs 2.4%, Log rank test: p = 0.01, Figure 3B). The discriminative power between HBsAg < 100 IU/mL (AUROC = 0.71, 95% CI 0.55–0.87) and detectable sPD-1 (AUROC = 0.70, 95% CI 0.57–0.82) was no significant difference in predicting HBsAg loss, as assessed by DeLong’s test (p = 0.789, Figure 3C). In addition, patients with detectable sPD-1 had remarkably higher rates of HBsAg decline to HBsAg < 10 IU/mL (38.6% vs 9.7%, Log rank test: p = 0.003, Supplementary Figure 3A), and HBsAg < 100 IU/mL (55.2% vs 26.7%, Log rank test: p = 0.008, Supplementary Figure 3B), than those with undetectable sPD-1, respectively.
 |
Table 3 Cox Proportional Hazard Model for HBsAg Loss (n=115) |
Correlations Between Plasma sPD-1 Levels and Other Factors
Spearman correlations between plasma sPD-1 levels and other factors are shown in Table 4. The sPD-1 levels correlated weakly with ALT levels at EOT, week 4, 8, 12, 24, and 48 with correlation coefficient of 0.347, 0.238, 0.408, 0.282, 0.308 and 0.184, respectively (all p < 0.05), while showed moderately correlation coefficient of 0.674 for ALT levels at time of clinical relapse. At most off-treatment time points, no significant correlation was found between sPD-1 and HBsAg, HBV DNA. Other factors including age, sex, start-of-treatment HBeAg status, consolidation therapy duration, treatment duration were not statistically associated with sPD-1 (all p > 0.05, data not shown).
 |
Table 4 Spearman Correlation Between sPD-1 Level and ALT, HBsAg, HBV DNA at the End of Treatment and After Treatment Discontinuation |
Discussion
In this study of 115 NA discontinued patients with chronic HBV infection, 56.6% (62/115) and 23.4% (14/115) of patients experienced 8-year cumulative clinical relapse or HBsAg loss, respectively. Importantly, detectable sPD-1 was shown for the first time to correlate with a remarkably reduced risk of clinical relapse. Notably, high sPD-1 was significantly associated with increased HBsAg loss in patients following NA discontinuation. Thus, sPD-1 is a novel biomarker linked to clinical relapse and loss of HBsAg.
“Stop-to-cure” is an interesting strategy to consider, in addition to further exploring the recommendation to discontinue NAs before HBsAg loss in patients receiving long-term NA therapy.26,27 The incidence of off-therapy HBsAg loss ranges from zero to 25% in different studies.4,5 This discrepancy may be due to study-specific differences in design, patient ethnicity, HBV genotype, and duration of follow-up. However, off-therapy HBsAg loss remains a phenomenon that is not well understood. The outcome of chronic HBV infection is dependent on the equilibrium between the host and virus,28 so parameters that silence HBV transcription and restore immune control may help to explain the mechanism. Low serum HBsAg is a useful predictor of subsequent HBsAg loss. Liu et al29 showed that patients with EOT HBsAg < 100 IU/mL can safely discontinue NA therapy and have a high probability of achieving HBsAg loss. The findings reported in the current study support a cut-off value of 100 IU/mL, since 8 of 23 patients with HBsAg < 100 IU/mL lost HBsAg expression after stopping NA. However, while patients in most studies had HBsAg levels < 100 IU/mL at the time of NA discontinuation, this was only true for 20% (23/115) of the patients in this study. Notably, six of the 14 patients who achieved HBsAg loss had HBsAg levels > 100 IU/mL. If HBsAg < 100 IU/mL is used as the only standard for NA cessation in clinical practice, some patients may not have the opportunity for HBsAg loss. As a result, identification of other factors associated with HBsAg loss should be considered and may help to promote a “stop-to-cure” strategy for individuals with chronic HBV infection who receive long-term treatment.
Consistent with a small scale study that high sPD-1 levels were predictive of HBsAg loss during treatment.20 In the present study, detectable sPD-1 at EOT was negatively associated with clinical relapse and positively associated with subsequent HBsAg loss. The AUROC values for EOT sPD-1 were not perfect to predict clinical relapse and HBsAg loss, at 0.65 and 0.70, respectively. However, the discriminative power of sPD-1 is comparable to HBsAg for HBsAg loss. Patients with undetectable sPD-1 at EOT could identify 67.7% and only 2.4% incidence of clinical relapse and HBsAg loss, respectively. Therefore, discontinuation of NA in these patients is not recommended.
Several arguments explain these findings. First, T cell dysfunction during chronic hepatitis B infection is an obstacle for viral control and functional cure, a hallmark associated with increased expression of inhibitory receptors like PD-1.30 Inhibiting PD-1/PD-L1 signaling may enhance anti-viral specific T cell responses,31 which is a key to achieve HBsAg clearance. Notably, sPD-1, a monomeric protein generated from spliced PD-1 exon 3,32 exhibits functional antagonism toward membrane -bound PD-1 on T cells.33 Thus, sPD-1 may rescue the functional exhaustion of T cells by inhibiting PD-1/PD-L1 signaling. Patients with higher sPD-1 levels may have sustained responses, while those relatively low sPD-1 levels may experience clinical relapse.
For patients experiencing HBsAg loss, early FINITE studies show that off-treatment hepatitis flares trigger immune-mediated functional cure.34 It is possible that the inflammation is involved in furthering HBV control and maintaining loss of HBsAg. Studies have investigated the profiles of sPD-1 among chronic HBV infected patients in different stages of the disease, during NA treatment, and in other relative diseases.18–21 The present studies demonstrated the controversial correlations between sPD-1 and HBsAg. Zhou L et al19 reported that sPD-1 positively correlated with HBsAg levels during antiviral treatment in CHB patients with treatment naive. However, Tian N et al35 recently showed that sPD-1 levels negatively correlated with HBsAg levels in CHB patients with antiviral treatment. Our study showed that sPD-1 levels were negatively correlated with HBsAg level, but weakly or not statistically significant. This can be explained by the relatively small number of patients with lower EOT HBsAg levels (91.3% patients with a distribution of 1–3 log10 IU/mL) after long-term treatment. Differences in the patient populations also account for the observed discrepancy. It suggests that sPD-1 may play an immunomodulatory role in disease development, rather than a virus-related marker.
Specifically, sPD-1 levels were significantly elevated during chronic hepatitis B and an association between sPD-1 and hepatitis indicators was identified. Wang et al36 showed that sPD-1 is associated with pathological injury during chronic hepatitis C virus infection. The current study found weak correlations between plasma sPD-1 and ALT at most early off-therapy time points, which probably due to low and normal levels of ALT in this period, while a stronger correlation between sPD-1 and ALT was found with a correlation coefficient of o.674. In addition, increasing levels of sPD-1 seen in patients with clinical relapse explains why off-treatment sPD-1 did not predict clinical relapse. This finding is contradictory that high EOT levels of sPD-1 related to a favorable outcome. We hypothesis that this paradoxical phenomenon is most likely due to the multifaceted functions of sPD-1 in HBV infection. We learned that if the concentrations of sPD-1 are too low, it’s not sufficient to support immune response against virus replication, for example, patients in the immune tolerance phase have the highest levels of HBV DNA levels but the lowest sPD-1 levels.18,21 However, if the concentrations of sPD-1 are too high, an overactive immunity may lead to severe liver injuries, such as hepatitis flare and liver fibrosis.19 Thus, patients with low EOT sPD-1 levels are at high risk of clinical relapse after NA discontinuation. Whereas an increase in sPD-1 may imbalance the immune system, leading to hepatitis flares in patients with clinical relapse. As mentioned, off-treatment hepatitis flares may trigger immune-mediated functional cure, it remains unclear whether the increasing sPD-1 in clinical relapse would be involved in the process. Nonetheless, it was beyond our scope to explore the inner mechanism of interplay between sPD-1, hepatitis flare, and HBsAg loss.
The association between sPD-1 and disease prognosis is not fully established.37 Several studies have shown that higher sPD-1 levels correlate with active and advanced disease, in addition to poor prognosis.21,38–40 However, stable or increased sPD-1 levels are associated with better outcomes after treatment of nontuberculous mycobacterial lung disease, renal cell carcinoma, lung cancer, hepatocellular carcinoma.41–44 To our knowledge, this is the first report demonstrating the role of sPD-1 as a possible biomarker for clinical relapse and HBsAg loss in patients who discontinue NA treatment.
This study has several limitations. First, the study was a single-center cohort study with a limited number of noncirrhotic patients, so the results need to be validated in a larger prospective cohort. Second, all the patients were re-treated if they experienced clinical relapse, so it was not possible to compare HBsAg decline or HBsAg loss between patients who were or were not re-treated. Moreover, only Chinese populations, who were likely infected with HBV genotype B or C, were included in this study, therefore further investigation is needed to assess responses in individuals infected with other genotypes. Finally, sPD-1 levels were only divided into two groups, detectable and undetectable, to assess how sPD-1 correlated with clinical outcomes. Because sPD-1 was only detected in a limited number of patients in this study, a more sensitive detection kit is needed for further research.
Conclusion
Measuring sPD-1 expression may help to select optimal candidates to stop NA treatment. The detectable sPD-1 at the end of treatment appears to predict subsequent HBsAg loss. This may be related to a bioactive function of sPD-1 and should by further examined and confirmed in larger cohorts.
Abbreviations
HBV, hepatitis B virus; CHB, chronic hepatitis B; ALT, serum alanine aminotransferase; NA, nucleos(t)ide analogue; EOT, end-of-treatment; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; BcrAg, hepatitis B core-related antigen; cccDNA, intrahepatic covalently closed circular DNA;ULN, the upper limit of normal; LLOD, the lower limit of detection; ELISA, enzyme-linked immunoassay; SD, standard deviation; HR, hazard ratio; CI, confidence interval, IQR, interquartile range; ROC, receiver operating characteristic; SR, sustained response; CR, clinical relapse; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1, sPD-1, soluble programmed cell death 1.
Data Sharing Statement
Part of the data used during the study has been public available in ResMan (http://www.medresman.org.cn) and ChiCTR (http://www.chictr.org.cn/index.aspx), including age, values of ALT/AST, HBV DNA, HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, AFP, ultrasonography, and liver stiffness.
Acknowledgments
The authors would like to thank all the patients included in this study, and the nurses who assisted in patient management and collection of serum samples.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Guichan Liao, Ziying Liu and Muye Xia share co-first authorship. Shaohang Cai, Xiaoyong Zhang and Jie Peng share co-senior authorship.
Funding
This study was supported by grants from the National Science Foundation of China (No.81971949, No.86878446), Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (No.LC2016PY003) and Clinical Research Program of Nanfang Hospital, Southern Medical University (No.2018CR026). The funding sources did not have any influence on the study design, data collection, analysis and interpretation of the data, writing of the manuscript, or decision to submit for publication.
Disclosure
All authors have no competing interests to disclose.
References
1. Terrault NA, Bzowej NH, Chang K, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi:10.1002/hep.28156
2. European Association for the Study of the Liver. Electronic address:[email protected]; European Association for the Study of the Liver: EASL2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
3. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi:10.1007/s12072-015-9675-4
4. van Boemmel F, Berg T. Risks and Benefits of discontinuation of nucleos(t)ide analogue treatment: a treatment concept for patients with HBeAg-negative chronic hepatitis B. Hepatol Commun. 2021;5:1632–1648. doi:10.1002/hep4.1708
5. Papatheodoridi M, Papatheodoridis G:. Emerging diagnostic tools to decide when to discontinue nucleos(t)ide analogues in chronic hepatitis B. Cells-Basel. 2020;9:493.
6. Xie Y, Li M, Ou X, et al. HBeAg-positive patients with HBsAg < 100 IU/mL and negative HBV RNA have lower risk of virological relapse after nucleos(t)ide analogues cessation. J Gastroenterol. 2021;56:856–867. doi:10.1007/s00535-021-01812-0
7. Mak LY, Wong DKH, Cheung KS, et al. Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharm Ther. 2018;47:43–54. doi:10.1111/apt.14376
8. Rivino L, Le Bert N, Gill US, et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668–681. doi:10.1172/JCI92812
9. Park J, Wong DK, Wahed AS, et al. Hepatitis B virus-specific and global T-cell dysfunction in chronic hepatitis B. Gastroenterology. 2016;150:684.
10. Chang K, Traum D, Park J, et al. Distinct phenotype and function of circulating V1(+) and V2(+) T-cells in acute and chronic hepatitis B. PLoS Pathog. 2019;15(4):e1007715. doi:10.1371/journal.ppat.1007715
11. Peng G, Li S, Wu W, et al. PD-1 upregulation is associated with HBV specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45(4):963–970. doi:10.1016/j.molimm.2007.07.038
12. Rinker F, Zimmer CL, Honer ZSC, et al. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69(3):584–593. doi:10.1016/j.jhep.2018.05.004
13. Pen JJ, Keersmaecker BD, Heirman C, et al. Interference with PD-L1/PD-1 co-stimulation during antigen presentation enhances the multifunctionality of antigen-specific T cells. Gene Ther. 2014;21:262–271. doi:10.1038/gt.2013.80
14. Khan M, Zhao Z, Arooj S, Fu Y, Liao G, Soluble PD-1. Predictive, Prognostic, and Therapeutic Value for Cancer Immunotherapy. Front Immunol. 2020;11:11. doi:10.3389/fimmu.2020.00011
15. Meyo MT, Jouinot A, Giroux-Leprieur E, et al. Predictive value of soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: a case-control study. Cancers. 2020;12:473.
16. Dong MP, Enomoto M, Thuy LTT, et al. Clinical significance of circulating soluble immune checkpoint proteins in sorafenib-treated patients with advanced hepatocellular carcinoma. Sci Rep-UK. 2020;10:1–10.
17. Cheng H, Kang P, Chuang Y, et al. Circulating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS One. 2014;9(11):e95870. doi:10.1371/journal.pone.0095870
18. Xia J, Huang R, Chen Y, et al. Profiles of serum soluble programmed death-1 and programmed death-ligand 1 levels in chronic hepatitis B virus-infected patients with different disease phases and after anti-viral treatment. Aliment Pharm Ther. 2020;51(11):1180–1187. doi:10.1111/apt.15732
19. Zhou L, Li X, Huang X, et al. Soluble programmed death-1 is a useful indicator for inflammatory and fibrosis severity in chronic hepatitis B. J Viral Hepatitis. 2019;26(7):795–802. doi:10.1111/jvh.13055
20. Tan N, Luo H, Kang Q, et al. Soluble programmed death-1 is predictive of hepatitis B surface antigen loss in chronic hepatitis B patients after antiviral treatment. World J Clin Cases. 2021;9(21):5812–5821. doi:10.12998/wjcc.v9.i21.5812
21. Li N, Zhou Z, Li F, et al. Circulating soluble programmed death-1 levels may differentiate immune-tolerant phase from other phases and hepatocellular carcinoma from other clinical diseases in chronic hepatitis B virus infection. Oncotarget. 2017;8(28):46020–46033. doi:10.18632/oncotarget.17546
22. Wang D, Du Q, Luo G, et al. Aberrant production of soluble inducible T cell co-stimulator and soluble programmed cell death protein 1 in patients with chronic hepatitis B. Mol Med Rep. 2017;16(6):8556–8562. doi:10.3892/mmr.2017.7630
23. Cao J, Chi H, Yu T, et al. Off-treatment hepatitis B virus (HBV) DNA levels and the prediction of relapse after discontinuation of nucleos(t)ide analogue therapy in patients with chronic hepatitis B: a prospective stop study. J Infect Dis. 2017;215(4):581–589. doi:10.1093/infdis/jix025
24. Xia M, Liao G, Chen H, et al. Plasma CXCL13 is a predictive factor for HBsAg loss and clinical relapse after discontinuation of nucleos(t)ide analogue treatment. Clin Immunol. 2019;198:31–38. doi:10.1016/j.clim.2018.11.016
25. Jeng W, Chen Y, Chien R, Sheen I, Liaw Y. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68(2):425–434. doi:10.1002/hep.29640
26. Berg T, Lampertico P. The times they are a-changing - A refined proposal for finite HBV nucleos(t)ide analogue therapy. J Hepatol. 2021;75:474–480. doi:10.1016/j.jhep.2021.04.040
27. Bert F, Rossol S. Acute exacerbation of reactivated chronic hepatitis-b after stopping antiviral medication followed by loss of HBsAg. Deut Med Wochenschr. 2020;145:552–554.
28. Boni C, Laccabue D, Lampertico P, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–973.e9. doi:10.1053/j.gastro.2012.07.014
29. Liu J, Li T, Zhang L, Xu A. The role of hepatitis B surface antigen in nucleos(t)ide analogues cessation among asian patients with chronic hepatitis B: a systematic review. Hepatology. 2019;70(3):1045–1055. doi:10.1002/hep.30474
30. Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;641:S71–S83. doi:10.1016/j.jhep.2016.01.026
31. Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61:1212–1219. doi:10.1016/j.jhep.2014.07.005
32. Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD-1 gene. Cell Immunol. 2005;235(2):109–116. doi:10.1016/j.cellimm.2005.07.007
33. Song M, Park S, Nam HJ, Choi D, Sung Y. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J Immunother. 2011;34:297–306. doi:10.1097/CJI.0b013e318210ed0e
34. Berg T, Simon K, Mauss S, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918–924. doi:10.1016/j.jhep.2017.07.012
35. Tan N, Luo H, Kang Q, et al. High levels of soluble programmed death-1 are associated with virological response in chronic hepatitis B patients after antiviral treatment. Virus Res. 2022;309:198660. doi:10.1016/j.virusres.2021.198660
36. Wang D, Zhou D, Du Q, et al. Aberrant production of soluble inducible T-cell co-stimulator (sICOS) and soluble programmed cell death protein 1 (sPD-1) in patients with chronic hepatitis C. Mol Med Rep. 2013;7:1197–1202. doi:10.3892/mmr.2013.1326
37. Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer. 2018;6(1):1–8. doi:10.1186/s40425-018-0317-y
38. Greisen SR, Rasmussen TK, Stengaard-Pedersen K, et al. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol. 2014;43(2):101–108. doi:10.3109/03009742.2013.823517
39. Bian B, Fanale D, Dusetti N, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology. 2019;8:e1561120.
40. Zhao Y, Jia Y, Li C, Shao R, Fang Y. Predictive value of soluble programmed death-1 for severe sepsis and septic shock during the first week in an intensive care unit. Shock. 2019;51(3):289–297. doi:10.1097/SHK.0000000000001171
41. Pan S, Su W, Chan Y, et al. Disease progression in patients with nontuberculous mycobacterial lung disease of nodular bronchiectatic (NB) pattern: the role of cavitary NB and soluble programmed death protein-1. Clin Infect Dis. 2021:ciab929. PMID: 34726741. doi:10.1093/cid/ciab929
42. Incorvaia L, Fanale D, Badalamenti G, et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology. 2020;9:1832348.
43. Sorensen SF, Demuth C, Weber B, Sorensen BS, Meldgaard P. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer. 2016;100:77–84. doi:10.1016/j.lungcan.2016.08.001
44. Chang B, Huang T, Wei H, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immun. 2019;68:353–363. doi:10.1007/s00262-018-2271-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.