Back to Journals » Drug Design, Development and Therapy » Volume 17
Solanine Represses Gastric Cancer Growth by Mediating Autophagy Through AAMDC/MYC/ATF4/Sesn2 Signaling Pathway
Authors Tang X, Guo Y, Zhang S , Wang X, Teng Y , Jin Q, Jin Q, Shen W, Wang R
Received 19 September 2022
Accepted for publication 31 January 2023
Published 8 February 2023 Volume 2023:17 Pages 389—402
DOI https://doi.org/10.2147/DDDT.S389764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Xiaolong Tang,1,2,* YingYing Guo,2,* Sijia Zhang,2 Xin Wang,2 Yuhao Teng,3 Qingjiang Jin,2 Qinglei Jin,2 Wei Shen,2,* Ruiping Wang1,3,*
1The First Clinical Medical College, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China; 2Department of Oncology, Suzhou Hospital of Integrated Traditional Chinese and Western Medicine, Suzhou, Jiangsu, People’s Republic of China; 3Department of Oncology, the Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Shen, Department of Oncology, Suzhou Hospital of Integrated Traditional Chinese and Western Medicine, 39 Xiashatang Road, Wuzhong District, Suzhou, Jiangsu, People’s Republic of China, Email [email protected] Ruiping Wang, Department of Oncology, the Affiliated Hospital of Nanjing University of Chinese Medicine, 155 Hanzhong Road, Qinhuai District, Nanjing, Jiangsu, People’s Republic of China, Tel +13815883181, Email [email protected]
Purpose: Solanine is the main component of the plant Solanum, which has been shown to provide growth-limiting activities in a variety of human cancers. However, little is known about its function in gastric cancer (GC).
Methods: We investigated the effect of solanine on GC in vivo and in vitro. The inhibition rate of solanine on the tumor was observed by constructing a subcutaneous tumor in nude mice. Morphological changes were analyzed with H&E staining. The expression of ATF4 was detected by IF analysis. MTT assays, EdU staining, and colony formation assays were used to detect the inhibition rate of solanine on GC cells. Matrigel transwells were used to detect the invasion of GC cells. Cell migration was measured using the wound healing assay. The flow cytometric analysis was used to monitor changes in the cell cycle and cell apoptosis. Western blotting was used to detect major proteins in cells and tumors.
Results: Solanine suppressed gastric tumorigenesis. Solanine also inhibited the proliferation, invasion and mitigation of GC cells, and induced cell cycle arrest and apoptosis in vitro. Moreover, the growth-limiting activities of solanine in gastric cancer were related to the suppression of the AAMDC/MYC/ATF4/Sesn2 pathway-mediated autophagy. Overexpression of AAMDC reversed the inhibitory effect of solanine on autophagy and gastric cancer.
Conclusion: In summary, our findings indicate that solanine confers growth-limiting activities by deactivating the AAMDC-regulated autophagy in gastric cancer.
Keywords: gastric cancer, autophagy, AAMDC, solanine
Introduction
Gastric cancer is the fifth-leading cause of global cancer-related mortality, and over 1 million new gastric cancer cases occurred in 2018.1 During the early stages, the symptoms of gastric cancer are nonspecific. It starts with general discomfort in the stomach or abdominal region and progresses to vomiting, anorexia, weight loss, trouble swallowing, excessive belching, and a sensation of early slumber. Due to this, the condition is often identified in its advanced stages.2 Given that the rate of median survival is less than 12 months for the advanced stage, gastric cancer still constitutes a global health problem.3 Currently, the commonly used therapies for treating gastric cancer include chemotherapy, radiation therapy, and gastrectomy. However, side effects, toxicity, and drug resistance restrict their application.4 Thus, there is an urgent need to identify new treatments.
According to previous research, various molecular pathways are implicated in the development of gastric cancer. Lei et al, for instance, discovered three subtypes of gastric cancer, including a proliferative profile linked with high levels of genomic instability, TP53 mutations, and DNA hypomethylation, a metabolic profile characterized by enhanced anaerobic glycolysis that ultimately results in tumor cells that are more susceptible to therapy with 5-fluorouracil, and a mesenchymal stem cell profile especially responsive to PI3K-AKT-mTOR inhibitors.5,6 Other mechanisms such as fatty acid metabolism, cell adhesion and proliferation, and carbohydrate and protein metabolism are also crucial to the progression of gastric cancer.6
Macroautophagy, referred to here as autophagy, is a highly conserved eukaryotic cellular recycling process that initiates the formation of autophagosomes that capture intracellular proteins and organelles and then fuse with lysosomes to recycle.7 Autophagy maintains proper cell function and cellular homeostasis.8 Its disruption may cause neurological, metabolic, infectious, cardiovascular, musculoskeletal, and renal disorders, as well as cancer.9 For example, the degradation of NBR1 by autophagy inhibits the metastasis of breast cancer.10 Gastric cancer upregulates autophagy, surviving microenvironmental stress and increasing its growth and aggressiveness.11 Through the activation of autophagy, the long noncoding RNA (lncRNA) EIF3J-DT is able to generate chemoresistance in gastric cancer.12 Thus, autophagy inhibition may be beneficial for gastric cancer therapy.
Autophagy is linked to several molecular pathways. AMPK is able to inhibit the mTOR signaling pathway, triggering autophagy; phagophore formation requires the PI3K/VPS34 complex that is comprised of Beclin-1, VPS34, VPS15, and ATG14L; light chain 3 (LC3)-I is converted to LC3-II by lipidation, mediating autophagosome formation; autophagosome-lysosome fusion is initiated by proteins such as SNAREs and LAMP2.13 The MYC proto-oncogene, which undergoes chromosomal translocation and gene amplification in many human cancers, is an essential regulator of autophagy.14 MYC upregulates the stress-induced transcription factor ATF4 that activates the cysteine sulfinyl reductase Sesn2 to trigger autophagy.15 Recently, MYC was reported to be controlled by the oncogene AAMDC.16
Solanine is a glycoalkaloid that has been found in various plants, including potatoes and Solanum nigrum.17 Emerging evidence suggests that solanine stimulates ATF4-mediated autophagy, which ultimately influences cell apoptosis.18 Solanine exerts an anticancer effect on multiple types of cancer, such as colorectal cancer, esophageal cancer, prostate cancer and pancreatic cancer.19–22 However, the function of solanine in gastric cancer has rarely been studied.
In this study, we investigated the anticancer activity of solanine in gastric cancer. We showed that solanine inhibited the growth of gastric cancer by suppressing the AAMDC pathway of autophagy in vivo and in vitro. Furthermore, overexpression of AAMDC compromised solanine-induced preventive effects in gastric cancer. Collectively, our findings revealed the anti-cancer property of solanine in gastric cancer.
Materials and Methods
Animals and Treatment
Male BALB/c nude mice (16−18 g body weight) were purchased from Shanghai SIPPR-BK Laboratory Animal Co. Ltd. (Shanghai, China) and housed in a pathogen-free animal facility. Animals received food and water ad libitum. All animal procedures were approved by Suzhou Hospital of Integrated Traditional Chinese and Western Medicine and performed in accordance with the regulations of experimental animal administration issued by the State Committee of Science and Technology of the People’s Republic of China. The SGC-7901 cell line was purchased from China Center for Type Culture Collection. Each mouse was subcutaneously injected with SGC-7901 cells (1 × 106 cells in 0.1 mL PBS) into the right armpit. When the tumor diameter≥0.5cm, the mice were randomly divided into three groups (n=12/group): (i) control group (ctr), (ii) Solanine 5 mg/kg group (low-so), (iii) Solanine 10 mg/kg group (high-so). Solanine was intraperitoneally injected daily for 14 days. Then animals were sacrificed by cervical dislocation, and tumors were collected for further analysis.
Cell Culture and Treatment
Human gastric epithelial cell line GES-1 and human gastric cancer cells SGC-7901 and MGC-803 were purchased from the Cell Bank of the Chinese Academy of Sciences and cultured in Dulbecco’s modified Eagle medium (DMEM) medium supplemented with 10% fetal bovine serum (FBS; GIBCO) at 37 °C in a humidified chamber with 5% CO2. After 24 h, solanine (0, 1, 2, 4, 6, 8, 10, 15, or 20 μM) was added to the cells for 48 h. For the overexpression of AAMDC, cell transfection was performed prior to solanine administration, as described below.
Cell Transfection
The SGC-7901 cells were seeded in a 24-well plate until they reached 80% confluency. Then AAMDC-overexpressing vectors and the negative controls were transfected into the cells using Lipofectamine 3000 (Invitrogen) following the manufacturer’s protocol. After transfection for 24 h, cells were harvested for further investigation. The AAMDC-overexpressing vectors and the negative controls were constructed by General Biosystems (Hefei) Co., Ltd.
Histological Evaluation
Morphological changes were analyzed with hematoxylin and eosin (H&E) staining, as reported elsewhere.23 The tumor tissues were collected, fixed in 4% formaldehyde, embedded in paraffin, and cut into 5-μm-thick sections. Subsequently, the slices were stained with hematoxylin and eosin and observed and imaged using an Olympus microscope.
Immunofluorescence Assay
The immunofluorescence assay was performed as described previously.24 Tumor tissues and cells were fixed in 4% formaldehyde for 20 min and permeabilized with 0.1% Triton X-100 for 10 min. After being blocked with 1% BSA for 30 min, the samples were incubated with primary antibodies against Ki-67 and ATF4 at 4 °C overnight. Subsequently, the samples were incubated with Alexa Fluor® 488 goat anti-rabbit IgG (ab150077, Abcam) and Alexa Fluor® 594 goat anti-rabbit IgG (ab150080, Abcam) for 2 h at 37 °C. The nuclei were stained with DAPI for 15 min. Fluorescent images were acquired by a fluorescence microscope (BX53, Olympus).
Western Blotting
The Western blotting was performed as previously described.25 Protein extraction from gastric cancer tissues and cells was performed using the RIPA buffer containing a protease and phosphatase inhibitor cocktail. The concentration of protein lysate was determined using a BCA Protein Assay Kit (Beyotime Biotechnology). Equal amounts of protein were resolved by SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk for 1 h. After that, the blots were incubated with primary antibodies against cleaved Caspase3, Caspase3, Bax, Bcl-2, LC3A/B, AAMDC, MYC, ATF4, Sesn2 and Tubulin overnight at 4 °C, followed by incubation with the appropriate secondary antibodies for 90 min at room temperature. Finally, immunoreactive signals were detected using an enhanced chemiluminescence kit (Beyotime Biotechnology). Band intensity was analyzed by ImageJ software. The antibodies used were purchased from Cell Signaling Technology, Abcam, Affinity, or Proteintech.
Cell Proliferation Assays
For MTT assays, cells were plated in 96-well plates and treated with solanine for 48 h. MTT solution (5 mg/mL, 20 μL per well) was then added and incubated at 37 °C for 4 h. Dimethyl sulfoxide (DMSO, 150 μL) was further used to dissolve formazan crystals. Finally, the absorbance was measured by a microplate reader at 490 nm.
The 5-Ethynyl-2′-deoxyuridine (EdU) staining was performed to measure cell proliferation as described.26 In brief, solanine-treated cells were incubated with 50 μM EdU for 2 h at 37 °C, fixed in 4% formaldehyde for 30 min, and permeabilized with 0.5% TritonX-100 for 10 min at room temperature. Afterwards, 1 × Apollo® reaction cocktail (400 μL) was used to react with the EdU for 30 min. Nuclei were stained with Hoechest33342 (400 μL) for 30 min, and cells were visualized under a fluorescence microscope.
For colony formation assays, cells were seeded in six-well culture dishes and treated with solanine. The medium was replaced with new medium every 4 days. After being cultured for 2 weeks, the colonies were fixed in methanol for 15 min, and stained with 0.1% crystal violet for 20 min to visualize colonies. Finally, colonies were photographed and counted.
Cell Invasion Assay
Matrigel transwell was used to detect the invasion of cells.27 In brief, cells were resuspended in the upper chamber of transwell inserts coated with Matrigel and supplemented with DMEM (serum-free). Then, 750 μL of DMEM containing 10% FBS was added to the lower chamber. After incubation for 24 h, cells on the upper side of the membrane were removed with a cotton swab, while cells attached to the underside of the membrane were fixed with methanol and stained with 0.1% crystal violet. Invading cells were observed and counted under light microscopy.
Cell Wound Healing Assay
Cell migration was measured using the wound healing assay.28 Gastric cancer cells were grown in a six-plate and wounds were scratched with 200 μL micropipette tips. Plates were washed with PBS buffer to remove non-adherent cells. Representative images were captured at 0, 48 h after culture in serum-free medium.
Cell Cycle and Cell Apoptosis Analysis
The flow cytometric analysis was used to monitor changes in cell cycle and cell apoptosis.29
For cell cycle analysis, cells were harvested and fixed in 70% ethanol at −20 °C overnight. Afterwards, cells were rinsed with PBS, treated with RNase A (100 μg/mL) for 30 min, and stained with propidium iodide (PI; 50 μg/mL) for 10 min at 4 °C before being subjected to a flow cytometer.
Cell apoptosis analysis was performed using an Annexin V-FITC Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s instructions. Briefly, cells were harvested and resuspended in a binding buffer. After double staining with FITC-Annexin V and propidium iodide (PI), the cells were analyzed by a flow cytometer.
Statistical Analysis
All experimental results were summarized as mean ± standard deviation (SD). Statistical analyses were performed using one-way or two-way ANOVA followed by Dunnett or Tukey’s post hoc tests in GraphPad Prism (version 6.0). A P value of less than 0.05 was considered statistically significant.
Results
Solanine Inhibits Gastric Tumorigenesis in vivo
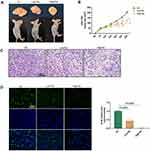
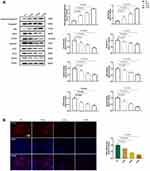
To evaluate the in vivo effect of solanine on tumorigenicity, we injected nude mice with SGC-7901 cells. Images of tumors from the three groups are shown in Figure 1A, and evident differences were observed between the control group and solanine-treated groups. Moreover, solanine treatment significantly reduced the tumor size relative to the saline-treated controls over time (Figure 1B). The H&E staining revealed that the gastric tumor from the control group had irregular nuclei, inconspicuous nucleoli, a high nuclear-cytoplasmic ratio, and few mitotic phenomena (Figure 1C). In contrast, the tumor tissues from the solanine-treated groups had fewer tumor cells; the nuclei are pyknotic, fragmented, dissolved, and deeply stained. Using the immunofluorescence assay, we noticed that solanine greatly inhibited the proliferation of gastric cancer cells, as evidenced by lower Ki-67 expression levels in solanine groups when compared with those of the controls (Figure 1D). In the Western blot analysis, solanine upregulated the levels of pro-apoptotic proteins cleaved Caspase3 and Bax, and downregulated the expression of the anti-apoptotic protein Bcl-2 (Figure 2A). Collectively, these results suggest that solanine prevented gastric carcinogenesis in vivo.
Solanine Suppresses AAMDC-Mediated Autophagy in vivo
Next, we explored the role of AAMDC-mediated autophagy in the solanine-induced anti-gastric tumor effect. During autophagy, LC3 is converted from its cytosolic form, LC3-I into the autophagy-related form, LC3-II.30 Thus, detecting LC3 conversion is a reliable method of monitoring autophagy. As shown in Figure 2A, solanine-treated groups displayed a lower LC3 II/I ratio and downregulated AAMDC, MYC, ATF4 and Sesn2 protein expression relative to the control group. Meanwhile, solanine decreased the ATF4 levels in the immunofluorescence assay (Figure 2B). These findings indicated that solanine inhibited gastric cancer cells by deactivating AAMDC-mediated autophagy in vivo.
Solanine Suppresses the Growth of Gastric Cancer Cells in vitro
We further examine the effect of solanine on gastric cancer in vitro. MTT results showed that solanine did not cause cell viability reduction in normal human gastric epithelial cells, GES-1 (Figure S1). Moreover, given that the half-maximal inhibitory concentration (IC50) value against SGC-7901 cells was 10.52 μM, 10 μM was chosen as the medium dose in further studies (Figure 3A). To assess the proliferation of gastric cancer cells, MTT, EdU staining and colony formation assays were conducted (Figure 3B-E). The results showed that cell proliferation was inhibited in the solanine-treated groups. We also investigated the effect of solanine on cell invasion and mitigation using transwell and wound healing assays, respectively. As shown in Figure 4A and B, solanine reduced the invasion and migration of gastric cancer cells. The flow cytometric analysis indicated that solanine induced S cell cycle arrest and apoptosis in comparison with control cells (Figure 4C and D). Furthermore, the Western blotting analysis confirmed the occurrence of gastric cell apoptosis following solanine intervention, as evidenced by the upregulation of cleaved Caspase3 and Bax, and the downregulation of Bcl-2 (Figure 5A). Overall, the data suggested that solanine inhibited the growth of gastric cancer cells in SGC-7901 cells. This result was further validated in the human gastric carcinoma cell line MGC-803, where solanine suppressed cell viability in a dose-dependent manner and decreased cell invasion and proliferation by deactivating the ATF4 signal (Figure S2).
Solanine Decreases AAMDC-Mediated Autophagy in vitro
The function of solanine on AAMDC-mediated autophagy was also investigated in vitro. Consistent with in vivo results, solanine greatly downregulated the protein expression levels of the AAMDC pathway and reduced the ATF4 immunoreactivity, leading to a decrease in the LC3 II/I percentage (Figure 5A and B). The above results indicated that solanine inhibited gastric cancer via deactivating AAMDC-mediated autophagy in vitro.
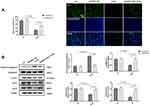
AAMDC Overexpression Compromised Solanine-Induced Inhibitory Effect in Gastric Cancer
To investigate the role of AAMDC in solanine-induced anti-gastric cancer effects. We transfected SGC-7901 cells with AAMDC-overexpressing plasmids. As depicted in Figure 6, overexpression of AAMDC greatly weakened solanine-induced cell proliferation inhibition and cell apoptosis in gastric cancer. Meanwhile, the solanine-induced decrease in LC3 II/I ratio and protein levels of MYC and ATF4 were reversed by AAMDC-overexpressing vectors. These findings confirmed that solanine prevented gastric cancer via the AAMDC pathway.
Discussion
Gastric cancer is a cancer of the digestive tract, characterized by high incidence and mortality. The first-line drugs for targeting gastric cancer in clinical practice are highly toxic and lack efficacy.31 Hence, novel drugs with better efficacy and safety are needed for treating gastric cancer. In the present study, we identified that solanine exhibited growth-limiting activities in murine and cellular models of gastric cancer. This effect was related to the suppression of AAMDC/MYC/ATF4/Sesn2 signaling-mediated autophagy. Furthermore, overexpression of AAMDC reversed the inhibitory effect of solanine on autophagy and gastric cancer.
Glycoalkaloids are nitrogen-containing secondary plant metabolites abundant in a variety of solanaceous plants and possess significant anti-cancer properties.32 Solamargine reduces the development of metastatic and primary melanoma cells (WM239 and WM115) by causing necrosis and cell rupture.33 Kim et al tested α-tomatine in vivo on CT-26 colon cancer cells and found that 14 days of α-tomatine treatment resulted in a 38% reduction in tumor growth in mice.34 Another research showed that α-tomatine dramatically decreased tumor take rate, tumor size and weight, as well as proliferative and angiogenic markers while promoting cell apoptosis in mice with breast tumor.35 Solanine was demonstrated to boost the immune response against liver cancer by reducing CD4+CD25+ Treg, Foxp3, and TGF.36 Solanine also considerably prevents prostate cancer development in xenograft athymic nude mice and suppresses cell proliferation in the cultured prostate cancer cell line DU145.21 In our work, solanine suppressed gastric tumorigenesis in vivo, hindered the proliferation, invasion and mitigation of gastric cancer cells, and induced cell cycle arrest and apoptosis in vitro, indicating the therapeutic potential of solanine in gastric cancer.
Autophagy, a survival-promoting pathway that supports nutrient recycling and metabolic adaptation, is a key player in cancer development.37 Autophagy has been demonstrated to facilitate tumorigenesis by providing cancer cells with energy and vital compounds upon various stress stimuli.37 Inhibition of autophagy via the MCOLN1 pathway not only decreases melanoma metastasis in mice but also reduces migration and invasion in malignant melanoma and glioma cell lines in vitro.38 Hepatocellular carcinoma cells were more susceptible to apigenin-induced apoptosis and proliferation inhibition when autophagy was repressed with 3-MA or siRNA targeting Atg5.39 Selective autophagy of NLRC5 enhances endometrial cancer immune evasion.40
Autophagy plays an important role in gastric cancer. Ahn et al determined the levels of Beclin-1 (an autophagy initiation factor) in 60 gastric carcinoma tissues using immunohistochemistry and found 83% of the gastric carcinomas had Beclin-1 expression, whereas normal gastric mucosa cells showed no or very weak expression of Beclin-1.41 lncRNA SNHG11 was demonstrated to promote gastric cancer progression by activating the Wnt/β-catenin pathway of autophagy.42 AQP3 facilitated chemoresistance to cisplatin (a first-line chemotherapeutic agent for gastric cancer) in gastric cancer cells via autophagy, while chloroquine, an autophagy inhibitor, enhanced cisplatin chemosensitivity in gastric cancer cells.43 In our study, solanine administration boosted the conversion of LC3 to its autophagy-related form and suppressed gastric cancer development in vivo and in vitro. Moreover, inhibition of autophagy by AAMDC overexpression blunted the preventive effect of solanine in gastric cancer cells, supporting the involvement of autophagy in regulating gastric cancer.
The MYC-ATF4-Sesn2 pathway is an important signal regulating autophagy in cancer. It has been documented that upregulating MYC promotes preferential translation of ATF4 and induces endoplasmic reticulum stress-controlled autophagy, which ultimately promotes tumorigenesis.14 Jin et al reported that ATF4-Sesn2 signaling activation is required for the induction of autophagy in cancer cells by ginseng, an herb with anti-cancer properties.44 The work of Ambrosio and his colleagues suggests that Sesn2 overexpression enhances autophagy in neuroblastoma cells, while loss of Sesn2 expression decreases autophagy induced by LSD1 depletion.45 Recently, MYC signaling was demonstrated to be regulated by AAMDC, an oncogene that controls the translation of MYC and ATF4.16 This finding was verified in our study, as evidenced by solanine-induced AAMDC downregulation resulting in the deactivation of the MYC-ATF4-Sesn2 pathway in nude mice and gastric cancer cells. On the other hand, solanine-induced upregulation of AAMDC promoted autophagy and apoptosis in gastric tumor tissues and gastric cancer cells, indicating the critical role of AAMDC in autophagic progress and the treatment of gastric cancer. This role was further supported by the findings that the inhibitory effects of solanine on autophagy and gastric cancer cell growth were weakened by the overexpression of AAMDC.
In conclusion, our data suggest that solanine could limit gastric cancer growth through suppressing the AAMDC pathway of autophagy in murine and cellular models of gastric cancer. The present study identified the potent inhibitory activities of solanine in gastric cancer and revealed its molecular mechanisms of anti-cancer action (Figure 7). These findings suggest that solanine may serve as a promising therapy for the treatment of gastric cancer.
 |
Figure 7 The mechanism of solanine against gastric cancer. |
Abbreviations
GC, Gastric cancer; ATF4, Activating Transcription Factor 4; AAMDC, Adipogenesis Associated Mth938 Domain Containing; MYC, MYC Proto-Oncogene, BHLH Transcription Factor; FBS, fetal bovine serum; Bax, BCL2 Associated X, Apoptosis Regulator; LC3A, Microtubule Associated Protein 1 Light Chain 3 Alpha; LC3B, Microtubule Associated Protein 1 Light Chain 3 Beta; SNHG11, Small Nucleolar RNA Host Gene 11; AQP3, Aquaporin 3; LSD1, Lysine Demethylase 1A.
Acknowledgments
This study were supported by the Science and Technology Development Plan of Suzhou (SYSD2018182, SYSD2020057, SYKXD2022073), and the Scientific Research Project of Jiangsu Provincial Health Commission (Z2022064). Thanks to all the authors’ families for their support.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. doi:10.5114/pg.2018.80001
2. Abbas M, Faggian A, Sintali DN, et al. Current and future biomarkers in gastric cancer. Biomed Pharmacother. 2018;103:1688–1700. doi:10.1016/j.biopha.2018.04.178
3. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21(11):4012. doi:10.3390/ijms21114012
4. Mao QQ, Xu XY, Shang A, et al. Phytochemicals for the Prevention and Treatment of Gastric Cancer: effects and Mechanisms. Int J Mol Sci. 2020;21(2):570. doi:10.3390/ijms21020570
5. Chen DL, Sheng H, Zhang DS, et al. The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p. Mol Cancer. 2021;20(1):166. doi:10.1186/s12943-021-01475-8
6. Alessandrini L, Manchi M, De Re V, Dolcetti R, Canzonieri V. Proposed Molecular and miRNA Classification of Gastric Cancer. Int J Mol Sci. 2018;19(6):1683. doi:10.3390/ijms19061683
7. Yun CW, Lee SH. The Roles of Autophagy in Cancer. Int J Mol Sci. 2018;19(11):3466. doi:10.3390/ijms19113466
8. Ashrafizadeh M, Paskeh MDA, Mirzaei S, et al. Targeting autophagy in prostate cancer: preclinical and clinical evidence for therapeutic response. J Exp Clin Cancer Res. 2022;41(1):105. doi:10.1186/s13046-022-02293-6
9. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. doi:10.15252/embj.2021108863
10. Marsh T, Debnath J. Autophagy suppresses breast cancer metastasis by degrading NBR1. Autophagy. 2020;16(6):1164–1165. doi:10.1080/15548627.2020.1753001
11. White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42–46. doi:10.1172/JCI73941
12. Luo Y, Zheng S, Wu Q, et al. Long noncoding RNA (lncRNA) EIF3J-DT induces chemoresistance of gastric cancer via autophagy activation. Autophagy. 2021;17(12):4083–4101. doi:10.1080/15548627.2021.1901204
13. Paskeh MDA, Entezari M, Clark C, et al. Targeted regulation of autophagy using nanoparticles: new insight into cancer therapy. Biochim Biophys Acta Mol Basis Dis. 2022;1868(3):166326. doi:10.1016/j.bbadis.2021.166326
14. Dey S, Tameire F, Koumenis C. PERK-ing up autophagy during MYC-induced tumorigenesis. Autophagy. 2013;9(4):612–614. doi:10.4161/auto.23486
15. Kim HJ, Joe Y, Kim SK, et al. Carbon monoxide protects against hepatic steatosis in mice by inducing sestrin-2 via the PERK-eIF2alpha-ATF4 pathway. Free Radic Biol Med. 2017;110:81–91. doi:10.1016/j.freeradbiomed.2017.05.026
16. Golden E, Rashwan R, Woodward EA, et al. The oncogene AAMDC links PI3K-AKT-mTOR signaling with metabolic reprograming in estrogen receptor-positive breast cancer. Nat Commun. 2021;12(1):1920. doi:10.1038/s41467-021-22101-7
17. Lin LT, Choong CY, Tai CJ. Solanine Attenuated Hepatocarcinoma Migration and Invasion Induced by Acetylcholine. Integr Cancer Ther. 2020;19:1534735420909895. doi:10.1177/1534735420909895
18. Hasanain M, Bhattacharjee A, Pandey P, et al. alpha-Solanine induces ROS-mediated autophagy through activation of endoplasmic reticulum stress and inhibition of Akt/mTOR pathway. Cell Death Dis. 2015;6:e1860. doi:10.1038/cddis.2015.219
19. Ni X, Chen J, Lu F, et al. Anti-Cancer Effect of alpha-Solanine by Down-Regulating S100P Expression in Colorectal Cancer Cells. Recent Pat Anticancer Drug Discov. 2018;13(2):240–247. doi:10.2174/1574892813666180329163945
20. Wu J, Wang L, Du X, et al. Alpha-solanine enhances the chemosensitivity of esophageal cancer cells by inducing microRNA138 expression. Oncol Rep. 2018;39(3):1163–1172. doi:10.3892/or.2018.6187
21. Pan B, Zhong W, Deng Z, et al. Inhibition of prostate cancer growth by solanine requires the suppression of cell cycle proteins and the activation of ROS/P38 signaling pathway. Cancer Med. 2016;5(11):3214–3222. doi:10.1002/cam4.916
22. Sun H, Lv C, Yang L, et al. Solanine induces mitochondria-mediated apoptosis in human pancreatic cancer cells. Biomed Res Int. 2014;2014:805926. doi:10.1155/2014/805926
23. Ba MC, Ba Z, Long H, et al. LncRNA AC093818.1 accelerates gastric cancer metastasis by epigenetically promoting PDK1 expression. Cell Death Dis. 2020;11(1):64. doi:10.1038/s41419-020-2245-2
24. Li Q, Li B, Li Q, et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9(9):854. doi:10.1038/s41419-018-0928-8
25. Mao W, Chen J, Peng TL, Yin XF, Chen LZ, Chen MH. Downregulation of gastrokine-1 in gastric cancer tissues and restoration of its expression induced gastric cancer cells to apoptosis. J Exp Clin Cancer Res. 2012;31:49. doi:10.1186/1756-9966-31-49
26. Li Q, Li Z, Wei S, et al. Overexpression of miR-584-5p inhibits proliferation and induces apoptosis by targeting WW domain-containing E3 ubiquitin protein ligase 1 in gastric cancer. J Exp Clin Cancer Res. 2017;36(1):59. doi:10.1186/s13046-017-0532-2
27. Zhang YQ, Pei JH, Shi SS, et al. CRISPR/Cas9-mediated knockout of the PDEF gene inhibits migration and invasion of human gastric cancer AGS cells. Biomed Pharmacother. 2019;111:76–85. doi:10.1016/j.biopha.2018.12.048
28. Ding X, Huang R, Zhong Y, et al. CTHRC1 promotes gastric cancer metastasis via HIF-1alpha/CXCR4 signaling pathway. Biomed Pharmacother. 2020;123:109742. doi:10.1016/j.biopha.2019.109742
29. Hu Q, Hou YC, Huang J, Fang JY, Xiong H. Itraconazole induces apoptosis and cell cycle arrest via inhibiting Hedgehog signaling in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):50. doi:10.1186/s13046-017-0526-0
30. Rakovic A, Ziegler J, Martensson CU, et al. PINK1-dependent mitophagy is driven by the UPS and can occur independently of LC3 conversion. Cell Death Differ. 2019;26(8):1428–1441. doi:10.1038/s41418-018-0219-z
31. Zhang Q, Wang X, Cao S, et al. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed Pharmacother. 2020;128:110245. doi:10.1016/j.biopha.2020.110245
32. Friedman M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J Agric Food Chem. 2015;63(13):3323–3337. doi:10.1021/acs.jafc.5b00818
33. Al Sinani SS, Eltayeb EA, Coomber BL, Adham SA. Solamargine triggers cellular necrosis selectively in different types of human melanoma cancer cells through extrinsic lysosomal mitochondrial death pathway. Cancer Cell Int. 2016;16:11. doi:10.1186/s12935-016-0287-4
34. Kim SP, Nam SH, Friedman M. The Tomato Glycoalkaloid alpha-Tomatine Induces Caspase-Independent Cell Death in Mouse Colon Cancer CT-26 Cells and Transplanted Tumors in Mice. J Agric Food Chem. 2015;63(4):1142–1150. doi:10.1021/jf5040288
35. Mohsenikia M, Alizadeh AM, Khodayari S, et al. The protective and therapeutic effects of alpha-solanine on mice breast cancer. Eur J Pharmacol. 2013;718(1–3):1–9. doi:10.1016/j.ejphar.2013.09.015
36. Gao J, Ying Y, Wang J, Cui Y. Solanine Inhibits Immune Escape Mediated by Hepatoma Treg Cells via the TGFbeta/Smad Signaling Pathway. Biomed Res Int. 2020;2020:9749631. doi:10.1155/2020/9749631
37. Amaravadi RK, Kimmelman AC, Debnath J. Targeting Autophagy in Cancer: recent Advances and Future Directions. Cancer Discov. 2019;9(9):1167–1181. doi:10.1158/2159-8290.CD-19-0292
38. Xing Y, Wei X, Liu Y, et al. Autophagy inhibition mediated by MCOLN1/TRPML1 suppresses cancer metastasis via regulating a ROS-driven TP53/p53 pathway. Autophagy. 2022;18(8):1932–1954. doi:10.1080/15548627.2021.2008752
39. Yang J, Pi C, Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother. 2018;103:699–707. doi:10.1016/j.biopha.2018.04.072
40. Zhan L, Zhang J, Wei B, Cao Y. Selective autophagy of NLRC5 promotes immune evasion of endometrial cancer. Autophagy. 2022;18(4):942–943. doi:10.1080/15548627.2022.2037119
41. Ahn CH, Jeong EG, Lee JW, et al. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. APMIS. 2007;115(12):1344–1349. doi:10.1111/j.1600-0463.2007.00858.x
42. Wu Q, Ma J, Wei J, Meng W, Wang Y, Shi M. lncRNA SNHG11 Promotes Gastric Cancer Progression by Activating the Wnt/beta-Catenin Pathway and Oncogenic Autophagy. Mol Ther. 2021;29(3):1258–1278. doi:10.1016/j.ymthe.2020.10.011
43. Dong X, Wang Y, Zhou Y, Wen J, Wang S, Shen L. Aquaporin 3 facilitates chemoresistance in gastric cancer cells to cisplatin via autophagy. Cell Death Discov. 2016;2:16087. doi:10.1038/cddiscovery.2016.87
44. Jin HR, Du CH, Wang CZ, Yuan CS, Du W. Ginseng metabolite Protopanaxadiol induces Sestrin2 expression and AMPK activation through GCN2 and PERK. Cell Death Dis. 2019;10(4):311. doi:10.1038/s41419-019-1548-7
45. Ambrosio S, Sacca CD, Amente S, Paladino S, Lania L, Majello B. Lysine-specific demethylase LSD1 regulates autophagy in neuroblastoma through SESN2-dependent pathway. Oncogene. 2017;36(48):6701–6711. doi:10.1038/onc.2017.267
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.