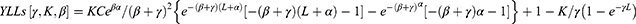Back to Journals » Infection and Drug Resistance » Volume 17
Socioeconomic Burden of Pyogenic Liver Abscess Caused by Klebsiella Pneumoniae from a Teaching Hospital in East China
Authors Wu Z, Li J, Fang P, Pan C, Chen Y
Received 21 November 2023
Accepted for publication 10 April 2024
Published 23 April 2024 Volume 2024:17 Pages 1589—1598
DOI https://doi.org/10.2147/IDR.S447506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhenzhu Wu,1 Jie Li,1 Peipei Fang,1 Chenwei Pan,1 Yi Chen2
1Department of Infectious Disease, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, 325000, People’s Republic of China; 2Department of Gastroenterology, Wenzhou People’s Hospital, Wenzhou, Zhejiang, 325000, People’s Republic of China
Correspondence: Yi Chen; Zhenzhu Wu, Email [email protected]; [email protected]
Background: The prevalence of pyogenic liver abscess (PLA) is increasing worldwide. However, evaluation on its economic burden is still lack.
Methods: A retrospective study that included all patients identified PLA from 2017 to 2020 was conducted. Clinical information and hospital costs were collected through the electronic medical records. We evaluated the economic burden using disability-adjusted life years (DALYs). Differences in socioeconomic burdens between Klebsiella pneumoniae-caused liver abscesses (KPLA) and non-Klebsiella pneumoniae-caused liver abscesses (non-KPLA) were compared.
Results: We found 327 patients identified PLA in the study, including 146 with KPLA and 181 with non-KPLA. The demographic characteristics, median hospital stay, severity, and in-hospital mortality were similar between the two groups. The median total in-hospital cost was higher in the non-KPLA than in the KPLA group, although no statistical difference was found ($3607.2 vs $3424.6; P = 0.446). The median DALY loss was significantly higher in the KPLA than in the non-KPLA group [1.49 (0.97– 2.30) vs 1.27 (0.87– 1.89); P = 0.033)], and male patients presented a higher average DALY loss than female patients. KPLA had a substantially greater median indirect economic loss than the non-KPLA group [$1442.8 (915.9– 17,221.5) vs $1232.5 (764.6– 15,473.0); P = 0.028], and indirect economic loss exhibited a significant increase from 2017 to 2020 in patients with PLA. No differences were found in the socioeconomic burden between the two groups [$8019.6 (4200.3– 21,832.1) vs $7436.4 (4023.2– 19,063.9); P = 0.172].
Conclusion: The economic burden of PLA is significant, particularly in patients with KP. Patients with KPLA experienced increased DALY loss and indirect economic loss than non-KPLA. PLA must be prioritized as the indirect economic burden rises annually.
Keywords: pyogenic liver abscess, Klebsiella pneumoniae, socioeconomic burden, disability-adjusted life years
Introduction
Pyogenic liver abscess (PLA) is an intrahepatic purulent infection caused by various microorganisms. With the rise of diabetic patients, abdominal invasive surgeries for hepatobiliary diseases, longer life expectancies, and widespread use of immunosuppressive drugs in organ transplants and malignancy patients, the incidence of PLA has increased globally with regional variations.1–3 According to previous studies, the incidence of PLA increased from 11 to 15 cases per 100,000 population between 2000 and 2011 in Taiwan.2 The crude annual incidence in China mainland increased from 0.9 to 5.3 cases per 100,000 population between 2000 and 2011.1,3,4 Therefore, the social and economic burden cannot be overlooked.
DALY is a metric widely used for evaluating disease burden. It was first proposed in the Global Burden of Disease (GBD) study5 and has been widely used since then.5–8 It is the summation of the lives lost due to death and the time lived in disabled states due to the disease.9
Previous articles on PLA have focused on clinical and microbiological aspects10–14 and ignored the socioeconomic burden of PLA, specifically KP-related PLA. Liu et al15 explored the economic burden of PLA but only focused on the direct hospitalization costs. No reports have explained the indirect socioeconomic costs of PLA yet. In this study, we retrospectively evaluated the direct hospitalization costs, indirect socioeconomic burden, and disability-adjusted life years (DALYs) in patients with PLA and their dynamic changes in recent years. This may improve our understanding of the socioeconomic status of patients with PLA and enhance infection prevention strategies. Although the problem of KPLA has raised widespread concern, little is known about the influence of KP on economic burden. Therefore, we aimed to clarify the potential clinical and economic cost of KPLA.
Materials and Methods
Study Population and Design
A retrospective cohort study of adult inpatients with PLA at the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (Wenzhou, China) was conducted from January 1, 2017, to December 31, 2020. The inclusion criteria were as follows: (1) clinical manifestations or imaging findings of liver abscess; (2) complete clinical data and hospital costs for analysis; (3) age ≥16 years old. Exclusion criteria included the following: (1) amoebic or tuberculosis liver abscess; (2) cases with incomplete clinical data; (3) age<16 years old. During this period, patients with PLA hospitalized more than once for the same pathogen were considered as one case. Then, the enrolled cases were divided into two groups according to the results of blood or pus culture: a monomicrobial KPLA and a non-KPLA group.
Identification of KP and determination of antimicrobial susceptibility were performed using the broth microdilution method and interpreted following Clinical and Laboratory Standards Institute (CLSI) guidelines.16
Data Collection and Definition
The data of all cases included in the study were reviewed from the electronic medical record, including demographics (age and gender), comorbidities, Charlson comorbidity index, intensive care unit (ICU) admission, hospital length of stay(LOS), severity of illness (Acute Physiology and Chronic Health Evaluation (APACHEII) score), clinical outcome, 30-day readmission, and hospitalization costs.
Hospitalization costs included drugs, laboratory tests, imaging and surgery costs. We converted all the costs into US dollars ($) with annual exchange rate according to the National Bureau of Statistics from 2017 to 2020.17
The direct economic burdens were the sum of all expenditures during hospitalization in our study.
The indirect economic burden of PLA was evaluated by DALYs and human capital; therefore, the indirect economic burden= DALYs × GDP per capita × productivity weight.18,19 The productivity weights were different among different age groups. The weights of the 0–14 years group without social wealth created was 0.15; the weights of the 15–44 and 45–59 years groups with more wealth created were 0.75 and 0.80, respectively; for people aged ≥ 60 years, the weight dropped to 0.1.18,19
DALYs for PLA were the sum of the years of life lost (YLL) for all deaths due to this disease and years lost due to disability (YLD) for people living in unhealthy conditions caused by this disease, and the formula was DALYs = YLLs + YLDs. The calculations for YLLs and YLDs were as follows:
where γ, β, and C are fixed constants. The values in the formula we used were recommended by the World Health Organization (WHO).20 γ is the discount rate (0.03), β is the age weighting coefficient (0.04) and C is the age weight adjustment factor (0.1658).9 For other parameters, D is the disability duration.9 According to the WHO’s Global Burden of Disease (GBD) template,21 the value of disability weight (D) ranges from 0.006, 0.051, and 0.133 based on mild, moderate, and severe infections.18
The socioeconomic burden including direct and indirect economic burden in our study. According to Jo C’s article,22 economic burden of disease is stratified into three categories-direct, indirect, and intangible costs. Since the intangible costs have seldom been quantified in studies due to the measurement difficulties and related controversies, socioeconomic burden mainly focus on the first two cost categories.
Statistical Analysis
We used IBM SPSS Statistics 23.0 software to complete all statistical analyses. Categorical variables were compared using Pearson’s χ2 test or two-tailed Fisher’s exact test. Continuous variables were compared using Student’s t-test or Mann–Whitney U-test as appropriate following the data distribution types. Categorical variables were reported as numbers and percentages. Continuous variables were reported as mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate. We considered P < 0.05 was statistically significant.
Ethical Considerations
This study was approved by the Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (approval number:YJ-2022-K-103-01), and followed the ethical principles of the Declaration of Helsinki 1964. Written informed consent was waived due to the retrospective nature of the study, which was approved by the ethics committee. All patient data were anonymized prior to the analysis.
Results
Patient Characteristics
From January 1, 2017, to December 31, 2020, 327 patients were enrolled in the study. These included 146 cases with KPLA and 181 cases with non-KPLA.
No differences were found in the sex, age, median LOS, antibiotic susceptibility, abscess site, APACHE II score, or in-hospital mortality rate between the two groups (Table 1). Patients with KPLA were more likely to be admitted to the ICU (9.6% vs 3.9%, P = 0.036) but less likely to receive 30-day readmission for recurrence (4.1% vs 13.3%, P = 0.004) than those in the non-KPLA group. As for comorbidities, diabetes mellitus was more common in the KPLA group (58.9% vs 33.1%, P < 0.001), while biliary tract diseases were more common in the non-KPLA group (43.6% vs 26.7%, P = 0.002). However, the Charlson Comorbidity Index between the two groups had no significant difference (P = 0.591). With regard to complications, sepsis was more common in the KPLA group (41.1% vs 26.0%, P= 0.004), whereas no differences were found in metastatic complications and septic shock (P>0.05). The clinical characteristics of the two groups are summarized in Table 1.
 |
Table 1 Features and Socioeconomic Burden of the Patients with PLA Caused by KP or Non-KP |
Direct Economic Burden
The median of total in-hospital cost in non-KPLA patients was higher than that in KPLA patients, although the difference was not statistically significant ($3607.2 vs $3424.6; P = 0.446). Medical costs constituted the largest part of the in-hospital cost accounting for 50.3% of in-hospital costs in the non-KPLA group and 50.0% in the KPLA group. KPLA patients had higher median laboratory test and operation costs than non-KPLA patients [($379.5 (279.0–554.5) vs $303.4 (224.2–416.9); P < 0.001, $81.7 (71.9–93.8) vs $72.2 (0–125.5); P < 0.001) (Table 1).
Disability-Adjusted Life Year (DALY) Loss
The median DALY loss in the KPLA group was significantly higher than that in the non-KPLA group [1.49 (0.97–2.30) vs 1.27 (0.87–1.89); P = 0.033)] (Table 1). The average DALY loss for patients with PLA was 1.7–1.9 years, decreasing first and then increasing from 2017 to 2020 (Table 2). The average DALY loss in males was significantly higher than that in females every year, while it presented a more obvious increase in females (Table 2 and Figure 1). The average DALY loss was the highest in patients aged 30–39 years and the lowest in those aged ≥80 years (Table 3).
 |
Table 2 Comparison of disability-adjusted life year (DALY) loss from 2017 to 2020 |
 |
Table 3 Socioeconomic Burden to Patients Caused by PLA in Different Age Groups |
 |
Figure 1 Average DALYs from 2017 to 2020 of patients with PLA by sex. |
Indirect Economic Burden and Socioeconomic Burden
The median indirect economic burden was significantly higher in the KPLA group than the non-KPLA group [$1442.8 (915.9–17,221.5) vs $1232.5 (764.6–15,473.0); P = 0.028, but no differences were found in the socioeconomic burden between the two groups [$8019.6 (4200.3–21,832.1) vs $7436.4 (4023.2–19,063.9); P = 0.172 (Table 1). The indirect economic burden increased significantly from 2017 to 2020 in patients with PLA, while the socioeconomic burden first decreased and then increased during this period (Table 4). Every year, males had a higher socioeconomic load than females, but this increased more in females (Table 4 and Figure 2). The average indirect economic burden was the highest in patients aged 30–39 years and lowest in those aged ≥80 years. However, the socioeconomic burden was highest in patients aged 30–39 years and lowest in those aged 70–79 years (Table 3).
 |
Table 4 Yearly Socioeconomic Burden per Patient in Patients with PLA from 2017 to 2020 |
 |
Figure 2 Average socioeconomic burden from 2017 to 2020 of patients with PLA by sex. |
Discussion
Researches on socioeconomic impact of PLA are lack at present. To the best of our knowledge, this is the first study assessing the indirect and direct economic losses of PLA. Furthermore, the current study introduced DALY to assess the economic burden more comprehensively, making it possible to compare it with other diseases. No significant differences were found in the baseline information of patients in our study. Thus, we can make a comparison directly in economic burden between the two groups without considering confounding factors.
We observed that the cost of laboratory tests and operations was higher in the KPLA. This was mainly due to the higher rate of ICU admission and percutaneous drainage in the KPLA group. But no significant increase was observed in total direct costs. This may owing to the rapid and effective treatment for all patients, and the length of hospital stay were similar between the two groups. We observed that the largest part of the direct cost was the medicine cost, and antimicrobial costs accounting for nearly half of all medical costs in patients with PLA. We considered that the etiology of liver abscess may have a significant impact on subsequent treatment as well as the hospitalization cost, especially for biliary diseases, biliary tumors and diabetes mellitus. But, the number of patients with biliary tumors and biliary diseases needed treatment in our study were few which made the analysis impossible. A large samples study is needed in the future.
In this study, we used DALY to analyze indirect economic burden. DALY includes the reduction in working time and social productivity due to a patient’s condition like illness, disability, or death.9 It is commonly used in many diseases to compare different disease burdens.5,21 We found that the median DALYs were higher in KPLA than non-KPLA (1.49 vs 1.27 DALYs). Moreover, the average DALYs ranged from 1.7 to 1.9 years, with a stable trend from 2017 to 2020. It showed a significantly lower burden than other infectious diseases reported.23 According to previous studies23 that focused on analyzing DALYs in infectious diseases, the medians per case loss of influenza, hepatitis B, tuberculosis, and invasive pneumococcal disease were 0.01, 2.79, 3.58, and 2.74 DALYs, respectively.23 The DALY values of PLA are low in our analysis, but we think they are reasonable. DALY is the sum of the years of life lost for all deaths due to this disease and years lost due to disability for people living in unhealthy conditions caused by this disease. Death and longer lost years are heavily weighed. PLA has a shorter disease course and lower fatality rate than other chronic diseases; hence, small values are reasonable.
We found that indirect economic burden increased annually from 2017 to 2020 in our study. We inferred that the indirect economic burden caused by PLA will increase continuously in the future as the incidence of PLA rises. Besides, patients with PLA aged 30–39 years showed the highest indirect economic loss, which may due to the high productivity weight of younger people.18,19 The productivity weight and DALYs of young cases with mortality were exceptionally high; therefore, fewer such individuals may cause a considerable amount of indirect economic loss.
In our study, we found that direct economic loss decreased annually from 2017 to 2020. And, the direct economic loss increased with age. Patients with PLA aged ≥80 years showed the highest indirect economic loss. This may be linked to the longer length of stay for older patients according to previous study.24
What’s more, we also found that the socioeconomic burden actually decreased with age in our study. This is because socioeconomic burden includes direct and indirect economic burden. And its results depend on the one with the larger share. In our study, in patient ≤ 60 year old, indirect economic burden accounts for a larger proportion and the socioeconomic burden share the same trend that decreased with age. While, in patient> 60 year old, though direct economic burden accounts for a larger proportion but there were no significant difference among different age groups. Therefore, the socioeconomic burden showed a decreasing trend with age. Moreover, we found that the socioeconomic burden showed a significant gender difference. It was higher in males than females every year, possibly due to the higher incidence and mortality of PLA in males.
This study indicated that the rate of diabetes mellitus was higher in the KPLA group than that in the non-KPLA group. According to previous reports,4,25–27 diabetes mellitus was more common in the KPLA, accounting for 56.8%–75.0%. It has been reported that KPLA patients with diabetes mellitus had higher metastatic complications.27 While, we found no difference in metastatic complications between the two groups in our study. This may be because it was not the diabetes mellitus itself but the level of glycemia plays a key role in metastatic complications according the Lin et al’s research.28 However, the data of glycemia was unavailable in our study. Therefore, further research on this aspect is required in the future.
We found no significant differences in imaging features like abscess site and gas-forming, except for a larger abscess size in KPLA cases. This was different from other research,27 which showed that KPLA was associated with a lower incidence of multiple abscesses and bilobar abscesses, as well as higher metastatic complications. We found that more patients with KPLA underwent percutaneous drainage compared to non-KPLA in our research. We considered that the larger size of abscess and more serious complications in the KPLA group were the main reasons.
In this study, we observed that patients with KPLA were more likely to be admitted to ICU, consistent with previous reports.29 This may be due to the higher rate of sepsis in patients with KPLA in our study. According to previous research, KPLA was frequently associated with serious complications like metastatic infections and sepsis,4,28 specifically in hypervirulent Klebsiella pneumoniae (hvKP) infections.25,26,30 But patients with KPLA did not cost more in the direct economic burden and social economic burden than patients with non-KPLA. This phenomenon may be attributed to effective and timely treatment. But further investigation on the economic burden of hvKp-PLA is required for a more comprehensive understanding.
Our study exists several limitations. First, bias are difficult to avoid completely due to its retrospective nature. Our study identified no baseline differences; however, the disease prevalence and treatment choices may differ from those of other medical institutions. Second, due to the difficulties in data collection, we ignored the direct non-medical costs such as transportation costs, food costs and other costs, as well as costs in outpatient or other medical institutions, and all these may cause the results not fully representing the direct economic burden of disease. Third, patients with hvKP were not investigated because the information was unavailable, which may underestimate the economic impact of PLA caused by these bacteria.
Conclusions
In conclusion, the loss of DALYs caused by KPLA is greater than that in non-KPLA. Patients with KPLA are more likely to experience increased indirect economic loss. PLA requires more attention as the indirect economic burden increases annually.
Abbreviations
KP, Klebsiella pneumoniae; PLA, Pyogenic liver abscess; KPLA, Klebsiella pneumoniae-caused liver abscesses; ICU, Intensive care unit; DALY, Disability-adjusted life years; E. coli, Escherichia coli; GBD, Global Burden of Disease; CLSI, Clinical and Laboratory Standards Institute; APACHE, Acute Physiology and Chronic Health Evaluation; GDP, Gross domestic product; YLL, Years of life lost; YLD, Years lost due to disability; WHO, World Health Organization; IQR, Interquartile range; SD, Standard deviation; LOS, Length of stay; hvKP, hypervirulent Klebsiella pneumoniae; ESBL, Extended-spectrum beta-lactamase producing.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. All patient data accessed complied with the relevant data protection and privacy regulations.
Acknowledgments
We would like to thank Tingting Xiao (First Affiliated Hospital, School of Medicine, Zhejiang University) for calculating the indirect economic loss. We also thank Home for Researchers editorial team for language editing service.
Author Contributions
All authors contributed significantly to the work reported, including conception, study design, execution, data acquisition, analysis, and interpretation, or all these areas; they have also written, revised, or critically reviewed the manuscript. They reviewed and consented to all versions of the manuscript before submission, gave final approval for the published version, agreed on the journal submission, and accepted responsibility for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Yin D, Ji C, Zhang S, et al. Clinical characteristics and management of 1572 patients with pyogenic liver abscess: a 12-year retrospective study. Liver Int. 2021;41(4):810–818. doi:10.1111/liv.14760
2. Chen Y, Lin C, Chang S, et al. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000–2011. J Microbiol Immunol Infect. 2016;49(5):646–653. doi:10.1016/j.jmii.2014.08.028
3. Law S, Li K. Older age as a poor prognostic sign in patients with pyogenic liver abscess. Int J Infect Dis. 2013;17(3):e177–84. doi:10.1016/j.ijid.2012.09.016
4. Qian Y, Wong C, Lai S, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep. 2016;6:38587. doi:10.1038/srep38587
5. Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi:10.1001/jamaoncol.2019.2996
6. Cassini A, Plachouras D. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. doi:10.1371/journal.pmed.1002150
7. Zhu Y, Xiao T, Wang Y, et al. Socioeconomic burden of bloodstream infections caused by carbapenem-resistant enterobacteriaceae. Infect Drug Resist. 2021;14:5385–5393. doi:10.2147/IDR.S341664
8. Yang K, Xiao T, Shi Q, et al. Socioeconomic burden of bloodstream infections caused by carbapenem-resistant and carbapenem-susceptible Pseudomonas aeruginosa in China. Antimicrob Resist Infect Control. J Glob Antimicrob Resist. 2021;26:101–107. doi:10.1016/j.jgar.2021.03.032
9. Kim YE, Jung YS, Ock M, et al. DALY estimation approaches: understanding and using the incidence-based approach and the prevalence-based approach. J Prev Med Public Health. 2022;55(1):10–18. doi:10.3961/jpmph.21.597
10. Xiao T, Zhu Y, Zhang S, et al. A retrospective analysis of risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteremia in nontransplant patients. J Infect Dis. 2020;221(Suppl 2):S174–83. doi:10.1093/infdis/jiz559
11. Cho H, Lee ES, Lee YS, et al. Predictors of septic shock in initially stable patients with pyogenic liver abscess. Scand J Gastroenterol. 2017;52(5):589–594. doi:10.1080/00365521.2017.1288757
12. Chen W, Chen CH, Chiu KL, et al. Clinical outcome and prognostic factors of patients with pyogenic liver abscess requiring intensive care. Crit Care Med. 2008;36(4):1184–1188. doi:10.1097/CCM.0b013e31816a0a06
13. Peris J, Bellot P, Roig P, et al. Clinical and epidemiological characteristics of pyogenic liver abscess in people 65 years or older versus people under 65: a retrospective study. BMC Geriatr. 2017;17(1):161. doi:10.1186/s12877-017-0545-x
14. Chen S, Lee Y, Yen C, et al. Pyogenic liver abscess in the elderly: clinical features, outcomes and prognostic factors. Age Ageing. 2009;38(3):271–6; discussion. doi:10.1093/ageing/afp002
15. Liu JQ, Liu Y, Fu L, et al. Economic burden of 495 patients with liver abscess and influencing factors. Chin J Nosocomiol. 2021;31(01):91–94. doi:10.11816/cn.ni.2021-201756
16. Humphries R, Bobenchik AM, Hindler JA, et al. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st Edition. J Clin Microbiol. 2021;59(12):e0021321. doi:10.1128/JCM.00213-21
17. National data. National Bureau of Statistics. Available from: https://data.stats.gov.cn/easyquer-y.htm?cn=C01.
18. Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–723.
19. Barnum H. Evaluating healthy days of life gained from health projects. Soc sci med. 1987;24(10):833–841.
20. Murray CJ, Salomon JA, Mathers C. A critical examination of summary measures of population health. Bull World Health Organ. 2000;78(8):981–994.
21. Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi:10.1016/S0140-6736(16)31678-6
22. Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20(4):327–337. doi:10.3350/cmh.2014.20.4.327
23. Cassini A, Colzani E, Pini A, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the burden of communicable diseases in Europe study, European Union and European economic area countries, 2009 to 2013. Euro Surveill. 2018;23(16):17–00454. doi:10.2807/1560-7917.ES.2018.23.16.17-00454
24. Chan KS, Junnarkar SP, Low JK, et al. Aging is associated with prolonged hospitalisation stay in pyogenic liver abscess-a 1:1 propensity score matched study in elderly versus non-elderly patients. Malays. J Med Sci. 2022;29(5):59–73. doi:10.21315/mjms2022.29.5.7
25. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi:10.4161/viru.22718
26. Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi:10.1016/S1473-3099(12)70205-0
27. Chan KS, Chia CTW, Shelat VG. Demographics, radiological findings, and clinical outcomes of Klebsiella pneumonia vs. non-klebsiella pneumoniae pyogenic liver abscess: a systematic review and meta-analysis with trial sequential analysis. Pathogens. 2022;11(9):976. doi:10.3390/pathogens11090976
28. Lin Y, Wang F, Wu P, et al. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis. 2013;13:56. doi:10.1186/1471-2334-13-56
29. Lee J, Jang Y, Ahn S, et al. A retrospective study of pyogenic liver abscess caused primarily by Klebsiella pneumoniae vs. non-Klebsiella pneumoniae: CT and clinical differentiation. Abdom Radiol. 2020;45(9):2669–2679. doi:10.1007/s00261-019-02389-2
30. Zhang S, Zhang X, Wu Q, et al. Clinical, microbiological, and molecular epidemiological characteristics of Klebsiella pneumoniae-induced pyogenic liver abscess in southeastern China. Antimicrob Resist Infect Control. 2019;8:166.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.