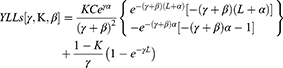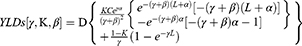Back to Journals » Infection and Drug Resistance » Volume 14
Socioeconomic Burden of Bloodstream Infections Caused by Carbapenem-Resistant Enterobacteriaceae
Authors Zhu Y, Xiao T, Wang Y, Yang K, Zhou Y, Luo Q , Shen P, Xiao Y
Received 28 September 2021
Accepted for publication 25 November 2021
Published 14 December 2021 Volume 2021:14 Pages 5385—5393
DOI https://doi.org/10.2147/IDR.S341664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yunying Zhu,* Tingting Xiao,* Yuan Wang, Kai Yang, Yanzi Zhou, Qixia Luo, Ping Shen, Yonghong Xiao
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, 310003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonghong Xiao
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, 310003, People’s Republic of China
Tel/Fax +86 571 87236421
Email [email protected]
Background: Although infection with carbapenem-resistant Enterobacteriaceae (CRE) has become an urgent public health threat worldwide, the socioeconomic burden of CRE bloodstream infection (BSI) remains to be clarified.
Methods: This retrospective study included all patients infected with Escherichia coli or Klebsiella pneumoniae who were hospitalized for BSI from 2013 to 2015. Socioeconomic burden, including direct and indirect economic burden, was compared in patients infected with carbapenem-sensitive Enterobacteriaceae (CSE) and CRE following 1:1 propensity score matching (PSM) to control for confounding variables.
Results: Data from 879 patients with Enterobacteriaceae BSI were evaluated, including 152 (17.3%) patients infected with CRE and 727 (82.7%) infected with CSE. PSM yielded 112 pairs of 224 patients. Median hospital length of stay did not differ significantly in the CRE and CSE groups (35 vs 29 days, P = 0.089), but in-hospital 28-day mortality rate was significantly higher in patients infected with CRE than with CSE (45.5% vs 32.1%, P = 0.040). Median direct economic burden was significantly greater in patients with CRE-BSI than with CSE-BSI during hospitalization ($24,940.1 vs 16,864.0, P = 0.017) but not during the period after infection ($10,403.4 vs 8498.0, P = 0.178). Drug expenditure accounted for the largest proportion of costs in both groups. The median disability-adjusted life year (DALY) was higher in CRE-BSI than in CSE-BSI patients, but the difference was not statistically significant (7.9 vs 6.7 years, P = 0.190). Median indirect economic burden did not differ significantly in these two groups ($3848.5 vs 1139.9, P = 0.304), although indirect economic burden increased significantly from 2013 to 2015 in patients with CRE-BSI.
Conclusion: Carbapenem resistance had a major impact on the clinical and socioeconomic burden of patients with Enterobacteriaceae BSI. The higher mortality rate in patients with CRE-BSI was associated with increased direct healthcare burden and indirect socioeconomic loss.
Keywords: carbapenem resistant, Enterobacteriaceae, Escherichia coli, Klebsiella pneumonia, socioeconomic burden, disability-adjusted life years
Introduction
Infections caused by antibiotic-resistant bacteria are a huge threat to modern healthcare systems and have triggered the development of comprehensive international plans to deal with them.1 In particular, bacterial resistance to carbapenems has a deleterious effect on patient safety. Carbapenems are atypical β-lactam antibiotics characterized by potent, broad-spectrum antibacterial activity and are often used to treat infections with multidrug-resistant (MDR) Enterobacteriaceae, including Escherichia coli and Klebsiella pneumoniae.2–4 However, the incidence of carbapenem-resistant Enterobacteriaceae (CRE) has increased worldwide with great regional variability. Previous study has shown different incidence of CRE BSI in three main adult acute-care hospitals of the metropolitan area of Italy, indicating the significance of management in different regions.5 The emergence and dissemination of CRE seriously threatened world public health security.6 Carbapenem resistance has been associated with increased length of hospital stay (LOS) and mortality in patients with CRE bloodstream infections (BSI).7–9 So that particular attention should be given to the patients with CRE BSI, especially in China.10 The mortality rates in patients infected with CRE were found to range from 26% to 44%, about 2-fold higher than in patients infected with carbapenem sensitive Enterobacteriaceae (CSE).7,8 Take Klebsiella pneumoniae for example, previous study revealed that the patients with bacteremia due to carbapenem-resistant Klebsiella pneumoniae (CRKP) had a 3-fold higher risk of death.11 Carbapenem resistance has also been associated with increased economic burdens on patients and healthcare systems so that control measures of CRE were economically worthwhile.12 For example, the cost of CRE infections was found to be higher than the annual costs of many chronic and acute diseases.13 The median direct costs of a single CRE infection can range from $22,484 to $66,031 for hospitals, $10,440 to $31,621 for third-party payers, and $37,778 to $83,512 for society. Studies analyzing the socioeconomic burden of disease tend to focus only on direct costs while overlooking indirect economic costs.13
Disability-adjusted life year (DALY) is a widely used metric for estimating disease loss.14–17 In a previous study, DALY was used to assess the harm caused by antimicrobial resistant in the European Union and European Economic Area (EU/EEA), the results showed the cumulative burden was estimated at 501 disability-adjusted life years (DALYs) per 100,000 general population each year.18 To provide information enabling strategic decisions on infection control measures, antibiotic stewardship, and resistance containment, this study analyzed the direct medical costs and indirect socioeconomic costs by measuring DALY in patients with CRE-BSI.
Methods
Patients
Our study was conducted at a tertiary teaching hospital which is a 2500-bed teaching hospital in Eastern China. Clinical records, microbiological laboratory results and economic costs of patients with Enterobacteriaceae bacteremia (including E. coli and K. pneumoniae BSI) were reviewed between January 2013 to December 2015. All patients aged >16 years hospitalised with Enterobacteriaceae bacteremia were included at the time of the first episode of infection. If the same patient had more than two episodes of Enterobacteriaceae bacteremia within 6 months, this study only included the first bloodstream infection data. Only patients with complete clinical microbiological and cost data for analysis were included in this study.
Enterobacteriaceae were identified and their antimicrobial susceptibility was determined using the Vitek 2 system (bioMérieux, Marcy-l’ Etoile, France). Patients with CRE were identified in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines.19 The study protocol was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University, which waived the requirement for informed consent due to the retrospective nature of this study.
The electronic medical records of all patients were reviewed. Data on expenditures were collected from the Hospital Information System. Medical costs were converted into US dollars ($) according to the average exchange rate (1$= 6.22 Renminbi) issued by the Bank of China from 2013 to 2015.20
Definition
CRE infection was defined as the first blood culture with a carbapenem non-susceptible organism (minimum inhibitory concentration (MIC) of ≥4 μg/mL for meropenem or imipenem). Disease severity was assessed by measuring the Acute Physiology and Chronic Health Evaluation (APACHE) II scores and the Pitt bacteremia scores21,22 and comorbid conditions were determined by the Charlson comorbidity index (CCI).23 DALY, an indicator of overall burden of disease, is a sum of ’years of life lost’ (YLLs), an indicator of premature death, and ’years lived with disability’ (YLDs), an indicator of loss of healthy living due to living under conditions worse than perfect health.24
Propensity Score Matching
To control for confounding variables, the two groups of patients infected with CRE and CSE were subjected to 1:1 propensity score matching (PSM). Variables used in PSM included patients’ demographic characteristics (age and sex), transplantation during hospital stay, admission to the intensive care unit (ICU), disease severity (APACHE II and Pitt bacteremia scores), and comorbid conditions (CCI), but not clinical outcomes (LOS and mortality rates) or economic burden.
Medical Costs
The direct economic burden is the direct economic costs of disease prevention and treatment, whereas the indirect economic burden refers to the current and future loss of value to the patient’s family and society caused by the reduced working time or capacity due to the disease itself or to disability or death. Direct medical costs included the costs of hospitalization (room and board), nursing care, drugs, laboratory and imaging tests, blood products, consultation with doctors, surgery, and other costs. In order to eliminate the impact of pre-infection costs, we collected overall direct medical costs and direct medical costs before/after the infection. To analyze DALYs, YLL and YLD were calculated using the equations.25
where K is the age weighting modulation factor; C is a constant; γ is the discount rate; a is age at onset of disability; β is a parameter of the age weighting function; L is the duration of disability; and D is the disability weight.
These formulas included the values recommended by the World Health Organization (WHO).26 The international standard discount rate was set at 0.03; K-values were set at 0 when no age weights were used and 1 when age weights are used; and the standard age weights used a β of 0.04 and a constant of 0.1658.25 According to the Global Burden of Disease (GBD) template provided by the WHO,27–29 D ranged from 0 to 1. D was based on the severity of acute infection, with mild, moderate, and severe infections having D values of 0.006, 0.051, and 0.133, respectively.25 Productivity weights for individuals aged 15–44, 45–59, and >60 years were 0.75, 0.80, and 0.1,30 respectively. Per capita gross domestic product (GDP) in 2013, 2014, and 2015 in China were $7023.15, $7584.08, and $8076.69, respectively.31
Statistical Analysis
Categorical variables were compared by the χ2 test or two-tailed Fisher’s exact test, as appropriate. Normally distributed continuous variables were expressed as mean ± standard deviation (SD) and compared using Student’s t tests, whereas non-normally distributed continuous variables were expressed as median and interquartile range (IQR) and compared using Mann–Whitney U-tests. All statistical analyses were performed using SPSS version 23.0 software (IBM Corporation, Armonk, NY, USA), with P-values ≤0.05 considered statistically significant.
Results
Patient Characteristics and Outcomes
Between January 2013 and December 2015, 879 patients hospitalized with Enterobacteriaceae BSIs qualified for this study; of these, 727 (82.7%) had CSE-BSI and 152 (17.3%) had CRE-BSI. Median LOS was significantly longer (35 vs 20 days, P < 0.001) and mortality rate significantly higher (57.2% vs 18.3%, P < 0.001) in the CRE than in the CSE group. However, median LOS after BSI in two groups had no significant difference (13 vs 13 days, P = 0.979). Patients with CRE-BSI were also more likely to undergo organ transplantation, be admitted to the ICU while hospitalized, and have more severe disease than patients with CSE-BSI (Table 1). PSM yielded 112 pairs of patients with largely balanced potential confounding factors (Table 2).
 |
Table 1 Demographic and Clinical Characteristics of the Patients with Bacteremia Caused by CRE or CSE |
 |
Table 2 Features of the Patients with Bacteremia Caused by CRE or CSE After PSM for Potential Confounding Variables |
Following PSM, median LOS was longer (35 vs 29 days, P = 0.089) and overall mortality rate was higher (50.1% vs 38.4%, P = 0.060) in patients with CRE-BSI than with CSE-BSI, but the differences were not statistically significant. While the median LOS after BSI in two groups had also no significant difference (12.5 vs 16.5 days, P = 0.493). However, mortality rates 7 (27.7% vs 16.1%, P = 0.036), 14 (47.5% vs 22.3%, P = 0.013), and 28 (45.5% vs 32.1%, P = 0.040) days after infection were significantly higher in the CRE than in the CSE group (Table 2).
Direct Economic Burden
Median in-hospital total direct healthcare burden was significantly higher in patients with CRE-BSI than with CSE-BSI ($24,940.1 vs 16,864.0, P = 0.017). After eliminating costs before infection, however, the direct economic burden after BSI onset was higher in the CRE-BSI than in the CSE-BSI group, but the difference was not statistically significant ($10,403.4 vs 8498.0, P = 0.178). Expenditures for medicine accounted for the largest proportion of costs in both groups. The median cost of antibiotics during the entire hospital stay was significantly higher in the CRE-BSI than in the CSE-BSI group ($5904.9 vs 3693.0, P = 0.001) but did not differ during the post-infection hospital stay ($2647.5 vs 1836.3, P = 0.190) (Table 3).
 |
Table 3 Socioeconomic Burden of the Patients with BSI Caused by CRE or CSE After PSM for Potential Confounding Variables |
Indirect Economic Loss
Median DALY was higher in patients with CRE-BSI than with CSE-BSI, but the difference was not significant (7.9 vs 6.7 years, P = 0.190). In addition, the median indirect economic loss was higher in patients infected with CRE than with CSE ($3848.5 vs 1139.9, P = 0.340) (Table 3). DALY and indirect economic loss were highest in patients aged 16–29 years and lowest in patients aged >74 years (Table 4). Moreover, indirect economic loss increased significantly from 2013 to 2015 in patients infected with CRE-BSI and Enterobacteriaceae (Table 5).
 |
Table 4 Indirect Economic Loss to Patients Caused by Enterobacteriaceae Bacteremia in Different Age Groups |
 |
Table 5 Yearly Socioeconomic Burden per Patient in Patients with Bacteremia from 2013 to 2015 |
Discussion
Infections caused by drug resistant gram-negative bacteria, particularly CRE, are becoming increasingly prevalent and constitute a serious threat to public health worldwide because they are difficult to treat and are associated with higher morbidity and mortality rates.32 The incidence of CRE infection in China was reported to be 4.0 per 10,000 discharges,33 and the mortality rates from CRE bacteremia have ranged from approximately 19% to 70%.34,35 Infection with carbapenem-resistant gram-negative bacteria may also increase medical costs.13 To better understand the burden of CRE infection, our study explored the direct economic costs and indirect economic loss of CRE-BSI infection and its dynamic changes in recent years.
The most important finding of this study was that carbapenem resistance had major effects on patient outcomes and socioeconomic burden of Enterobacteriaceae BSI. Compared with patients in the CSE-BSI group, those in the CRE-BSI group were more likely to have nosocomial infections, undergo organ transplantation, be admitted to the ICU, and have more severe disease (APACHEII and Pitt scores) and more comorbidities (Charlson score). To eliminate potential confounding factors for outcomes, the two groups of patients were subjected to PSM, minimizing possible sources of bias, such as demographic characteristics and disease severity. After eliminating confounding factors, the poorest clinical outcomes were also observed in this study. Patients with CRE-BSI had higher mortality than patients with CSE-BSI at 7, 14 or 28 days after infection (P < 0.05), which was similar to previous study13 and underscored the magnitude of the damage carbapenems did. This study found that the median per capita total direct medical costs ($24,940.1 vs 16,864.0, P = 0.017) and indirect economic loss ($3848.5 vs 1139.9, P = 0.304) were higher in patients with CRE-BSI than with CSE-BSI, confirming that carbapenem resistance increased the economic burden of disease. This finding was consistent with studies showing that carbapenem resistance was associated with higher medical costs in patients infected with K. pneumoniae36 and E. coli.37 In addition, the median economic burden for hospitals of a single patient infected with CRE was about $59,366.2, far more than the average person in China can afford.
The differences in direct economic burden between the two groups were caused primarily by the higher costs of antibiotics in the CRE group. Similarly, the cost of antibiotics, especially broad-spectrum antibiotics, has been reported to contribute to the high direct medical expenses in patients with CRE.38 Another study, however, reported that the cost of antibacterial drugs accounted for a small part of the total cost of hospitalization.39 However, we found that the cost of medicine was the largest single direct medical cost, with antibiotics accounting for 23.7% ($5904.9/$24,940.1) of all direct medical costs in CRE infected patients. Thus, although other factors were more responsible for direct medical costs in these patients, the contribution of antibiotics to all costs should not be underestimated. Implementation of antibiotic stewardship practices may therefore reduce the economic burden on individuals, hospitals, and society. Besides, we indeed found that CRE infections were associated with higher medicine cost, but increased cost mainly occurred before the infection. This may be related to delayed correct diagnosis or premature antibiotic intervention or some underlying condition such as other kinds of infection. On the other hand, unlike the United States or other developed countries, the cost of room and board in China was very cheap. So that the cost of room and board played a trivial role in all total direct medical expenses, which was inseparable from Chinese national conditions.
The indirect loss analyzed in this study consisted primarily of reduced working time and socially creative productivity resulting from a patient’s illness, disability, or death. Although these costs are frequently determined using a capital- or output-accounting approach, this method has certain limitations.40 The indirect economic loss due to disease in this study was analyzed by determining DALY, a new disease burden index. The present study found that DALY and indirect economic loss were higher in patients infected with CRE than with CSE, but the differences were not statistically significant. Analysis of indirect economic loss in different age groups showed that this loss was highest in patients aged 16–29 years, which may be related to different productivity weights. However, the results remained uncertain due to smaller sample sizes in some age groups (eg, 16–29 years group) and there were little data based on the distribution of indirect burdens by age groups worldwide. And it was important to note that these burdens were mainly paid by individuals but did not reduced by the healthcare provision. Therefore, healthcare providers may be able to reduce the economic burden of society to the greatest extent by changing the proportion of medical reimbursements for different age groups. Notably, indirect economic loss in this group increased yearly. Combined with the increasing incidence of CRE, these findings suggest that the indirect economic loss caused by CRE infection may continue to increase over time.
Similar to previous findings,14 the present study found that mortality rates were significantly higher in patients with CRE-BSI than with CSE-BSI (P < 0.05), emphasizing the clinical effects of carbapenem resistance. Although hospital LOS was greater in the CRE-BSI than in the CSE-BSI group, the difference was not statistically significant. PSM that included LOS as a potential confounding variable resulted in a similar median LOS in the two groups, indicating that the effect of carbapenem-resistance on hospital LOS was due to longer hospital LOS before infection.36 These findings suggest that hospital LOS was unrelated to the increase in medical costs.
This study had several limitations. First, this study was a retrospective analysis, which has certain selection and recall biases. Although PSM was performed to control for potential confounding factors, some unmeasured confounders remained present. Second, the socioeconomic burden of disease also includes indirect medical expenses and intangible economic burdens such as psychosocial costs. Because these costs are difficult to estimate, they were omitted from the cost estimates in this study. Finally, this single-center study only included patients infected with K. pneumoniae and E. coli. Thus, our findings may not represent the economic burden of infection with other types of Enterobacteriaceae.
In conclusion, infection with carbapenem-resistant bacteria resulted in higher direct and indirect economic costs. Although CRE-BSI did not significantly affect hospital LOS, mortality rates were higher in patients with CRE-BSI than with CSE-BSI. Medicines accounted for the largest proportion of direct medical expenses in both groups, suggesting the need for stricter regulation of antibiotic use. But the fundamental thing was that we could spend more effort to prevent the occurrence of nosocomial infection, which may reduce the cost of infection effectively.
Ethics
This study protected relevant data of all patients and received approval from the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Reference number 2019693) in accordance with the Declaration of Helsinki. All data in relation to transplantations performed in the People’s Republic of China were obtained after the modification of the transplant law banning the use of organs from executed prisoners (year 2015) which was conducted in accordance with the Declaration of Istanbul. All organs were donated voluntarily with written informed consent. The study was registered at http://www.ClinicalTrials.gov (ID: ChiCTR1900025064).
Acknowledgments
We would like to thank Professor Hengjin Dong, Zhejiang University, for directing economic data analysis. We also thank Jinru Ji and Chaoqun Ying of the First Affiliated Hospital, School of Medicine, Zhejiang University, for helping to identify bacterial species; as well as Ni Cao of the First Affiliated Hospital, School of Medicine, Zhejiang University, for help with data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. These authors contributed equally to this work and should be considered co-first authors: Yunying Zhu and Tingting Xiao.
Funding
This work was partially supported by grants from The National Key Research and Development Program of China (2017YFC1200203), the National Natural Science Foundation of China (81971984) and Investigator Initiated Studies (IIS) Clinical Status Update Report IISP #(58449).
Disclosure
None of the authors reports any conflicts of interest for this work.
References
1. World Health Organization. Global action plan on antimicrobial resistance. Available from: http://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1;2015.
2. Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–1798. doi:10.3201/eid1710.110655
3. Elshamy AA, Aboshanab KM. A review on bacterial resistance to carbapenems: epidemiology, detection and treatment options. Future Sci OA. 2020;6:FSO438. doi:10.2144/fsoa-2019-0098
4. Chiu SK, Chan MC, Huang LY, Lin YT, Yeh KM. Tigecycline resistance among carbapenem-resistant Klebsiella Pneumoniae: clinical characteristics and expression levels of efflux pump genes. PLoS One. 2017;12:e0175140. doi:10.1371/journal.pone.0175140
5. Cristina ML, Alicino C, Sartini M, et al. Epidemiology, management, and outcome of carbapenem-resistant Klebsiella pneumoniae bloodstream infections in hospitals within the same endemic metropolitan area. J Infect Public Health. 2018;11(2):171–177. doi:10.1016/j.jiph.2017.06.003
6. Spagnolo AM, Orlando P, Panatto D, Perdelli F, Cristina ML. An overview of carbapenem-resistant Klebsiella pneumoniae: epidemiology and control measures. Rev Med Microbiol. 2014;25:7–14. doi:10.1097/MRM.0b013e328365c51e
7. Stewardson AJ, Marimuthu K, Sengupta S, et al. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): a multinational prospective cohort study. Lancet Infect Dis. 2019;19:601–610. doi:10.1016/S1473-3099(18)30792-8
8. Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas AKZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20:1170–1175. doi:10.3201/eid2007.121004
9. Ara-Montojo MF, Escosa-García L, Alguacil-Guillén M, et al. Predictors of mortality and clinical characteristics among carbapenem-resistant or carbapenemase-producing Enterobacteriaceae bloodstream infections in Spanish children. J Antimicrob Chemother. 2021;76(1):220–225. doi:10.1093/jac/dkaa397
10. Li C, Li Y, Zhao Z, Liu Q, Li B. Treatment options and clinical outcomes for carbapenem-resistant Enterobacteriaceae bloodstream infection in a Chinese university hospital. J Infect Public Health. 2019;12(1):26–31. doi:10.1016/j.jiph.2018.08.002
11. Cristina ML, Sartini M, Ottria G, et al. Epidemiology and biomolecular characterization of carbapenem-resistant Klebsiella pneumoniae in an Italian hospital. J Prev Med Hyg. 2016;57(3):E149–E156.
12. Bartsch SM, Huang SS, McKinnell JA, et al. The economic value of the centers for disease control and prevention carbapenem-resistant Enterobacteriaceae toolkit. Infect Control Hosp Epidemiol. 2018;39(5):516–524. doi:10.1017/ice.2018.49
13. Bartsch SM, McKinnell JA, Mueller LE, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23:
14. Polinder S, Haagsma JA, Stein C, Havelaar AH. Systematic review of general burden of disease studies using disability-adjusted life years. Popul Health Metr. 2012;10:21. doi:10.1186/1478-7954-10-21
15. Lamberti LM, Fischer Walker CL, Black RE. Systematic review of diarrhea duration and severity in children and adults in low- and middle-income countries. BMC Public Health. 2012;12:276. doi:10.1186/1471-2458-12-276
16. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi:10.1016/S0140-6736(12)61680-8
17. Jia H, Li W, Hou T, et al. The Attributable Direct Medical Cost of Healthcare Associated Infection Caused by Multidrug Resistance Organisms in 68 Hospitals of China. Biomed Res Int. 2019;2019:7634528. doi:10.1155/2019/7634528
18. Friedrich AW. Control of hospital acquired infections and antimicrobial resistance in Europe: the way to go. Wien Med Wochenschr. 2019;169(Suppl 1):25–30. doi:10.1007/s10354-018-0676-5
19. CLSI. Performance Standards for Antimicrobial Susceptibility Testing——Twenty-Ninth Edition: M100. CLSI; 2019.
20. Bank of China. Available from: https://srh.bankofchina.com/search/whpj/search_cn.jsp.
21. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi:10.1097/00003246-198510000-00009
22. Rhee JY, Kwon KT, Ki HK, Sang YS, Song JH. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009;31:146–150. doi:10.1097/SHK.0b013e318182f98f
23. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8
24. World Health Organization. Health statistics and information systems, metrics: disability-adjusted life year (DALY). Available from: https://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/.
25. Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001;16:326–331. doi:10.1093/heapol/16.3.326
26. World Health Organization. Disability weights, discounting and age weighting of DALYs. Available from: https://www.who.int/healthinfo/global_burden_disease/daly_disability_weight/en/.
27. Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–e723. doi:10.1016/S2214-109X(15)00069-8
28. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602.
29. WHO methods and data sources for global burden of disease estimates 2000-2015. Available from: http://www.who.int/healthinfo/global_burden_disease/GlobalDALYmethods_2000_2015.pdf?ua=1.
30. Barnum H. Evaluating healthy days of life gained from health projects. Soc Sci Med. 1987;24:833–841. doi:10.1016/0277-9536(87)90184-5
31. National Bureau of Statistics of China. Available from: http://data.stats.gov.cn/easyquery.htm?cn=C01.
32. Kaye KS, Pogue JM. Infections Caused by Resistant Gram-Negative Bacteria: epidemiology and Management. Pharmacotherapy. 2015;35:949–962. doi:10.1002/phar.1636
33. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: report from the China CRE Network. Antimicrob Agents Chemother. 2018;62:e01882–17. doi:10.1128/AAC.01882-17
34. Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi:10.1128/CMR.05035-11
35. Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58:2322–2328. doi:10.1128/AAC.02166-13
36. Huang W, Qiao F, Zhang Y, et al. In-hospital Medical Costs of Infections Caused by Carbapenem-resistant Klebsiella pneumoniae. Clin Infect Dis. 2018;67:S225–S230. doi:10.1093/cid/ciy642
37. Meng X, Liu S, Duan J, et al. Risk factors and medical costs for healthcare-associated carbapenem-resistant Escherichia coli infection among hospitalized patients in a Chinese teaching hospital. BMC Infect Dis. 2017;17:82. doi:10.1186/s12879-016-2176-9
38. Vargas-Alzate CA, Higuita-Gutiérrez LF, López-López L, Gallet AVC, Quicenoet JNJ. High excess costs of infections caused by carbapenem-resistant Gram-negative bacilli in an endemic region. Int J Antimicrob Agents. 2018;51:601–607. doi:10.1016/j.ijantimicag.2017.12.012
39. Zilberberg MD, Nathanson BH, Sulham K, Fan M, Shorr AF. 30-day readmission, antibiotics costs and costs of delay to adequate treatment of Enterobacteriaceae UTI, pneumonia, and sepsis: a retrospective cohort study. Antimicrob Resist Infect Control. 2017;6:124. doi:10.1186/s13756-017-0286-9
40. Muller A, Reutzel TJ. Willingness to pay for reduction in fatality risk: an exploratory survey. Am J Public Health. 1984;74:808–812. doi:10.2105/AJPH.74.8.808
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.