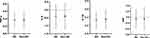Back to Journals » Psychology Research and Behavior Management » Volume 16
Smartphone Use and Inflammation at 2-Year Follow-Up in College Students: The Mediating Role of Physical Activity
Authors Li R , Li T, Xie Y, Zhai S, Qu Y, Zhang D, Zou L, Yang Y, Wu X, Tao F, Tao S
Received 4 March 2023
Accepted for publication 22 April 2023
Published 27 April 2023 Volume 2023:16 Pages 1509—1519
DOI https://doi.org/10.2147/PRBM.S411043
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Renjie Li,1 Tingting Li,1 Yang Xie,1 Shuang Zhai,1 Yang Qu,1 Dan Zhang,1 Liwei Zou,2 Yajuan Yang,2 Xiaoyan Wu,1,2 Fangbiao Tao,1,2 Shuman Tao2,3
1Department of Maternal, Child & Adolescent Health, School of Public Health, Anhui Medical University, Hefei, 230032, People’s Republic of China; 2MOE Key Laboratory of Population Health Across Life Cycle; Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, 230032, People’s Republic of China; 3Department of Ophthalmology, The Second Hospital of Anhui Medical University, Hefei, 230601, People’s Republic of China
Correspondence: Shuman Tao, Email [email protected]
Purpose: Smartphone use could lead to being physically inactive and a greater risk for health problems, such as inflammation. However, the associations between smartphone use, physical activity (PA), and systemic low-grade inflammation remained unclear. This study aimed to examine the potential mediating effect of PA on the association between smartphone use and inflammation.
Patients and Methods: A two-year follow-up study was conducted between April 2019 and April 2021. Duration of smartphone use, smartphone dependence and PA were assessed by a self-administered questionnaire. Laboratory analysis of blood samples was performed to evaluate the levels of TNF-α, IL-6, IL-1β, and CRP as biomarkers of systemic inflammation. The correlations between smartphone use, PA, and inflammation were analyzed using Pearson correlation. Structural equation modelling was used to analyze the potential mediating effect of PA on the associations between smartphone use and inflammation.
Results: A total of 210 participants were included with a mean (standard deviation) age of 18.7 (1.0) years, 82 (39%) of whom were males. Smartphone dependence was negatively associated with the total PA level (r=− 0.18, P< 0.01). PA mediated the associations between the duration of smartphone use and smartphone dependence with inflammatory markers. Specifically, as PA decreased, the duration of smartphone use was more negatively associated with TNF-α (ab=− 0.027; 95% CI: − 0.052, − 0.007) and more positively correlated to IL-6 (ab=0.020; 95% CI: 0.001, 0.046) and CRP (ab=0.038; 95% CI: 0.004, 0.086); smartphone dependency was more negatively associated with TNF-α (ab=− 0.139; 95% CI: − 0.288, − 0.017) and more positively related to CRP (ab=0.206; 95% CI: 0.020, 0.421).
Conclusion: Our study illustrates that there are no direct associations between smartphone use and systemic low-grade inflammation, however, PA level plays a weak but significant mediating effect on the associations between smartphone use and inflammation among college students.
Keywords: smartphone, inflammation, physical activity, mediating role, follow-up
Introduction
Over the past decade, several studies have shown that the number of smartphone users has been growing rapidly.1–3 In 2019, global smartphone penetration reached approximately 41.5% of the global population.4 Young people (18–22 years old) are the largest and fastest-growing group of smartphone users.5 There is a growing body of research examining the impacts of smartphone overuse on health. Elhai et al6 indicated that mental health problems such as depression and anxiety were associated with excessive smartphone use. Another study showed that smartphone use was positively associated with perceived stress levels.7 In addition, studies have shown that media devices could negatively affect sleep quality.8 However, the evidence for the associations between smartphone use and physical health remains limited. A few studies have examined the association between smartphone use and neck pain9 or between social media use and inflammation10–12 supporting the hypothesis that smartphone use hurts health.
Systemic low-grade inflammation describes the persistent production of proinflammatory factors, as opposed to an acute inflammatory state, chronic inflammation is considered integral to the development of serious systemic diseases such as type 2 diabetes mellitus, cardiovascular diseases, gastrointestinal disorders, and rheumatoid arthritis.13 This inflammatory state is indicated by elevated levels of circulating inflammation markers, tumor necrosis factor-alpha (TNF-α),14 interleukin-6 (IL-6)15 and interleukin-1β (IL-1β)16 are important cytokines involved in the inflammatory response. C-reactive protein (CRP) is an acute phase protein that is secreted from the liver upon IL-6 stimulation,17 which plays a crucial role in the regulation of the inflammatory process along with other inflammation cytokines. Studies suggested there was a positive association between social media use and systemic pro-inflammatory status,10 but there was no clear pattern to illustrate a specific association between the two.
Many features of smartphone use have been reported to be associated with sedentary behaviors.18 Additional studies have shown a positive association of sedentary behavior with higher body mass index (BMI) and lower PA levels.19,20 Moreover, the negative impact of problematic smartphone use on PA levels was addressed.21 Although some studies proposed the ability of smartphones to promote PA through some apps,22,23 the relationships between excessive smartphone use and the symptoms of health were well-established,24,25 which also inevitably lead to an increase in screen-based sedentary behaviors and a decrease in PA. There are some discrepancies in the previous literature regarding the association between PA and inflammation. Concerning the anti-inflammatory effects of exercise, one study suggests that the likely mechanism is a reduction of visceral fat,26 that regular exercise leads to increased circulating levels of adiponectin and decreased levels of several circulating proinflammatory adipokines, including IL-6, TNF, retinol-binding protein 4, and leptin,27–29 and that increased physical activity may therefore reduce systemic inflammation by decreasing proinflammatory adipokine secretion.30 In addition, exercise leads to a significant increase in cellular and circulating levels of IL-6 in contracted skeletal muscle,31 and transient elevations in IL-6 are responsible for subsequent increases in circulating levels of the anti-inflammatory cytokine IL-10 and IL-1 receptor antagonist (IL-1RA).32 Meanwhile, IL-1RA is secreted mainly by monocytes and macrophages and inhibits the pro-inflammatory effects of IL-1β effects.33 However, a recent mouse study showed that intensive exercise training led to an increased response of anti-inflammatory cytokines (IL-10) to antigen exposure.34 Similar findings have been reported in population experiments.35 A large body of evidence from mouse and human studies suggestted that IL-10 production generally imposed some limitations on the effectiveness of pathogen-specific immune responses.36,37 These studies suggest that high-intensity training loads induce an anti-inflammatory state that increases the risk of minor infections.
Although there were independent associations between PA or smartphone use and inflammatory status, studies illustrating a specific link between the three were limited. Based on the foregoing, we hypothesized a mediating effect of PA on the associations between smartphone use, especially the duration of smartphone use and the symptoms of dependence, and systemic low-grade inflammation. Our study was conducted in a 2-year follow-up design among healthy college students and aimed to explore the mediating role of PA in the associations between smartphone use and inflammation, which could contribute to the development of intervention strategies on PA among youth with excessive media use.
Materials and Methods
Participants and Procedure
Participants were recruited from a medical university in Hefei, Anhui Province and a comprehensive normal college in Shangrao, Jiangxi Province between April and May 2019. All first-year students from two faculties of each university were selected by cluster random sampling. A 4-wave follow-up at 6-month intervals in 2 years was conducted from October 2019 to April 2021. The baseline survey consisted of an electronic questionnaire scanned with a smartphone and a physical examination. A total of 1135 valid questionnaires were received, with a response rate of 98.6%. Blood samples were collected from 771 students at baseline. Because of the COVID-19 epidemic and the health status of students, blood samples were collected from 339 students at wave 4. Finally, 210 valid respondents who provided both the questionnaire and blood samples at baseline and wave 4 were analyzed. The research protocol was approved by the Ethics Committee of Anhui Medical University (approval number: 20170291). All respondents signed informed consent before completing the research, and the study was conducted following the principles of the Declaration of Helsinki.
Measures
Duration of Smartphone Use and the Smartphone Dependence
Duration of smartphone use was assessed by asking “How long did you use your smartphone per day?” The answers were counted in hours and recorded as less than 2 hours (1), 2–3.99 hours (2), 4–5.99 hours (3), and greater than or equal to 6 hours (4).38
Smartphone dependence was assessed by using the Self-rating Questionnaire for Adolescent Problematic Mobile Phone Use (SQAPMPU).39 The questionnaire consists of 13 items containing 3 dimensions named withdrawal symptoms, craving, and physical and psychological effects, and uses a 5-point scale with scores ranging from 1 (never) to 5 (always). The total scores ranged from 13 to 65, with higher scores indicating a higher level of dependence. Smartphone dependence was defined as a total score greater than or equal to the 75th percentile of the whole group.40 The Cronbach’s alpha coefficient was 0.92 in the present study.
Physical Activity
PA was assessed using the Chinese version of the International Physical Activity Questionnaire Short Form (IPAQ-SF).41 The questionnaire contains 7 questions covering 3 types of PA: walking, moderate PA (MPA; eg, lifting light objects, cycling at normal speed, or playing tandem tennis), and vigorous PA (VPA; eg, lifting heavy objects, digging, aerobic exercise, or fast cycling). Participants were asked to recall the frequency (days per week, d/w) and duration (minutes per day, min/d) of varying intensity of PA during the last 7 days. The amount of PA is calculated in metabolic equivalent (MET). The METs for VPA, MPA and walking are 8.0, 4.0 and 3.3, respectively. The MET-min/w scores of each activity were calculated by its METs × frequency (d/w) × duration (min/d). The total PA was a sum of (VPA+ MPA +walking) MET-min/w. The total PA level was classified into three groups:
High: (a) VPA on at least 3 days per week with a minimum total PA level of 1500 MET-min/w.(b) A total of VPA, MPA and walking at least 7 days per week with a minimum total PA of 3000 MET-min/w.
Medium: (a) VPA at least 20 minutes per day for a minimum total of 3 days per week. (b) MPA or walking at least 30 minutes per day for a total of 5 days or more. (c) VPA, MPA and walking for 5 days or more per week with a minimum total PA level of 600 MET-min/w.
Low: Those respondents who did not meet the high or medium criteria mentioned above were defined as at a low PA level.
Pro-Inflammatory Cytokines and C-Reactive Protein
Laboratory analysis of blood specimens was performed to evaluate TNF-α, IL-6, IL-1β, and CRP as markers of systemic inflammation. During the physical examination, 5 mL of fasting venous blood samples were collected from 6:00 to 8:00 in the morning using a vacuum blood collection tube with anticoagulant (EDTA). The blood samples were centrifuged for 10 min at a set speed of 3000 revolutions per minute within 2 hours. The upper plasma samples were stored at −80°C. Liquid-phase protein suspension chip (Luminex) was used in the detection process. Plasma inflammatory markers were detected by multi-bead enzyme-free analyzer MILLIPLEX ® MAP instrument (Merck Millipore). Within the analysis of variation, the intra-assay coefficient of variation of IL-1β, IL-6, and TNF-α were all <5%, and the inter-assay coefficients of variation were <15%, <20%, and <15%, respectively. The assay had a lower detection limit of IL-1β, IL-6, and TNF-α of 0.14 pg/mL, 0.11 pg/mL, and 0.16 pg/mL, respectively. All inflammatory markers were log-transformed to settle skewness. The level of CRP was measured by immunoturbidimetry using the serum on the day the blood samples were collected.
Socio-Demographic Information and Covariates
Baseline socio-demographic information was collected by electronic questionnaires, including age, gender (male or female), residence (rural or town), health status (poor, medium or good), socioeconomic status (SES; operationalized as the mean score derived from parental education level and self-reported family economy, each measured on a scale ranging from 1 to 5, with higher scores indicating higher SES) and body mass index (BMI). Moreover, PA levels at baseline were also assessed by IPAQ-SF; TNF-α, IL-6, IL-1β and CRP at baseline were detected; and duration of smartphone use and smartphone dependence scores at follow-up were also collected by questionnaires and adjusted.
Statistical Analyses
IBM SPSS Statistics 26.0 was used to perform descriptive analysis and correlation analysis between variables. Total PA levels, TNF-α, IL-6, IL-1β and CRP were all log-transformed. Normally distributed data were represented by the mean and standard deviation (SD). Categorical data were expressed as frequencies (n) and percentages (%). Additionally, to examine the differences in inflammation levels stratified by smartphone dependence or PA level groups, additional two independent samples t-test and one-way ANOVA were performed. Mplus version 8.3 statistical software was used for mediation analysis. To test our hypotheses, we conducted an overall structural equation model. The model can determine the total, direct and indirect effects between the duration of smartphone use and smartphone dependence and the levels of inflammation:42 the total effect was an unadjusted association between the duration of smartphone use, smartphone dependence and inflammation levels; The indirect effect was the associations between the duration of smartphone use, smartphone dependence and inflammation levels through PA levels; The direct effect is the association that remains after adjusting for the effects of PA level.
The full information maximum likelihood method was used to deal with missing data.43 The goodness of fit was assessed with the following fitting indexes: comparative fit index (CFI), Tucker-Lewis index (TLI), and root mean square error of approximation (RMSEA). Thresholds were considered as follows: for CFI and TLI excellent fit >0.95 and moderate fit >0.90; for RMSEA excellent fit <0.05 and moderate fit <0.08.44
Results
Descriptive Analyses
Table 1 presents demographic information for 210 college students (39.0% males and 61.0% females) aged 16–26 years old (M=18.7, SD=1.0). There were 182 (86.7%) college students from rural areas and 28 (13.3%) from urban areas. One hundred and eighteen (56.2%) college students described their health as good, 88 (41.9%) as medium, and 4 (1.9%) as poor. The mean SES scores were 8.27±2.14. The mean BMI was 21.00±2.51 kg/m2. The mean duration of smartphone use at baseline was 3.07±0.80 hours and 2.22±1.19 hours at the 2-year follow-up. The total scores of smartphone dependence at baseline were 23.91±8.30 and 21.95±8.95 at the two-year follow-up. The prevalence of smartphone dependence among college students was 30.5% at baseline and 21.4% at the two-year follow-up. The IPAQ-SF scores (log-transformed) were 3.34±0.37 at baseline and 3.29±0.45 at the two-year follow-up. At the follow-up after 2 years, 39%, 43.3%, and 17.6% of participants reported high, medium, and low PA levels, respectively. The log-transformed mean levels of TNF-α, IL-6, IL-1β, and CRP at baseline were 0.83±0.21 pg/mL, 0.76±0.35 pg/mL, 0.41±0.28 pg/mL, −0.39±0.58 pg/mL, respectively; and were 0.37±0.21 pg/mL, −0.40±0.75 pg/mL, 0.34±0.22 pg/mL, −0.06±0.36 pg/mL at the two-year follow-up. Demographic differences between the final included and excluded samples for the analysis are shown in Table S1.
 |
Table 1 Descriptive Statistics of the Study Samples (n=210) |
Correlation Analysis
Table 2 demonstrates the correlations between smartphone use, PA, and inflammation. The duration of smartphone use was significantly and positively correlated with smartphone dependence at baseline. Smartphone dependence at baseline was negatively correlated with PA measured at follow-up. Total PA levels were positively correlated with TNF-α and negatively correlated with IL-6 at the 2-year follow-up. The correlations between the duration of smartphone use and smartphone dependence measured both at baseline and follow-up were displayed in Table S2. There were significant correlations among inflammatory factors measured both at baseline and follow-up, shown in Table S3.
 |
Table 2 Bivariate Correlations Between Smartphone Use, Physical Activity and Inflammation |
The Inflammation Levels in Different Groups of Smartphone Dependence and PA
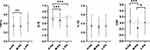
The inflammation levels at 2-year follow-up in different groups of smartphone dependence at baseline are shown in Figure 1. The results showed that there were no significant differences in TNF-α (P=0.59), IL-6 (P=0.93), IL-1β (P=0.41), and CRP (P=0.92) at follow-up between the college students with smartphone dependence and those without smartphone dependence at baseline. The differences in inflammation levels at follow-up between different levels of PA at follow-up are shown in Figure 2. TNF-α was significantly higher in the high PA level group than that of the medium PA level group (P=0.005). IL-6 was significantly higher in the high PA level group than in the medium PA level group (P<0.001) or the low PA level group (P<0.001). CRP levels were higher in the high PA level group than in the medium PA level group (P<0.001), but were lower in the medium PA level group than in the low PA level groups (P=0.001). IL-1β was not significantly different between high, medium and low PA level groups (P=0.74). We additionally explored the differences in the total PA levels at follow-up between students with and without smartphone dependence at baseline, which indicated the total PA levels at follow-up were significantly lower in the smartphone dependence group at baseline, as seen in Figure S1 (P=0.018).
The Mediating Effect of Follow-Up PA on the Associations Between Baseline Smartphone Use and Follow-Up Inflammation in College Students
Table 3 exhibits the mediating effect of PA at follow-up between smartphone use at baseline and inflammation levels at follow-up in college students. After adjusting for gender, total PA levels at baseline, inflammatory indicators at baseline, duration of smartphone use at follow-up, and symptoms of smartphone dependence at follow-up, the results showed a negative mediating effect of total PA level on the association between the duration of smartphone use and TNF-α (ab=−0.027, 95% CI: −0.052, −0.007), but the direct and total effects were not significant. Furthermore, there was a positive mediating effect of total PA level on the association between duration of smartphone use and IL-6 (ab=0.020, 95% CI: 0.001, 0.046) and CRP (ab=0.038, 95% CI: 0.004, 0.086), but the direct and total effects were not significant. The mediating effect of total PA level on the association between the duration of smartphone use and IL-1β was not significant, but there was a total effect (c=−0.049, P<0.05). When smartphone dependence was used as a predictor, the total PA levels showed a negative mediating effect on the association between smartphone dependence scores and TNF-α (ab=−0.139, 95% CI: −0.288, −0.017), and the direct and total effects were not significant. There was a positive mediating effect of total PA level on the association between smartphone dependence and CRP (ab=0.206, 95% CI: 0.020, 0.421), with a significant direct effect (c′=−0.565, P<0.05) and a non-significant total effect; The mediating, direct, and total effects of total PA level on the associations between smartphone dependence and IL-6 and IL-1β were not significant.
 |
Table 3 The Mediating Effect of Physical Activity on the Associations Between Smartphone Use and Inflammation Among College Students |
Discussion
To our knowledge, most previous studies have focused on the associations between problematic smartphone use and depression, anxiety, chronic stress, or low self-esteem.6 This is the first study to examine the relationships between smartphone use and inflammation among healthy college students. We also found a weak but significant mediating role of PA in the associations between smartphone use and inflammatory status.
Our results reported the percentage of smartphone dependence was 30.5% at baseline and 21.4% at the 2-year follow-up, which differed from that of college students in Spain (12.8%),45 Serbia (22.7%)46 and another city of China (29.8%).47 Moreover, our findings indicated smartphone dependence inevitably led to increasing screen time and decreasing the time spent on PA. A large number of studies have shown that increased media-based screen time can lead to sedentary behavior.48–50 Macías et al51 noted that television viewing, computer use, and other screen-based activities were associated with sedentary time spent in front of a screen. A Finnish study showed that a large amount of sedentary behavior was associated with lower PA.20 A systematic review identified that a surge in screen time was a major risk factor for sedentary behavior.52
To date, there are no studies that have specifically tested the association between smartphone dependence and inflammation. There was also no direct associations between smartphone dependence and inflammation observed in our study. However, significant differences in inflammation between groups of PA were found. The potential beneficial effects of exercise on inflammation are well-established anteriorly.53 Previous studies have shown that PA can prevent clinical conditions associated with systemic low-grade inflammation in adults.26,54 Otherwise, some evidence was consistent with our findings. A study showed that TNF-α levels were significantly lower in the moderate PA group compared to the low PA group, while IL-6 levels were significantly higher in the high PA group than in the moderate and low PA groups.55 This suggests that moderate PA may be the optimal intensity to reduce the pro-inflammatory state. In addition, some studies have shown that IL-1β may not be as sensitive as IL-6 and TNF-α,55,56 which confirmed that why did not find a mediating role of PA on the relationship between smartphone use and IL-1β.
Moderate physical activity plays a positive role in reducing anxiety, depression and complicated anxiety and depression symptoms.57 At the same time, the level of biomarkers of peripheral inflammation increased in patients with depression.58 Studies on animal models have also shown that the release of pro-inflammatory factors and the activation of microglia in the animal brain show signs of anxiety and depression.59,60 Therefore, anxiety and depression may be important factors leading to the development of inflammation due to the decrease in physical activity. In addition, TNF-α and IL-6 are usually overexpressed in adipose tissue cells and macrophages in the physically inactive and obese population, which could cause an inflammatory response.61,62 However, exercise-induced intramuscular IL-6 mRNA, which increases circulating IL-6, then acts as a trigger to activate hepatic glycogenolysis and lipolysis, providing additional energy to exercising muscles.63 This is consistent with the results observed in this study where IL-6 levels were higher in the high PA group than in the moderate and low PA groups, suggesting that high-intensity PA appear to play a pro-inflammatory role.64 A systematic review and meta-analysis of 83 randomized controlled trials involving 3769 participants suggested that exercise for more than 2 weeks can reduce CRP.65 In our study, the moderate PA group also showed a significant decrease in CRP levels compared to the low PA group. Thus, the mediating role of PA may be an important pathway for the associations between screen-based sedentary behaviors and inflammation. It is worth noting that PA played a negative mediating effect between smartphone use and TNF-α levels, which suggested that while smartphone use can certainly lead to altered PA, the level of PA intensity may be associated with inflammatory status. Paolucci et al55 showed that high-intensity intermittent exercise increased the concentration of TNF-α, which in turn leads to an increase in inflammatory levels. In addition, we found a positive mediating effect of PA on the association between the duration of smartphone use and IL-6 or CRP, and PA played a fully mediated role between smartphone use and inflammation. Preacher et al66 explained that fully mediated results are easily obtained when the total effect and sample size are small. Therefore, the sample population should be appropriately increased in future studies.
Several strengths of the present study should be addressed. First, a longitudinal design was used to examine the associations between smartphone use, PA, and inflammation over two years, and controlled for PA and inflammation at baseline, duration of smartphone use and smartphone dependence at follow-up in the study. Second, the combined measurement of several inflammatory biomarkers provided a more comprehensive picture of the systemic pro-inflammatory state. Otherwise, some limitations of this study should be acknowledged. First, self-report instruments were used to assess smartphone use and PA levels, which may introduce potential reporting bias, but they were well-verified with good reliability worldwide.39,67 Second, although smartphone use represents prior exposure, no clear conclusions can be drawn regarding the timing of the occurrence of elevated PA levels and inflammation. The temporal sequence of changes in PA levels and inflammation should be further clarified in future studies to establish potential pathways of associations between smartphone use, PA, and inflammation. Third, because of the impact of COVID-19, students devoted more time to taking courses online, which resulted in increased access to digital media devices and decreased physical activity time.68,69 The extrapolation of our results is limited and future studies should further track observations in a broader population. Finally, although our study has controlled for several confounding factors, there are still some confounders which are difficult to measure (eg, physical and chemical environment, immunization) that may interfere with the results, and future studies should set up better-designed protocols.
Conclusion
Our study illustrates that there are no direct associations between smartphone use and inflammation among college students, PA level plays a weak but significant mediating effect on the associations between smartphone use and inflammatory status among college students. These findings suggest that an appropriate increase in PA level has positive implications for reducing screen-based sedentary behaviors and systemic pro-inflammatory status.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No. 81803257, 82173542). The authors sincerely thank all participants involved in this study for their full support.
Disclosure
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
References
1. Csibi S, Griffiths MD, Demetrovics Z, et al. Analysis of problematic smartphone use across different age groups within the ‘components model of addiction’. Int J Ment Health Ad. 2021;19:616–631. doi:10.1007/s11469-019-00095-0
2. Vernon L, Modecki KL, Barber BL. Mobile phones in the bedroom: trajectories of sleep habits and subsequent adolescent psychosocial development. Child Dev. 2018;89:66–77. doi:10.1111/cdev.12836
3. Rideout V, Robb MB Social media, social life: teens reveal their experiences. San Francisco, CA: Common Sense Media; 2018. Available from: https://www.socialmediatoday.com/news/social-media-social-life-teens-reveal-their-experiences-infographic/532424/.
4. Ertemel AV, Ari E. A marketing approach to a psychological problem: problematic smartphone use on adolescents. Int J Environ Res Public Health. 2020;17:2471. doi:10.3390/ijerph17072471
5. Jiang ZC, Zhao XX. Self-control and problematic mobile phone use in Chinese college students: the mediating role of mobile phone use patterns. BMC Psychiatry. 2016;16:416. doi:10.1186/s12888-016-1131-z
6. Elhai JD, Dvorak RD, Levine JC, et al. Problematic smartphone use: a conceptual overview and systematic review of relations with anxiety and depression psychopathology. J Affect Disord. 2017;207:251–259. doi:10.1016/j.jad.2016.08.030
7. Pengpid S, Peltzer K. Vigorous physical activity, perceived stress, sleep and mental health among university students from 23 low-and middle-income countries. Int J Adolesc Med Health. 2020;2020:32.
8. Mireku MO, Barker MM, Mutz J, et al. Night-time screen-based media device use and adolescents’ sleep and health-related quality of life. Environ Int. 2019;124:66–78. doi:10.1016/j.envint.2018.11.069
9. Lee S, Kang H, Shin G. Head flexion angle while using a smartphone. Ergonomics. 2015;58:220–226.
10. Lee DS, Way BM. Social media use and systemic inflammation: the moderating role of self-esteem. Brain Behav Immun Health. 2021;16:100300. doi:10.1016/j.bbih.2021.100300
11. Afifi TD, Zamanzadeh N, Harrison K, et al. WIRED: the impact of media and technology use on stress (cortisol) and inflammation (interleukin IL-6) in fast paced families. Comput Human Behav. 2018;81:265–273. doi:10.1016/j.chb.2017.12.010
12. Lee DS, Jiang T, Crocker J, et al. Social media use and its link to physical health indicators. Cyberpsychol Behav Soc Netw. 2022;25:87–93. doi:10.1089/cyber.2021.0188
13. Pietzner M, Kaul A, Henning AK, et al. Comprehensive metabolic profiling of chronic low-grade inflammation among generally healthy individuals. BMC Med. 2017;15:15. doi:10.1186/s12916-017-0795-7
14. Rockstrom MD, Chen LY, Taishi P, et al. Tumor necrosis factor alpha in sleep regulation. Sleep Med Rev. 2018;40:69–78. doi:10.1016/j.smrv.2017.10.005
15. Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann Ny Acad Sci. 2012;1261:88–96. doi:10.1111/j.1749-6632.2012.06634.x
16. Rethorst C, Greer T, Toups M, et al. IL-1β and BDNF are associated with improvement in hypersomnia but not insomnia following exercise in major depressive disorder. Transl Psychiatry. 2015;5:e611–e611. doi:10.1038/tp.2015.104
17. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1 moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–156. doi:10.1161/CIRCRESAHA.115.306656
18. Rosenberg DE, Norman GJ, Wagner N, et al. Reliability and validity of the sedentary behavior questionnaire (SBQ) for adults. J Phys Act Health. 2010;7:697–705. doi:10.1123/jpah.7.6.697
19. Kautiainen S, Koivusilta L, Lintonen T, et al. Use of information and communication technology and prevalence of overweight and obesity among adolescents. Int J Obes. 2005;29:925–933. doi:10.1038/sj.ijo.0802994
20. Tammelin T, Ekelund U, Remes J, et al. Physical activity and sedentary behaviors among Finnish youth. Med Sci Sports Exerc. 2007;39:1067–1074. doi:10.1249/mss.0b13e318058a603
21. Lepp A, Barkley JE, Sanders GJ, et al. The relationship between cell phone use, physical and sedentary activity, and cardiorespiratory fitness in a sample of US college students. Int J Behav Nutr Phy. 2013;10:79. doi:10.1186/1479-5868-10-79
22. Bentley CL, Powell L, Potter S, et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. Jmir Mhealth Uhealth. 2020;8:e16203. doi:10.2196/16203
23. Romeo A, Edney S, Plotnikoff R, et al. Can smartphone apps increase physical activity? Systematic review and meta-analysis. J Med Internet Res. 2019;21:e12053. doi:10.2196/12053
24. Alageel AA, Alyahya RA, Bahatheq YA, et al. Smartphone addiction and associated factors among postgraduate students in an Arabic sample: a cross-sectional study. BMC Psychiatry. 2021;21:302. doi:10.1186/s12888-021-03285-0
25. Nishida T, Tamura H, Sakakibara H. The association of smartphone use and depression in Japanese adolescents. Psychiatry Res. 2019;273:523–527. doi:10.1016/j.psychres.2019.01.074
26. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi:10.1152/japplphysiol.00164.2004
27. Mujumdar PP, Duerksen-Hughes PJ, Firek AF, et al. Long-term, progressive, aerobic training increases adiponectin in middle-aged, overweight, untrained males and females. Scand J Clin Lab Invest. 2011;71:101–107. doi:10.3109/00365513.2011.554995
28. Ben Ounis O, Elloumi M, Lac G, et al. Two-month effects of individualized exercise training with or without caloric restriction on plasma adipocytokine levels in obese female adolescents. Ann Endocrinol-Paris. 2009;70:235–241. doi:10.1016/j.ando.2009.03.003
29. Lim S, Choi SH, Jeong IK, et al. Insulin-sensitizing effects of exercise on adiponectin and retinol-binding protein-4 concentrations in young and middle-aged women. J Clin Endocrinol Metab. 2008;93:2263–2268. doi:10.1210/jc.2007-2028
30. Yudkin JS. Inflammation, Obesity, and the Metabolic Syndrome. Horm Metab Res. 2007;39(10):707–709. doi:10.1055/s-2007-985898
31. Pedersen BK, Edward F. Adolph distinguished lecture: muscle as an endocrine organ: Il-6 and other myokines. J Appl Physiol. 2009;107(4):1006–1014. doi:10.1152/japplphysiol.00734.2009
32. Steensberg A, Fischer CP, Keller C, et al. Il-6 enhances plasma Il-1ra, Il-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–437. doi:10.1152/ajpendo.00074.2003
33. Freeman BD, Buchman TG. Interleukin-1 receptor antagonist as therapy for inflammatory disorders. Expert Opin Biol Ther. 2001;1:301–308. doi:10.1517/14712598.1.2.301
34. Wang J, Song H, Tang X, et al. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22:643–652. doi:10.1111/j.1600-0838.2010.01288.x
35. Gleeson M, Bishop N, Oliveira M, et al. Respiratory infection risk in athletes: association with antigen-stimulated Il-10 production and salivary iga secretion. Scand J Med Sci Sports. 2012;22:410–417. doi:10.1111/j.1600-0838.2010.01272.x
36. van der Sluijs KF, van Elden LJ, Nijhuis M, et al. Il-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi:10.4049/jimmunol.172.12.7603
37. Blackburn SD, Wherry EJ. Il-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi:10.1016/j.tim.2007.02.006
38. Woo KS, Bong SH, Choi TY, et al. Mental health, smartphone use type, and screen time among adolescents in South Korea. Psychol Res Behav Manag. 2021;14:1419–1428. doi:10.2147/PRBM.S324235
39. Tao S, Fu J, Wang H, et al. The development of self-rating questionnaire for adolescent problematic mobile phone use and the psychometric evaluation in undergraduates. Chin J Sch Health. 2013;34:26–29.
40. Tao S, Wu X, Yang Y, et al. The moderating effect of physical activity in the relation between problematic mobile phone use and depression among university students. J Affect Disord. 2020;273:167–172. doi:10.1016/j.jad.2020.04.012
41. Macfarlane DJ, Lee CC, Ho EY, et al. Reliability and validity of the Chinese version of IPAQ (short, last 7 days). J Sci Med Sport. 2007;10:45–51. doi:10.1016/j.jsams.2006.05.003
42. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi:10.1146/annurev.psych.58.110405.085542
43. Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi:10.1146/annurev.psych.58.110405.085530
44. Xia Y, Yang Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: the story they tell depends on the estimation methods. Behav Res Methods. 2019;51:409–428. doi:10.3758/s13428-018-1055-2
45. Lopez-Fernandez O. Short version of the smartphone addiction scale adapted to Spanish and French: towards a cross-cultural research in problematic mobile phone use. Addict Behav. 2017;64:275–280. doi:10.1016/j.addbeh.2015.11.013
46. Randjelovic P, Stojiljkovic N, Radulovic N, et al. Problematic smartphone use, screen time and chronotype correlations in university students. Eur Addict Res. 2021;27:67–74. doi:10.1159/000506738
47. Chen BF, Liu F, Ding SS, et al. Gender differences in factors associated with smartphone addiction: a cross-sectional study among medical college students. BMC Psychiatry. 2017;17:341. doi:10.1186/s12888-017-1503-z
48. Delfino LD, Silva DAD, Tebar WR, et al. Screen time by different devices in adolescents: association with physical inactivity domains and eating habits. J Sport Med Phys Fit. 2018;58:318–325.
49. Grimaldi-Puyana M, Fernandez-Batanero JM, Fennell C, et al. Associations of objectively-assessed smartphone use with physical activity, sedentary behavior, mood, and sleep quality in young adults: a cross-sectional study. Int J Environ Res Public Health. 2020;17:3499. doi:10.3390/ijerph17103499
50. Solomon-Moore E, Sebire SJ, Macdonald-Wallis C, et al. Exploring parents’ screen-viewing behaviours and sedentary time in association with their attitudes toward their young child’s screen-viewing. Prev Med Rep. 2017;7:198–205. doi:10.1016/j.pmedr.2017.06.011
51. Macias N, Espinosa-Montero J, Monterrubio-Flores E, et al. Screen-based sedentary behaviors and their association with metabolic syndrome components among adults in Mexico. Prev Chronic Dis. 2021;18:E95. doi:10.5888/pcd18.210041
52. Musa S, Elyamani R, Dergaa I. COVID-19 and screen-based sedentary behaviour: systematic review of digital screen time and metabolic syndrome in adolescents. PLoS One. 2022;17:e0265560. doi:10.1371/journal.pone.0265560
53. Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol. 2020;34:101504. doi:10.1016/j.berh.2020.101504
54. Nimmo MA, Leggate M, Viana JL, et al. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab. 2013;15:51–60. doi:10.1111/dom.12156
55. Paolucci EM, Loukov D, Bowdish DM, et al. Exercise reduces depression and inflammation but intensity matters. Biol Psychol. 2018;133:79–84. doi:10.1016/j.biopsycho.2018.01.015
56. Hirano S, Zhou Q, Furuyama A, et al. Differential regulation of IL-1β and IL-6 release in murine macrophages. Inflammation. 2017;40:1933–1943. doi:10.1007/s10753-017-0634-1
57. Schuch FB, Bulzing RA, Meyer J, et al. Associations of moderate to vigorous physical activity and sedentary behavior with depressive and anxiety symptoms in self-isolating people during the covid-19 pandemic: a cross-sectional survey in Brazil. Psychiatry Res. 2020;292:113339. doi:10.1016/j.psychres.2020.113339
58. Kofod J, Elfving B, Nielsen EH, et al. Depression and inflammation: correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur Neuropsychopharmacol. 2022;54:116–125. doi:10.1016/j.euroneuro.2021.09.006
59. Munshi S, Loh MK, Ferrara N, et al. Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats. Brain Behav Immun. 2020;84:180–199. doi:10.1016/j.bbi.2019.11.023
60. Wang YL, Han QQ, Gong WQ, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation. 2018;15:21. doi:10.1186/s12974-018-1054-3
61. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi:10.1172/JCI92035
62. Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. 2019;9:223. doi:10.3390/biom9060223
63. Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. doi:10.1038/nrrheum.2014.193
64. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi:10.1152/physrev.90100.2007
65. Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C-reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. 2017;51:670–676. doi:10.1136/bjsports-2016-095999
66. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi:10.3758/BRM.40.3.879
67. Lee PH, Macfarlane DJ, Lam TH, et al. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phy. 2011;8:115. doi:10.1186/1479-5868-8-115
68. Shuai L, He S, Zheng H, et al. Influences of digital media use on children and adolescents with ADHD during covid-19 pandemic. Global Health. 2021;17:1–9. doi:10.1186/s12992-020-00651-7
69. Lu Z, Mao C, Tan Y, et al. Trends in physical fitness and nutritional status among school-aged children and adolescents during the covid-19 pandemic in Shaanxi, China-a cross-sectional study. Nutrients. 2022;14:3016. doi:10.3390/nu14153016
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.