Back to Journals » International Journal of General Medicine » Volume 16
Small Intestinal Bacterial Overgrowth in Patients with Gallbladder Polyps: A Cross-Sectional Study
Authors Dong C, Xian R, Wang G, Cui L
Received 6 December 2022
Accepted for publication 1 February 2023
Published 1 March 2023 Volume 2023:16 Pages 813—822
DOI https://doi.org/10.2147/IJGM.S399812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Changhao Dong,1,2 Rui Xian,1,2 Guangxiang Wang,2,3 Lihong Cui1,2
1Department of Gastroenterology, School of Medicine, South China University of Technology, Guangzhou, People’s Republic of China; 2Department of Gastroenterology, The Sixth Medical Center of PLA General Hospital of Beijing, Beijing, People’s Republic of China; 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, People’s Republic of China
Correspondence: Lihong Cui, Department of Gastroenterology, the Sixth Medical Center of PLA General Hospital of Beijing, No. 6 Fucheng Road, Haidian Distract, Beijing, 100048, People’s Republic of China, Tel +86-10-66957541, Email [email protected]
Purpose: There is probably a high prevalence of small intestinal bacterial overgrowth (SIBO) in patients with gallbladder polyps (GBPs). To date, no study has evaluated the occurrence of SIBO in patients with GBPs. The aim of this study was to investigate the prevalence of SIBO in patients with GBPs and explore the possible association between these two conditions.
Patients and Methods: The hydrogen-methane breath test was used to diagnose SIBO, and patients were divided into GBPs and control groups based on whether GBPs were found under ultrasound. Clinical and paraclinical factors were compared between the two groups.
Results: A total of 297 subjects were included in this study. The prevalence of SIBO was significantly higher in the GBPs group than in the control group (50.0% vs.30.8%, p< 0.01). Multivariate logistic regression analysis showed that male (OR=2.26, 95% CI=1.12– 4.57, p=0.023), SIBO (OR=3.21, 95% CI=1.69– 6.11, p< 0.001), fatty liver (OR=2.91, 95% CI= 1.50– 5.64, p=0.002) and BMI (OR=1.13, 95% CI=1.01– 1.26, p=0.035) were independently associated with GBPs. And by subgroup analysis, we found that the association between SIBO and GBPs was stronger in females than in males (p for interaction< 0.001). In addition, SIBO (OR=5.11, 95% CI=1.42– 18.36, p=0.012) and fasting glucose (OR=3.04, 95% CI=1.27– 7.28, p=0.013) were found to be associated with solitary polyps.
Conclusion: SIBO was highly prevalent in patients with GBPs, and this association seemed to be stronger among females.
Keywords: gut microbiota, bacterial overgrowth, breath test, gallbladder polyps
Introduction
Polypoid lesions of the gallbladder are one of the most commonly detected gallbladder diseases in adults on abdominal ultrasonography. Gallbladder polyps (GBPs) are usually asymptomatic, but in a few cases, they may present with symptoms similar to those of acute cholecystitis due to their specific anatomical location or when polyp fragments detach and obstruct the bile duct.1 Most GBPs are generally considered benign, but a small percentage of GBPs were reported to have malignant potential.2 According to the European joint guidelines,3,4 cholecystectomy is suggested for individuals with GBPs ≥10 mm in diameter; moreover, cholecystectomy is also recommended if the patient has a 6–9 mm polyp and one or more risk factors. Although a single polyp larger than 10 mm is by far the most consistent predictor of potential malignancy, many cases of malignant transformation of small polyps have been reported.2,5,6 The currently reported risk factors for GBPs include male sex, obesity, hypertension, hyperlipidemia, fatty liver, etc.7–10 However, the conclusions of these studies are inconsistent, and the differences may be due to variations in geography, ethnicity and study design. Gallbladder cancer has a very low 5-year survival rate, and early detection is difficult due to the lack of obvious symptoms. Considering that GBPs constitute an important risk factor for gallbladder cancer, the identification of etiology and relevant factors of GBPs is crucial for early screening and developing rational clinical strategies.
Gut microbiota is one of the most complex and important micro-ecosystems in the human body. As the understanding of gut microbiota has grown, its role in human health is gradually being emphasized. Gut microbiota coexists with humans as commensals, and it plays an important role in participating in host nutrient metabolism, maintaining the integrity of the intestinal barrier, and regulating the immune function. There is growing evidence that gut microbes have many complex physiological connections to multiple organs in the body. Under normal physiological circumstances, gut microbes maintain a dynamic balance. However, this balance may be disrupted by the occurrence of some diseases, manifested by changes in the structure or quantity of the gut microbiota. On the other hand, gut microbes can be potentially harmful when the gut micro-ecosystem undergoes abnormal changes, and dysbiosis of the gut microbes may affect the host health in many ways.11 Small intestinal bacterial overgrowth (SIBO) is one of the most common forms of gut microbial dysbiosis, and studies have shown that it is associated with several diseases, such as irritable bowel syndrome, fatty liver, and metabolic syndrome.12–14 Gallbladder disease may be associated with SIBO. Abnormal gallbladder function can lead to an altered homeostasis of bile acids and a reduced antimicrobial effect, which may be one of the mechanisms of SIBO. In turn, SIBO might contribute to the development of gallbladder disease in a variety of ways. However, there is almost a gap in studies exploring the association between gallbladder diseases and SIBO. The purpose of this study was to investigate the prevalence of SIBO in patients with GBPs and to explore whether there is an association between these two conditions.
Materials and Methods
Study Design and Patients
From June 2021 to December 2021, patients with gastrointestinal discomfort (including abdominal pain, bloating, belching, dyspepsia, constipation, diarrhea, nausea and loss of appetite) from the Sixth Medical Center of the PLA General Hospital were enrolled in this study. The lactulose hydrogen-methane breath test was used to diagnose SIBO. All patients had an abdominal ultrasound as well as laboratory tests on the same day that they had the breath test. Patients were divided into GBPs group and control group according to whether GBPs were found under ultrasound. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Sixth Medical Center of PLA General Hospital. Written informed consent was obtained from all subjects.
Inclusion and Exclusion Criteria
Inclusion criteria were patients aged 18 years or older who underwent a lactulose hydrogen-methane breath test to assess SIBO. Exclusion criteria were patients who 1) had taken antibiotics, H2 receptor antagonists, proton pump inhibitors, probiotics, or medications that affect gastrointestinal motility within the last month; 2) had undergone vagotomy; 3) had a history of gastrointestinal surgery; 4) had a colonoscopy, barium enema, or any other test with a bowel preparation within one week; 5) had postprandial hypoglycemia; 6) were diagnosed with irritable bowel syndrome, inflammatory bowel disease, or chronic pancreatitis.
Demographic, Clinical and Laboratory Data
Clinical data were recorded including age, sex, height, weight, blood pressure, smoking, alcohol consumption, past medical history and medication history. Smoking was defined as smoking one cigarette per day for at least half a year, and drinking was defined as drinking at least once a week. Laboratory test data including fasting glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, total bile acids, albumin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, direct bilirubin, total bilirubin, and γ-glutamyl transpeptidase were also collected for each patient. Metabolic syndrome (MS) was defined according to the National Cholesterol Education Program Adult Treatment Panel III.15
Lactulose Hydrogen-Methane Breath Test
Each subject underwent a lactulose hydrogen-methane breath test, which was performed with reference to the North American Consensus.16 To improve the accuracy of the test, patients were required to strictly adhere to the following conditions: (1) avoid using antibiotics 4 weeks before the breath test; (2) fast for 8–12 hours before the breath test; (3) avoid consuming fermentable foods such as complex carbohydrates (milk, buckwheat, beans, oatmeal, sweet potatoes, corn, etc.) the day before the breath test; (4) avoid smoking on the day of the breath test (burning tobacco can produce large amounts of hydrogen gas); and (5) limit strenuous activity during the test.
The lactulose hydrogen-methane breath test apparatus (QuinTron, QuinTron Instrument Company, USA) was used to first measure the patients’ fasting H2 and CH4 values, and then the patients were instructed to take 10 g of lactulose with one cup of water. Exhaled hydrogen and methane levels were measured every 30 minutes for a total time of 210 minutes. The criteria for a positive diagnosis of SIBO were as follows: 1) an increase in hydrogen level ≥20 ppm from baseline within 90 minutes; and 2) a methane level ≥10 ppm during the test.
Ultrasonographic Examination
The subjects underwent an abdominal ultrasound examination in the fasting state, and a GBP was defined as a strongly or moderately echogenic nodule protruding from the gallbladder wall into the lumen, which did not move with body position and was not accompanied by a posterior acoustic shadow.3 The diagnosis of fatty liver was based on the Asia-Pacific Guidelines (diffusely enhanced echogenicity liver with liver echogenicity higher than kidney or spleen, vascular blurring, and deep beam attenuation).17 All procedures and diagnostics were performed by experienced ultrasound physicians who were blinded to the clinical and laboratory information of the subjects.
Statistical Analysis
SPSS version 26.0 (IBM, Armonk, NY, United States) was used for statistical analysis. Continuous variables were expressed as means ± standard deviations, and categorical variables were expressed as frequencies and percentages. Student’s t-test, chi-square test and Fisher’s exact test were used to analyze differences between groups. Multivariable logistic regression was performed to identify the factors associated with GBPs and solitary polyps. The results of logistic analysis were expressed as the odds ratios (ORs) and 95% confidence intervals (95% CIs). P values <0.05 were considered indicative of statistical significance.
Results
Among the 335 patients initially recruited, 18 patients met the exclusion criteria. Twenty patients were also ruled out due to a previous cholecystectomy (Figure 1). Eventually, a total of 297 subjects were included in this study (mean age, 56 years; range, 25–86 years), consisting of 60 patients with GBPs and 237 controls.
 |
Figure 1 Flow chart. |
Table 1 shows the characteristics of the GBPs group and the control group. SIBO was found in 30/60 patients with GBPs compared to 73/237 without GBPs (50.0% vs 30.8%, p<0.01; Figure 2). The mean age of the GBPs group was 54.9 years, including 46 males and 14 females, and the prevalence of GBPs was significantly higher in males than in females (25.6% vs 11.8%, p< 0.01). The GBPs group had higher BMI (p<0.01), blood pressure values (p<0.05), and prevalence of fatty liver (p<0.01) and metabolic syndrome (p<0.05) than the control group. There were no significant differences in age, smoking, alcohol consumption and prevalence of gallstones between the GBPs and the control group. In addition, in terms of laboratory data, the fasting glucose levels were higher in the GBPs group (p< 0.05), but the HDL-C levels were lower in the GBPs group than in the control group (p< 0.01), and there were no statistical differences between the two groups on other laboratory data.
 |
Table 1 Univariate Comparison Between the GBPs and Control Groups |
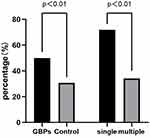 |
Figure 2 The prevalence of SIBO: comparison between GBPs and control groups, single and multiple polyps groups. |
The GBPs group was divided into two groups including single and multiple polyp groups. The characteristics of patients with single polyps and multiple polyps were compared in Table 2. The prevalence of SIBO (p<0.01) and gallstones (p<0.05) were higher in the group with single polyps than those with multiple polyps. The fasting glucose values were higher in the single polyp group (p<0.01).
 |
Table 2 Univariate Comparison Between the Single and Multiple Polyps Groups |
Multivariate regression analysis showed that male (OR=2.26, 95% CI=1.12–4.57, p=0.023), SIBO (OR=3.21, 95% CI=1.69–6.11, p<0.001), fatty liver (OR=2.91, 95% CI= 1.50–5.64, p=0.002) and BMI (OR=1.13, 95% CI=1.01–1.26, p=0.035) were independently associated with GBPs (Table 3). And fasting glucose (OR=3.04, 95% CI=1.27–7.28, p=0.013) and SIBO (OR=5.11, 95% CI=1.42–18.36, p=0.012) were independently associated with solitary polyps (Table 4).
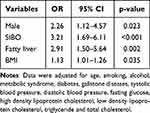 |
Table 3 Multivariate Analysis of Risk Factors Associated with GBPs |
 |
Table 4 Multivariate Analysis of Risk Factors Associated with Single Polyps |
Lastly, we further analyzed the association between SIBO and GBPs stratified for age, sex, and BMI using logistic regression (Table 5). In the subgroup analysis, we found a significantly stronger risk effect of SIBO in females than in males (OR = 3.87 vs 2.30, P for interaction< 0.001), but the effect did not modify by age (P for interaction= 0.70) or BMI (P for interaction= 0.22).
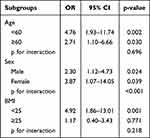 |
Table 5 Subgroup Analysis for Association Between SIBO and GBPs Stratified by Sex, Age and BMI |
Discussion
GBP is one of the most common gallbladder diseases, and its detection rate is increasing with the widely available imaging examinations. The risk factors associated with GBPs remain unclear, previous studies have suggested that the formation of GBPs is associated with several factors, such as male sex, BMI, fatty liver and metabolic syndrome. Gut microbiota is the largest micro-ecosystem in the human body and it plays an important role in regulating metabolism and maintaining the health of the body. SIBO is one of the most common forms of gut microbiota disorders.18 A bacterial colony count of more than 103 CFU/mL in small intestinal aspirates is considered the criteria for the diagnosis of SIBO,14 but this traditional diagnostic method is complex and painful for patients. The hydrogen-methane breath test has an acceptable level of accuracy, is simple and noninvasive, and is now widely used to diagnose SIBO in clinical practice. Increasing evidence reveals the role of SIBO in digestive diseases, however, the association between SIBO and gallbladder disease has not been fully investigated. To the best of our knowledge, the present study is the first to assess the association between GBPs and SIBO. We investigated the prevalence of SIBO in patients with GBPs and explored the association between these two conditions. The results showed a high prevalence of SIBO in patients with GBPs, and this association persisted after adjusting for potential confounders by multivariate regression analysis. In subgroup analysis, we found that the association between GBPs and SIBO was stronger in females than in males, although the reasons for this need to be further explored.
The pathogenesis of GBPs is complex and may involve a variety of factors, such as abnormal cholesterol metabolism and chronic inflammation. Excess cholesterol deposition on the gallbladder mucosa is one of the causes of the formation of GBPs, which can also promote gallbladder mucosal hyperplasia and impair the contractility of the gallbladder. Considering the antimicrobial properties of bile, the impaired gallbladder motility may contribute to the development of SIBO. In turn, SIBO can reduce the absorption of fat-soluble vitamins, such as vitamin D,19 and research has shown that vitamin D deficiency may further aggravate the cholestasis,20 thus starting a vicious cycle. In addition, the formation of GBPs may be associated with a disturbance of the bile acid pool21,which is common in SIBO. Previous studies have found that compared to SIBO-negative patients, the levels of unconjugated bile acids were higher in SIBO-positive patients.22,23 The possible explanation for that is the increased number of colon-specific bacteria in the small intestine under the condition of SIBO, which can lead to an increase in the deconjugation of bile acids. However, further studies are needed to elucidate the specific mechanisms of the association between SIBO and GBPs.
In the present study, male and BMI were independently associated with GBPs. And higher BMI appears to be related to SIBO.24 Previous studies have shown that distal small intestinal transit is slowed in obesity because of the presence of leptin resistance,25 and the reduced clearance capacity of the small intestine due to motility disorders is an important trigger for SIBO. The present study suggests that fatty liver is closely associated with GBPs, which is consistent with previous studies.9,26 The study by Ahn et al9 found a dose-dependent relationship between fatty liver and increased risk of GBPs, and that fatty liver may be a risk factor not only for cholesterol polyps but also for adenomatous gallbladder polyps. The association between SIBO and fatty liver has been reported several times. A meta-analysis including 10 studies showed that patients with SIBO had a significantly increased risk of developing fatty liver.27 The exact mechanism of the association between SIBO and fatty liver is still being explored, and it is now generally accepted that it may be related to the impaired intestinal barrier and increased translocation of bacteria and bacterial by-products in patients with SIBO.28 SIBO-positive patients have been shown to have a higher level of endotoxin and significantly increased expression of CD14, NF-κB and TLR4 compared to SIBO-negative patients.29 Notably, the production of various inflammatory cytokines can directly participate in the non-alcoholic steatohepatitis (NASH).29 In addition, this chronic inflammatory state can induce the insulin resistance, which is one of the important mechanisms of fatty liver.30
In the univariate analysis, we found that the prevalence of metabolic syndrome were higher in the GBPs group than in the control group, but this difference was no longer significant in the multivariate analysis, which we consider might be related to the small sample size. Previous studies have shown that metabolic syndrome is a risk factor for GBPs.31,32 Metabolic syndrome is a complex multifactorial metabolic disease that refers to a pathological state in which the body’s substances such as proteins, fats and carbohydrates are metabolically disordered. Studies suggested that there was an increased inflammatory response in metabolic syndrome patients, which may be associated with altered gut microbiota and increased penetration of impaired intestinal membranes by bacterial components.33 SIBO has been reported to be associated with the metabolic syndrome and its components. Fialho et al34 compared the prevalence of metabolic syndrome and its components in SIBO-positive versus SIBO-negative patients, they found that the prevalence of metabolic syndrome was higher in the SIBO-positive group than in the SIBO-negative group, and this association persisted after adjusting for confounding factors.
In the current study, reduced HDL-C was also a related factor for GBPs at univariate analysis, whereas not at multivariate analysis. Whether reduced HDL-C is a risk factor for GBPs is controversial. A study by Yang et al35 found that HDL-C was one of the risk factors for GBPs, and their results showed that subjects with lower HDL-C levels had a 3.346 times higher risk for having GBPs than control subjects. However, the study from Zheng et al36 found significant differences in LDL and cholesterol (TC) levels between the GBPs and control groups, and no differences in HDL and triglyceride (TG) levels.
We also found that SIBO and fasting glucose were associated with solitary polyps in this study (Table 4), but the mechanism of this association was not clear. Several previous studies reported that compared to benign polyps, malignant polyps were often solitary,37–39 although this view remains controversial. A recent study by Terzioglu et al40 also showed that isolated polyps were an independent predictor of neoplastic GBPs. However, for GBPs, it is difficult to make a definitive histological diagnosis except for postoperative pathological confirmation. Further prospective studies are needed to confirm whether SIBO is associated with the formation of neoplastic polyps or an increased risk of malignant transformation of the polyps.
There are still some limitations of this study. First, the breath test is not the gold standard for diagnosing SIBO, but it has an acceptable level of accuracy. Second, cross-sectional study prevents us from determining a causal link between SIBO and GBPs, and we could not ascertain the timing and sequence of the occurrence of SIBO and GBPs.
In conclusion, according to the above analysis, our study for the first time found an association between SIBO and GBPs, although this association may be a coincidental occurrence rather than a causal one. Further prospective studies are needed to verify these results and explain the exact mechanism. If confirmed, patients with GBPs, especially females, might benefit from the assessment of polyps and active control of their risk factors.
Data Sharing Statement
The data analyzed in this study can be obtained on reasonable request from the corresponding author.
Ethics Statement
This study was approved by the Ethics Committee of the Sixth Medical Center of the PLA General Hospital. Written informed consent was obtained from all subjects.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Capital Health Research and Development of Special Fund Program of China (grant number: 2020-2-5113) and the National Natural Science Foundation of China (grant number:82070553)
Disclosure
The authors declare that there are no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
1. McCain RS, Diamond A, Jones C, et al. Current practices and future prospects for the management of gallbladder polyps: a topical review. World J Gastroenterol. 2018;24(26):2844–2852.
2. Gautam A, Pandey A, Masood S, et al. Incidental gallbladder carcinoma in gallbladder polyps: challenges of gallbladder malignancy for an endemic population. Malays J Med Sci. 2021;28(1):27–34.
3. Wiles R, Thoeni RF, Barbu ST, et al. Management and follow-up of gallbladder polyps: joint guidelines between the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), European Association for Endoscopic Surgery and other Interventional Techniques (EAES), International Society of Digestive Surgery - European Federation (EFISDS) and European Society of Gastrointestinal Endoscopy (ESGE). Eur Radiol. 2017;27(9):3856–3866.
4. Foley KG, Lahaye MJ, Thoeni RF, et al. Management and follow-up of gallbladder polyps: updated joint guidelines between the ESGAR, EAES, EFISDS and ESGE. Eur Radiol. 2021;2021:1.
5. Babu BI, Dennison AR, Garcea G. Management and diagnosis of gallbladder polyps: a systematic review. Langenbecks Arch Surg. 2015;400(4):455–462.
6. Lu D, Radin R, Yung E, et al. Malignant transformation of a 5-mm gallbladder polyp over 2 years: a case report and review of current literature. Ultrasound Q. 2015;31(1):66–68.
7. Lin WR, Lin DY, Tai DI, et al. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: analysis of 34 669 cases. J Gastroenterol Hepatol. 2008;23(6):965–969.
8. Xu Q, Tao LY, Wu Q, et al. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB. 2012;14(6):373–381.
9. Ahn DW, Jeong JB, Kang J, et al. Fatty liver is an independent risk factor for gallbladder polyps. World J Gastroenterol. 2020;26(44):6979–6992.
10. Yamin Z, Xuesong B, Guibin Y, et al. Risk factors of gallbladder polyps formation in East Asian population: a meta-analysis and systematic review. Asian J Surg. 2020;43(1):52–59.
11. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–519.
12. Ghoshal UC, Baba CS, Ghoshal U, et al. Low-grade small intestinal bacterial overgrowth is common in patients with non-alcoholic steatohepatitis on quantitative jejunal aspirate culture. Indian J Gastroenterol. 2017;36(5):390–399.
13. Ghoshal UC, Shukla R, Ghoshal U. Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy. Gut Liver. 2017;11(2):196–208.
14. Pimentel M, Saad RJ, Long MD, et al. ACG clinical guideline: small intestinal bacterial overgrowth. Am J Gastroenterol. 2020;115(2):165–178.
15. Grundy SM. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421.
16. Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American Consensus. Am J Gastroenterol. 2017;112(5):775–784.
17. Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22(6):775–777.
18. Lema I, Araujo JR, Rolhion N, et al. Jejunum: the understudied meeting place of dietary lipids and the microbiota. Biochimie. 2020;178:124–136.
19. Miazga A, Osinski M, Cichy W, et al. Current views on the etiopathogenesis, clinical manifestation, diagnostics, treatment and correlation with other nosological entities of SIBO. Adv Med Sci. 2015;60(1):118–124.
20. Singla R, Dutta U, Aggarwal N, et al. Vitamin-D deficiency is associated with gallbladder stasis among pregnant women. Dig Dis Sci. 2015;60(9):2793–2799.
21. Wu L, Wang Y, Zhu S, et al. Changes in plasma bile acids are associated with gallbladder stones and polyps. BMC Gastroenterol. 2020;20(1):363.
22. Bolt MJ, Stellaard F, Sitrin MD, et al. Serum unconjugated bile acids in patients with small bowel bacterial overgrowth. Clin Chim Acta. 1989;181(1):87–101.
23. Bala L, Ghoshal UC, Ghoshal U, et al. Malabsorption syndrome with and without small intestinal bacterial overgrowth: a study on upper-gut aspirate using 1H NMR spectroscopy. Magn Reson Med. 2006;56(4):738–744.
24. Roland BC, Lee D, Miller LS, et al. Obesity increases the risk of small intestinal bacterial overgrowth (SIBO). Neurogastroenterol Motil. 2018;30(3):154.
25. Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med. 2013;1(2):14.
26. Lim SH, Kim D, Kang JH, et al. Hepatic fat, not visceral fat, is associated with gallbladder polyps: a study of 2643 healthy subjects. J Gastroenterol Hepatol. 2015;30(4):767–774.
27. Wijarnpreecha K, Lou S, Watthanasuntorn K, et al. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32(5):601–608.
28. Kanda T, Goto T, Hirotsu Y, et al. Molecular mechanisms: connections between nonalcoholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int J Mol Sci. 2020;21(4):57.
29. Kapil S, Duseja A, Sharma BK, et al. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31(1):213–221.
30. Vanni E, Bugianesi E. The gut-liver axis in nonalcoholic fatty liver disease: another pathway to insulin resistance? Hepatology. 2009;49(6):1790–1792.
31. Park EJ, Lee HS, Lee SH, et al. Association between metabolic syndrome and gallbladder polyps in healthy Korean adults. J Korean Med Sci. 2013;28(6):876–880.
32. Choi YS, Do JH, Seo SW, et al. Prevalence and risk factors of gallbladder polypoid lesions in a healthy population. Yonsei Med J. 2016;57(6):1370–1375.
33. Remely M, Aumueller E, Jahn D, et al. Microbiota and epigenetic regulation of inflammatory mediators in type 2 diabetes and obesity. Benef Microbes. 2014;5(1):33–43.
34. Fialho A, Fialho A, Thota P, et al. Higher visceral to subcutaneous fat ratio is associated with small intestinal bacterial overgrowth. Nutr Metab Cardiovasc Dis. 2016;26(9):773–777.
35. Yang HL, Kong L, Hou LL, et al. Analysis of risk factors for polypoid lesions of gallbladder among health examinees. World J Gastroenterol. 2012;18(23):3015–3019.
36. Yamin Z, Xuesong B, Zhen Z, et al. Correlation of dyslipidemias and gallbladder polyps-A large retrospective study among Chinese population. Asian J Surg. 2020;43(1):181–185.
37. Shinkai H, Kimura W, Muto T. Surgical indications for small polypoid lesions of the gallbladder. Am J Surg. 1998;175(2):114–117.
38. Yeh CN, Jan YY, Chao TC, et al. Laparoscopic cholecystectomy for polypoid lesions of the gallbladder: a clinicopathologic study. Surg Laparosc Endosc Percutan Tech. 2001;11(3):176–181.
39. Kwon W, Jang JY, Lee SE, et al. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24(3):481–487.
40. Terzioglu SG, Kilic MO, Sapmaz A, et al. Predictive factors of neoplastic gallbladder polyps: outcomes of 278 patients. Turk J Gastroenterol. 2017;28(3):202–206.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
