Back to Journals » Journal of Inflammation Research » Volume 15
Slightly Elevated Lymphocyte to Monocyte Ratio Predicting Favorable Outcomes in Patients with Spontaneous Intracerebral Hemorrhage
Authors Wang J, Wang W, Wang A, Zhang X, Bian L, Du Y , Lu J, Zhao X
Received 20 September 2022
Accepted for publication 1 December 2022
Published 16 December 2022 Volume 2022:15 Pages 6773—6783
DOI https://doi.org/10.2147/JIR.S390557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Jinjin Wang,1,2,* Wenjuan Wang,1,2,* Anxin Wang,1,2 Xiaoli Zhang,1,2 Liheng Bian,1,2 Yang Du,1,2 Jingjing Lu,1,2 Xingquan Zhao1– 4
1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2China National Clinical Research Center for Neurological Diseases, Beijing, People’s Republic of China; 3Research Unit of Artificial Intelligence in Cerebrovascular Disease, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 4Beijing Institute of Brain Disorders, Collaborative Innovation Center for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xingquan Zhao; Jingjing Lu, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, No. 119 South 4th Ring West Road, Fengtai District, Beijing, 100070, People’s Republic of China, Tel +86-10-59978555, Fax +86-10-83191171, Email [email protected]; [email protected]
Objective: This study was designed to determine the association between admission lymphocyte to monocyte ratio (LMR) values and clinical outcomes in patients with spontaneous intracerebral hemorrhage (ICH).
Methods: We used a prospective and registry-based database, and ICH patients were consecutively recruited in Beijing Tiantan Hospital between January 2014 and September 2016. All participants were stratified by quartiles of the LMR. Univariable and multivariable logistic regression analyses were plotted to evaluate the association between LMR levels and functional outcomes. Kaplan–Meier survival curves and Cox regression analysis were also performed to examine the relevance between different LMR quartiles and case fatality at follow-up.
Results: Six hundred and forty patients with spontaneous ICH were finally included in this study. Compared with the patients with LMR values in quartile 1 (Q1), slightly elevated LMR values showed a negative correlation with risks of poor short-term outcomes (adjusted ORs in Q2 were 0.572 [95% CI: 0.338– 0.968] at 1 month, 0.515 [95% CI: 0.305– 0.871] at 3 months). Patients with LMR values in Q1 had the highest cumulative death rate. A slightly elevated LMR was also independently relevant to a deduced mortality rate compared to that in Q1 (adjusted HRs in Q2 were 0.471 [95% CI: 0.274– 0.809] at 1 month, 0.474 [95% CI: 0.283– 0.793] at 3 months, 0.575 [95% CI: 0.361– 0.917] at 1 year). Additionally, a higher LMR value was associated with a lower risk of in-hospital infections.
Conclusion: This study suggests that a lower LMR value is associated with higher risks of in-hospital infections, poor functional outcomes, and follow-up mortality in patients with ICH. However, a slightly elevated LMR value, especially in Q2, relates to a favorable prognosis, which may reflect an inner balance between inflammation and immunodepression and thus provides a promising marker for predicting ICH prognosis.
Keywords: lymphocyte to monocyte ratio, outcomes, in-hospital infections, spontaneous intracerebral hemorrhage
Introduction
Intracerebral hemorrhage (ICH) is one of the main stroke subtypes, with a disproportionately high mortality rate in the acute stage and a significant morbidity rate among survivors.1,2 In China, the reported prevalence of ICH was 14.2% among 3,411,168 stroke patients admitted in 1672 tertiary public hospitals, while 82.6% of the population were diagnosed with ischemic stroke (IS).3 In China, the incidence and related mortality rates have fallen dramatically in the last 30 years. However, ICH still processed a noticeably higher proportion of discharges against medical advice (DAMA) or death, more extended stay, and higher total hospital bill fees than IS. Therefore, it is pivotal to develop new research for emerging treatments to improve prognosis and establish a dependable prognostic model to aid decision-making. A reliable prognostic model could help differentiate the risk of patients with ICH, design individual management, and predict accurate recovery.
Inflammation plays a vital role in the pathophysiological process of ICH.4 Once hemorrhagic stroke occurs, blood flows into the brain parenchyma and then triggers a cascade of inflammatory reactions, including activating inflammatory cells and releasing inflammatory factors. In the peripheral system, neutrophils and monocytes are rapidly activated and infiltrate into perihematomal tissue via the broken blood-brain barrier (BBB).5 Accumulating evidence has suggested that leukocyte count is a widely acknowledged biomarker for predicting ICH prognosis.6,7 In addition, immune response, especially immunosuppression, exerts a protective effect on restraining the overwhelming systemic inflammation characterized by lymphopenia.8 Current data also support an increased risk of infections and unfavorable prognosis in patients with lymphopenia.9,10 Several composite prognostic scores based on peripheral leukocyte counts have been proven to identify patients at high risks of unfavorable outcomes or death, for example, neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte count (PLR).11,12
Recent research has demonstrated that lymphocyte to monocyte ratio (LMR), an index reflecting inflammatory and immune balance, performs better in predicting prognosis in patients with acute ischemic stroke.13–15 However, little is known about the relevance of LMR and ICH prognosis. Thus, this study primarily aimed to determine the association between LMR values and clinical outcomes in patients with ICH at 1 month and 3 months. Besides, we designed this study to investigate the predictive value of LMR values for in-hospital infections and long-term prognosis at 1 year.
Methods
Study Populations
In this study, we used the data from a prospective and registry-based database that consecutively recruited patients diagnosed with acute ICH in Beijing Tiantan Hospital between January 2014 and September 2016. The protocol was approved by the Institutional Review Board (IRB) of Beijing Tiantan Hospital, Capital Medical University (IRB No. KY2014-023-02). All researchers performed this study in full accordance with the Declaration of Helsinki. Before participating in this study, each patient or their legal relatives must sign the informed content.
According to WHO standards, this study included ICH patients who arrived at the emergency room within 72 hours of symptoms onset.16 The exclusion criteria included: (1) age <18 years old; (2) known major comorbidities or late-stage diseases, such as malignant tumors, heart failure, and liver failure; (3) secondary ICH with causes including arteriovenous malformation, trauma, aneurysm, and coagulation disorders; (4) abnormal values of hematoma volumes and blood cell counts; and (5) missing inflammatory indexes or/and follow-up outcomes at 3 months.
Baseline Clinical and Imaging Characteristics Collection
Study investigators recorded baseline characteristics according to a standardized questionnaire, including age, sex, medical history, medication history, current smoking and drinking, and other clinical information, as previously reported.17 Trained researchers assessed and noted conscious status using the Glasgow Coma Scale (GCS) on admission. Blood pressure and other vital signs were also documented by searching emergency notes.
Before participating in this study, each patient had undergone a non-contrasted computed tomography (NCCT) scan according to standard parameters. The coordinating center centrally gathered imaging data in uncompressed DICOM formats. Finally, two experienced investigators analyzed ICH imaging data separately, including hematoma location (supratentorial or infratentorial hemorrhage), hematoma volume, and intraventricular hemorrhage (IVH). The hematoma volume was calculated by the ABC/2 method.18 These investigators were blinded to any other clinical data of included patients. ICH Score, a simple and composite clinical grading scale, was plotted to evaluate ICH severity on admission (Figure 1).19
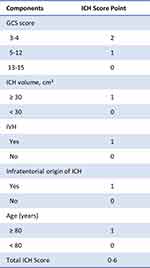 |
Figure 1 Components of ICH Score. Reproduced from Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. Open Access.19 Abbreviations: ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; IVH, intraventricular hemorrhage. |
Laboratory Data and Definition of LMR
Blood samples were taken from the median cubital vein and assayed in qualified laboratories to measure various serum indices. Blood cell counts, such as WBC counts, neutrophil counts, monocyte counts, lymphocyte counts, and platelet counts, were evaluated through a blood routine examination. The formula for calculating LMR is as follows: LMR = lymphocyte counts/monocyte counts. In addition to the baseline LMR value obtained within 72 hours (T1), we also recorded other LMR values assessed during the first week (T2) and the second week (T3).
Follow-Up and Outcomes Assessment
The modified Rankin Scale (mRS) score was used to evaluate the functional status of patients. An unfavorable outcome was defined as a modified Rankin Scale (mRS) score ≥4, usually representing a severe physical disability or death following ICH. At discharge, investigators conducted a face-to-face interview to assess the neurological deficits and functional states. We also recorded the co-existing infections by a full review of medical notes and discharge reports, which included in-hospital pneumonia and urinary tract infections that most commonly occurred after stroke and accounted for ≈10% of the patients.20 The diagnosis of complicated infections was identified based on previously established criteria.21,22 Researchers conducted telephone interviews to determine their functional status at 1 month, 3 months, and 1 year of ICH onset. All-cause deaths and corresponding data would be further recorded.
Statistical Analysis
SAS software (Version 9.4; SAS Institute, Cary, NC, USA) was used for statistical analysis in this study. All continuous variables were shown as medians (interquartile range [IQR]). These factors did not coincide with normal distribution and were compared by the Kruskal–Wallis test to determine intergroup differences. Categorical variables were presented as counts (percentage [%]), and the Chi-square test was used to compare groups. We conducted a univariable and multivariable-adjusted logistic regression analysis to analyze the association between different quartiles of LMR and composite ICH prognosis. Model 1 was adjusted for baseline epidemiological factors: age and sex. Model 2 was adjusted for age, sex, and other variables with statistical significance (p values <0.05) in the univariable analysis, including admission hematoma volume, GCS score, and IVH. Given ICH score is a composite index for ICH severity which contains GCS score, hematoma volume, hematoma location, and IVH, model 3 was adjusted for age, sex, and ICH score. Results are presented as odds ratio [OR], adjusted OR [aOR], and 95% confidence interval [CI]. The receiver operating characteristics (ROC) curve was plotted to test the discriminatory ability of LMR and ICH Score, combined or separately, to predict infections or unfavorable outcomes by calculating C-statistics (areas under ROC curves). Kaplan–Meier (K-M) survival analysis was also performed to analyze the association between different quartiles of LMR and mortality rate at 3 months. Cox regression analysis was also used to analyze the relevance between LMR and follow-up death rate. A two-tailed p-value <0.05 was considered statistically significant.
Results
A total of 640 patients were finally included in the study after excluding 593 patients according to the abovementioned criteria (Figure 2). The cohort was median 56.0 (48.0–64.0) years old, and 71.4% (457) were male patients. LMR’s median (IQR) value was 3.22 (2.17–4.39). All participants had a median ICH Score of 1.0 (0–2.0). Baseline characteristics among groups are summarized in Table 1. All patients were divided into four groups by the quartiles of LMR values. Among the four groups, there was a significant difference in the distribution of age and sex (p values <0.05). Regarding baseline ICH characteristics, there were larger hematoma volumes, higher proportions of IVH, and more severe ICH conditions in patients with LMR values in quartile 1 (Q1, all p values <0.05). Accordingly, leukocyte counts also showed a significant difference among four quartiles of patients (p values <0.05).
 |
Table 1 Baseline Characteristics of All Study Patients Stratified by the Quartiles of LMR |
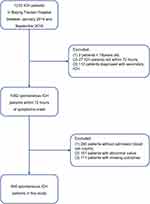 |
Figure 2 The flowchart of this study. Abbreviation: ICH, intracerebral hemorrhage. |
We also observed the time course of LMR values in patients with ICH (Figure 3). It was notably lower within the first week in patients with an ICH Score ≥3 (p values <0.05). Patients with in-hospital infections had a continuously decreased LMR value within two weeks after the onset of ICH symptoms (all p values <0.05). When stratifying patients by 1-month mRS score, we got a similar tendency to infections (all p values <0.05).
During the hospitalization, 219 (34.2%) patients were diagnosed with pneumonia or/and urinary infection. During a 1-month follow-up period, there were 353 (55.2%) patients with an mRS score ≥4, while the number was 266 (41.6%) at 3 months. In the univariable logistic regression analysis, higher LMR values were associated with a decreased risk of poor outcomes when LMR in Q1 was taken for reference. In model 1, we found similar results to the crude model. In model 2, no association was observed between higher LMR values and poor outcomes. After adjusting age, sex, and ICH Score in model 3, slightly elevated LMR values were independent predictors for reduced risks of unfavorable ICH prognosis (at 1 month: aOR [95% CI] in Q2, 0.572 [0.338–0.968]; at 3 months: aOR [95% CI] in Q2, 0.515 [0.305–0.871], aOR [95% CI] in Q3, 0.576[0.338–0.980]). However, this relevance was not found at 1 year. Compared with the LMR in Q1, higher LMR values were associated with a decreased risk of complicated infections, whether in the crude model or adjusted models (Model 3: aOR [95% CI] in Q2, 0.518 [0.319–0.842]; aOR [95% CI] in Q3, 0.398 [0.239–0.664]; aOR [95% CI] in Q4, 0.435 [0.264–0.718]. Table 2). Then, ROC curves were further used to compare the predictive ability of combined LMR and ICH Score, LMR, or ICH Score alone in predicting endpoint events (Supplementary Figure 1). C-statistic was significantly higher for the combined effect of LMR and ICH Score than LMR or ICH Score alone for in-hospital infections (all p values <0.05). We also observed a higher prognostic utility of combined LMR and ICH Score for poor outcomes at 1 month and 3 months, but these results were not statistically significant.
 |
Table 2 Univariable and Multivariable-Adjusted and/or 95% CI for Clinical Outcomes According to the Quartiles of LMR |
At 1 month of ICH onset, 120 (18.8%) patients died from all causes, increasing to 128 (20.0%) at 3 months. K-M curves indicated the highest death rate in patients with lower LMR values at 1 month and 3 months, compared to other quartiles (all p values <0.001, Figure 4). In the univariable Cox regression analysis, mildly higher LMR values in Q2 and Q3 were related to a declining mortality rate in contrast to Q1. There remained this relationship in model 1. In model 2, patients with LMR values in Q2 had a smaller proportion of death, only at 1 month (aHR [95% CI], 0.501 [0.256–0.979]). In model 3, we also found an independent association between LMR values in Q2 and a dropped fatality rate (at 1 month: aHR [95% CI], 0.471 [0.274–0.809]; at 3 months: aHR [95% CI], 0.474[0.283–0.793]; at 1 year: aHR [95% CI], 0.575 [0.361–0.917]. Table 3).
 |
Table 3 Univariable and Multivariable-Adjusted HR and 95% CI for Deaths According to the Quartiles of LMR |
 |
Figure 4 Cumulative incidence of follow-up mortality among ICH patients stratified by quartiles of LMR. (A) at 1 month; (B) at 3 months. Abbreviation: LMR, lymphocyte to monocyte ratio. |
Discussion
In this study, we found that (1) patients with lower LMR values had a more severe ICH condition on admission and a larger proportion of poor outcomes at follow-up; (2) a higher LMR value was associated with a reduced risk of infections during hospitalization; (3) a slightly elevated LMR value in Q2 was an independent predictor for favorable outcomes, compared to that in Q1; (4) K-M curves showed a higher cumulative incidence of death in patients with lower LMR values in Q1, whereas a lowest cumulative death rate in patients with a slightly elevated LMR value in Q2. These results showed an independent association between slightly LMR values and favorable outcomes in patients with ICH. Therefore, there is a potential for LMR to be used as a prognostic biomarker for ICH prognosis.
LMR, a composite index containing lymphocyte and monocyte count, has been wildly acknowledged as a reliable predictor for post-stroke prognosis. In a retrospective study of 121 patients undergoing mechanical thrombectomy (MT) after acute ischemic stroke (AIS), a lower LMR value at 24h post-MT was significantly associated with unfavorable outcomes.15 Gong reported an association between LMR and early neurological deterioration (END) in 1060 patients treated by intravenous thrombolysis.23 Admission LMR also performed well in predicting hemorrhagic transformation in AIS patients.13 In addition, the monocyte to lymphocyte ratio, a reverse ratio of LMR, was indicated to have a prognostic ability for stroke-associated pneumonia (SAP).24 However, limited research investigated the predictive value of LMR for ICH prognosis. A small-sample and retrospective study showed LMR as a predictor for ND and 90-day mortality.25 Our results are partly consistent with previous studies. This study observed a promising association between favorable outcomes and slightly elevated LMR value. It might represent an inner balance between immunosuppression and inflammation in the pathophysiological process of the disease.4
ICH is caused by the ruptured blood vessel bursting in the brain parenchyma and ensuing hematoma formation, then activating innate immunity. Various innate immune cells are stimulated and release large amounts of cell factors, contributing to harmful inflammatory effects of hemorrhagic injury after stroke. Many peripheral blood cell subtypes exert an extremely vital role in brain injury and functional recovery after ICH.26 Adaptive immune system is initiated to maintain homeostasis against post-stroke inflammation, including stroke-induced immunosuppression (SIIS).27 One of the main characteristics is a shift of lymphocyte phenotypes from T-helper (Th) 1 to a Th2, contributing to SIIS marked from a pro-inflammatory reaction to an anti-inflammatory response. Another trait of SIIS is splenic impairment, mainly including lymphocytopenia in blood, spleen, and lymph nodes.28 Therefore, stroke patients are more susceptible to stroke-associated infections, especially with certain clinical factors like dysphagia and being bedridden.20,28 ln patients with ICH, the percentage of lymphocytes was lower than in healthy controls.29 Clinical evidence has demonstrated an association between admission lymphopenia and increased risks of infectious complications or poor outcomes in patients with ICH.9,10
As for monocytes in the peripheral system, their activation is one of the most prominent characteristics after ICH. Monocyte response is complex and heterogeneous: it regulates inflammatory pathways and is associated with the repair.30 However, the traditional blood routine test could not identify the monocyte subtypes in the clinical laboratories. Monocyte-derived macrophages carry out a phagocytic function in the subacute stage and contribute to endogenous blood clearance at a later time window.5,31 A recent study using bioinformatics approaches also showed a decreased proportion of T lymphocytes while the percentage of monocytes increased in the early stage after ICH.5 Data from Walsh have supported that higher admission monocyte count was independently associated with 30-day case-fatality.7 A preclinical study also demonstrated that CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbated acute disability following ICH.32 In our present study, there were the lowest lymphocyte count and the highest monocyte count in patients with lower LMR values in Q1. LMR integrates lymphocyte and monocyte, reflecting a composite status on monocyte-related inflammation and lymphopenia-related immunodepression. Patients with lower LMR values showed a larger proportion of in-hospital infections, poor outcomes, and death during the follow-up periods.
We also found an association between higher LMR values and a decreased risk of infections during hospitalization, which is in line with previous studies.24,33 These findings provide a surrogate biomarker of the immune response induced by local cerebral hematoma and systemic inflammation, reflecting the likelihood of secondary brain injury and vulnerability to post-stroke infections. Accordingly, the early decrease of LMR values is driven by lymphocyte reduction and/or monocyte rise, which can enhance the reliable prediction of the risks of complicated infections, short-term mortality, and adverse functional outcomes. As a parameter integrating the innate and adaptive immunity compartments, a slightly elevated LMR value might represent a reasonable balance of the inner immune system, which restrains overwhelming proinflammatory injury and reduces immunosuppression-induced infections.
Besides, this study indicated a continuously lower LMR value in patients with infections or poor outcomes. Previous research has pointed out that ICH patients with permanent lymphocytopenia showed an even worse outcome, whereas lymphocyte counts returned to normal within 24 hours.9 In this respect, LMR may serve as an effective index for reflecting and synthesizing the inflammatory response related to the ICH course.
One of the key strengths of this study is its relatively large sample and prospective study design. Other strengths include a long-term follow-up period. We used clinical data to determine the prognostic value of LMR on admission, a serum index calculated by total blood cell counts based on standard laboratory measurements. It is routinely evaluated and easily obtained in the clinical setting, reflecting an inner balance between inflammation and immunodepression in the peripheral blood system. We also found an association between LMR values and risks of in-hospital infections after ICH, which few reports have clarified. Besides, we observed and compared the time course of the LMR at different endpoints, reflecting a dynamic concentration of blood cells and immune status.
However, our study has several limitations that we must admit. Firstly, the study used clinical data from a single center; this design may lead to a selection bias, although the hospital is a tertiary medical institution with mounting ICH patients. Secondly, our research showed an association between LMR and ICH prognosis, which is easily affected by numerous factors, such as the time to draw blood, current comorbidities, and drug usage. Thirdly, this study recruited ICH patients within 72 hours of symptoms onset, causing an omission of patients with severe hemorrhage, especially for ICH in the ultra-acute stage. Thus, it is worth noticing that the conclusion is more applied to populations with mild to moderate ICH when interpreting the results of this study.
Conclusion
In summary, this study suggests an independent association between admission LMR and short-term outcomes in patients with ICH, especially for mild to moderate ICH. A lower level of LMR value was associated with more severe ICH conditions on admission and an increased risk of case fatality at follow-up. In contrast, a slightly higher LMR value was an independent predictor for favorable short-term outcomes and decreased rates of in-hospital infections. As a readily obtained laboratory parameter, LMR is easily available and cost-effective, and it is widely acknowledged and usually used in routine medical practice. Thus, LMR has the potential to serve as a promising marker for predicting ICH prognosis, aiding early prognosis prediction, and making related decisions to improve outcomes in future clinical practice.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy/ethical restrictions.
Ethical Standards
The study protocol was approved by the Institutional Review Board (IRB) of Beijing Tiantan Hospital, Captial Medical University (IRB No. KY2014-023-02). Written informed consents were obtained from all participants or their legal relatives. The study was performed in full accordance with the Declaration of Helsinki.
Funding
The research was supported by the National Key Research and Development Program of the People’s Republic of China (Grant No.2018YFC1312200/2018YFC131224 & 2018YFC1705003), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-029) and Beijing Municipal Committee of Science and Technology (Z201100005620010).
Disclosure
All authors have no conflicts of interest to declare in this work.
References
1. O’Carroll CB, Brown BL, Freeman WD. Intracerebral hemorrhage: a common yet disproportionately deadly stroke subtype. Mayo Clinic Proceed. 2021;96:1639–1654. doi:10.1016/j.mayocp.2020.10.034
2. Feigin VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi:10.1016/S1474-4422(21)00252-0
3. Wang YJ, Li ZX, Gu HQ, et al. China stroke statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vascular Neurol. 2022;7:5. doi:10.1136/svn-2021-001374
4. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi:10.1016/S1474-4422(12)70104-7
5. Mei S, Shao Y, Fang Y, et al. The changes of leukocytes in brain and blood after intracerebral hemorrhage. Front Immunol. 2021;12:617163. doi:10.3389/fimmu.2021.617163
6. Gusdon AM, Thompson CB, Quirk K, et al. CSF and serum inflammatory response and association with outcomes in spontaneous intracerebral hemorrhage with intraventricular extension: an analysis of the CLEAR-III Trial. J Neuroinflammation. 2021;18:179. doi:10.1186/s12974-021-02224-w
7. Walsh KB, Sekar P, Langefeld CD, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke. 2015;46:2302–2304. doi:10.1161/STROKEAHA.115.009880
8. Saand AR, Yu F, Chen J, Chou SHY. Systemic inflammation in hemorrhagic strokes - A novel neurological sign and therapeutic target? J Cereb Blood Flow Metab. 2019;39:959–988. doi:10.1177/0271678X19841443
9. Giede-Jeppe A, Bobinger T, Gerner ST, et al. Lymphocytopenia is an independent predictor of unfavorable functional outcome in spontaneous intracerebral hemorrhage. Stroke. 2016;47:1239–1246. doi:10.1161/STROKEAHA.116.013003
10. Morotti A, Marini S, Jessel MJ, et al. Lymphopenia, infectious complications, and outcome in spontaneous intracerebral hemorrhage. Neurocrit Care. 2017;26:160–166. doi:10.1007/s12028-016-0367-2
11. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Translational Stroke Res. 2019;10:137–145. doi:10.1007/s12975-018-0649-4
12. Pikija S, Sztriha LK, Killer-Oberpfalzer M, et al. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J Neuroinflammation. 2018;15:319. doi:10.1186/s12974-018-1359-2
13. Song Q, Pan R, Jin Y, et al. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol Sci. 2020;41:2511–2520. doi:10.1007/s10072-020-04355-z
14. Ren H, Liu X, Wang L, Gao Y. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:2595–2602. doi:10.1016/j.jstrokecerebrovasdis.2017.06.019
15. Lux D, Alakbarzade V, Bridge L, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. 2020;17:60. doi:10.1186/s12974-020-01739-y
16. Greenberg SM, Ziai WC, Cordonnier C, et al. Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2022;53 :e282–e361. doi:10.1161/STR.0000000000000407
17. Wang D, Wang W, Wang A, Zhao X. Association of severity and prognosis with elevated homocysteine levels in patients with intracerebral hemorrhage. Front Neurol. 2020;11:571585. doi:10.3389/fneur.2020.571585
18. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi:10.1161/01.STR.27.8.1304
19. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi:10.1161/01.STR.32.4.891
20. Westendorp WF, Dames C, Nederkoorn PJ, Meisel A. Immunodepression, infections, and functional outcome in ischemic stroke. Stroke. 2022;53:1438–1448. doi:10.1161/STROKEAHA.122.038867
21. Johnson JR. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;39:873. doi:10.1086/423844
22. Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. 2015;46:2335–2340. doi:10.1161/STROKEAHA.115.009617
23. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18:51. doi:10.1186/s12974-021-02090-6
24. Cao F, Wan Y, Lei C, et al. Monocyte-to-lymphocyte ratio as a predictor of stroke-associated pneumonia: a retrospective study-based investigation. Brain Behav. 2021;11:e02141. doi:10.1002/brb3.2141
25. Qi H, Wang D, Deng X, Pang X. Lymphocyte-to-monocyte ratio is an independent predictor for neurological deterioration and 90-day mortality in spontaneous intracerebral hemorrhage. Med Sci Monit. 2018;24:9282–9291. doi:10.12659/MSM.911645
26. Xue M, Yong VW. Neuroinflammation in intracerebral haemorrhage: immunotherapies with potential for translation. Lancet Neurol. 2020;19:1023–1032. doi:10.1016/S1474-4422(20)30364-1
27. Jiang Q, Stone CR, Elkin K, Geng X, Ding Y. Immunosuppression and neuroinflammation in stroke pathobiology. Exp Neurobiol. 2021;30:101–112. doi:10.5607/en20033
28. Faura J, Bustamante A, Miró-Mur F, Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflam. 2021;18:127. doi:10.1186/s12974-021-02177-0
29. Jiang C, Wang Y, Hu Q, et al. Immune changes in peripheral blood and hematoma of patients with intracerebral hemorrhage. FASEB J. 2020;34:2774–2791. doi:10.1096/fj.201902478R
30. Cormican S, Griffin MD. Human monocyte subset distinctions and function: insights from gene expression analysis. Front Immunol. 2020;11:1070. doi:10.3389/fimmu.2020.01070
31. Puy L, Perbet R, Figeac M, et al. Brain peri-hematomal area, a strategic interface for blood clearance: a human neuropathological and transcriptomic study. Stroke. 2022;53:2026–2035. doi:10.1161/STROKEAHA.121.037751
32. Hammond MD, Taylor RA, Mullen MT, et al. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–3909. doi:10.1523/JNEUROSCI.4070-13.2014
33. Cheng HR, Song JY, Zhang YN, et al. High monocyte-to-lymphocyte ratio is associated with stroke-associated pneumonia. Front Neurol. 2020;11:575809. doi:10.3389/fneur.2020.575809
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

