Back to Journals » Nature and Science of Sleep » Volume 12
Sleep Time Duration Does Not Affect Oral Inflammation and Periodontal Health Status in Night-Shift Workers: A Cross-Sectional Study
Authors Roestamadji RI, Luthfi M, Surboyo MDC , Rumokoi RB, Khotimah FK
Received 15 September 2020
Accepted for publication 2 November 2020
Published 27 November 2020 Volume 2020:12 Pages 1083—1090
DOI https://doi.org/10.2147/NSS.S279088
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Steven A Shea
This paper has been retracted.
Retno Indrawati Roestamadji, 1 Muhammad Luthfi, 1 Meircurius Dwi Condro Surboyo, 2 Rauhansen Bosafino Rumokoi, 3 Fridaniyanti Khusnul Khotimah 3
1Department of Oral Biology, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia; 2Department of Oral Medicine, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia; 3Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia
Correspondence: Retno Indrawati Roestamadji
Department of Oral Biology, Faculty of Dental Medicine, Universitas Airlangga, Jln. Prof. Dr. Moestopo No. 47, Surabaya 60132, Indonesia
Email [email protected]
Background: Night-shift workers experience circadian rhythm disruption, changes in sleep time duration, and effects on their eating habits. All these factors may be related to the release of inflammatory mediators and may affect oral inflammation and periodontal health status. The objective of this study was to analyze the effects of sleep time duration on oral inflammation and periodontal health status in night-shift workers and non-night-shift workers.
Methods: This study involved two groups with 27 participants each: one group of night-shift workers and one group of non-night-shift workers. Examination of depth of pocket and bleeding on probing (BOP) was conducted with a periodontal probe. Non-stimulating saliva samples were collected to analyze the levels of melatonin, malondialdehyde (MDA), and tumor necrosis factor α (TNF-α) using ELISA. Comparisons for each parameter were performed using independent t-tests, and the relationships between duration of sleep and depth of pocket, BOP, salivary melatonin, MDA, and TNF-α were calculated using linear regression.
Results: The night-shift worker group had a short sleep time duration (p = 0.000). The salivary melatonin level of the night-shift workers was lower than that of the non-night-shift workers (p = 0.000). MDA, depth of pocket, and BOP were higher in the night-shift workers (p = 0.000). Only salivary melatonin showed a correlation with sleep time duration in the night-shift worker group (p < 0.05). Neither subject group showed an effect of sleep time duration on depth of pocket, BOP, salivary melatonin, MDA, or TNF-α (p > 0.05).
Conclusion: Night-shift workers showed higher rates of oral inflammation and periodontal health status, but there was no relationship between these factors and sleep time duration.
Keywords: night-shift worker, oral inflammation, periodontal health status, sleep time duration, melatonin, malondialdehyde
Introduction
Circadian rhythms are human physiological rhythms that regulate cycles in the body across a 24-hour period,1 including the dark–light cycle and the sleep–wake cycle, which are influenced by light.2 Light passes through the retinal–hypothalamic pathway to the suprachiasmatic nucleus. This brain area regulates the pineal gland’s production of the circadian neurohormone melatonin (N-acetyl-5-methoxytryptamine), which is secreted in large amounts at night and very small amounts during the day. Melatonin provides coordination of physiological functions including the sleep–wake cycle, food and water intake, hormone secretion, and metabolism. Changes in light intensity, duration, and spectral quality at certain times, such as those that occur in night-shift workers who are exposed to light during night-time hours, acutely suppress melatonin levels and can cause various diseases.3
Melatonin secretion levels can be enhanced in night-shift workers who are exposed to low-intensity light such as that emitted by technological devices including light-emitting diodes (LEDs), computer or television screens, and cellphones during night-shift working.4 These workers not only receive light exposure during working but also experience changes in their food and drink intake habits. Food and drink intake changes result from increased levels of ghrelin and decreased levels of leptin hormones, which can increase appetite; this occurs because the workers are still awake during the night and sometimes suffer from lack of sleep.5 Therefore, there is evidence that night-shift workers are at higher risk of developing diabetes because they have a higher body mass index (BMI), which can lead to obesity.6 This increased BMI can also contribute to a decreased salivary flow rate and an increased incidence of dental caries.7
Melatonin provides highly effective free-radical scavengers, protects cells from inflammatory processes, and reduces oxidative damage8 by scavenging reactive oxygen species (ROS) and reactive nitrogen species (RNS),9 and provides a protective effect by decreasing malondialdehyde (MDA), lipid peroxidation,10,11 and inhibiting cytokine production, especially tumor necrosis factor α (TNF-α).12 The MDA level in the circulation is also reflected in the salivary MDA level, which has a relationship with an increase of ROS activity in some oral diseases.13 It is also postulated that there is a relationship between salivary melatonin and salivary MDA.
Night-shift workers are at risk for fatigue, anxiety, and sleep disturbance. These conditions could exacerbate systemic inflammation and oxidative stress, which are risk factors for diabetes and periodontitis. Furthermore, periodontitis may portend systemic inflammation and oxidative stress, as locally produced pro-inflammatory cytokines from the periodontal tissue can spread to target organs via systemic circulation.14 Sleep disturbance and sleep time duration in night-shift workers may thus have a relationship with periodontal health status, and diabetes may modify this association.15
No previous evidence-based data exists regarding the effects of sleep time duration on oral inflammation and periodontal health status in night-shift workers. On this basis, an analysis of sleep time duration in night-shift workers in relation to salivary melatonin and its effects on salivary MDA and salivary TNF-α as oral inflammation indicators, together with depth of pocket and bleeding on probing (BOP) as periodontal health status indicators, need to be performed.
Materials and Methods
Ethical Approval
This study was conducted following the ethical standard and complied with the Declaration of Helsinki. Ethical approval was granted by the Universitas Airlangga Hospital (registration number 184/KEH/2018). The research was carried out as an observational analytical study. After receiving written and oral information about the study, all participants signed written informed consent forms.
A power calculation was used to determine group size based on an average difference in sleep time duration, depth of pocket, BOP, and salivary melatonin, MDA, and TNF-α levels between subject assuming a medium effect size (d = 0.12, p = 0.5, respectively) n = 27 would be required to achieve significance (p < 0.05) using an ANOVA test (95% statistical power).
Subject
The subjects of this study met the criteria of being male, being aged 26–50 years old, not having a smoking habit, and having worked for at least 1 year. All the subject originates from the same hospital. The exclusion criteria were patients with orthodontic treatment or a history of tuberculosis, hepatitis B, human immunodeficiency virus (HIV) infection, influenza, pneumonia, or diabetes mellitus. The selected subjects were then divided into two groups. The workers in the first group worked night shifts periodically, while those in the second group did not work night shifts.
Sleep Time Duration
The subjects were interviewed about how many hours they had slept every day for at least the previous three months.
Sampling of Saliva
A 6-mL sample of each subject’s saliva was collected: sampling was carried out between 07:30 am and 09:00 am local time. Saliva samples were collected using the passive drool method and stored in saliva collection equipment. All saliva samples collected were centrifuged at 13,000 rpm for 10 minutes at –4°C, divided into aliquots, and then stored at –80°C.
Melatonin, TNF-α, and MDA Levels in Saliva
The melatonin, TNF-α, and MDA levels in the saliva samples were measured using the ELISA technique. The primary antibody was human melatonin ELISA kits (E1013Hu, Bioassay Technology Laboratory, Shanghai, China), human TNF-α ELISA kits (E0082Hu, Bioassay Technology Laboratory, Shanghai, China), and human malondialdehyde (E1371Hu, Bioassay Technology Laboratory, Shanghai, China).
Periodontal Health Status
Periodontal health status was assessed based on depth of pocket and BOP. Periodontitis examinations were conducted using a periodontal probe. Measurements were taken of a minimum of six teeth (one tooth each anterior upper and lower jaw, one tooth each posterior right upper and lower jaw, one tooth each posterior maxillary and mandibular left). The pockets were classified as shown in Table 1.16,17
 |
Table 1 The Classification of Depth Pocket |
The measure of BOP was examined by checking all gingival tissue surrounding each tooth for gingival inflammation. Each tooth was probed in the area proximal both mesial and distal, buccal and palatal or lingual aspect. Each tooth was scored according to the following criteria:
If bleeding was found in at least one aspect, a score of 1 was assigned; and
If no bleeding was found, a score of 0 was assigned.
Statistical Analysis
The data for sleep time duration, depth of pocket, BOP, and salivary melatonin, MDA, and TNF-α levels were tabulated, and an independent sample t-test was performed to determine significant differences between shift workers and non-shift workers. The relationships between sleep time duration and periodontal status, and sleep time duration and melatonin, MDA, and TNF-α levels were analyzed by Pearson correlation. The relationships between sleep time duration and oral inflammation and periodontal health were calculated using linear regression. A significant difference was defined as a p-value of <0.05.
Results
Subject Demographics
Observations were made for each sample group. There were two groups in this study, with each group consisting of 27 participants. The data analysis in this study utilized data ratios. The characteristics of the subjects included age, duration of work, and shift frequency. The night-shift subjects had an average age of 33.07 years, and the non-night-shift workers had an average age of 32.26 years. Most of the night-shift subjects (14/27) and the non-night-shift subjects (15/27) had a work duration of >5 years. The most common night-shift frequency for the shift workers was once per month (12/27), followed by once a week (9/27), and then >3 times per week (6/27) (Table 2).
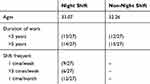 |
Table 2 Subject Characteristics |
Sleep Time Duration
The sleep time duration for each respondent was different. The night-shift workers had a shorter average sleep time duration than the non-night-shift workers (p = 0.000). There was at least a two-hour difference in sleep time duration (Table 3).
 |
Table 3 Sleep Time Duration of the Subjects |
Levels of Salivary Melatonin, TNF-α, and MDA
The level of salivary melatonin in the night-shift subjects (200.52 ± 35.72) was lower than that in the non-night-shift subjects (310.15 ± 81.22) (p = 0.000). The level of salivary MDA in the night-shift subjects (2.62 ± 1.54) was higher than that in the non-night-shift subjects (1.73 ± 0.79) (p = 0.010). There was no difference between the levels of salivary TNF-α in the night-shift or non-night-shift subjects (p = 0.140) (Table 4).
 |
Table 4 Subjects’ of Salivary Melatonin, TNF-α, and MDA Levels |
Periodontal Health Status
The periodontal health status measures consist of depth of pocket and BOP. The night-shift subjects had superior periodontal health status to the non-night-shift subjects. The depth of pocket in the night-shift subjects (3.48 ± 3.06) was deeper than in the non-night-shift subjects (0.19 ± 0.48) (p = 0.000), and the BOP score for the night-shift subjects (7.78 ± 0.85) was severe compared with the non-night-shift subjects (5.26 ± 1.85) (p = 0.000) (Table 5).
 |
Table 5 Subjects’ Periodontal Health Status |
The Correlation Between Sleep Time Duration and Periodontal Status
The results for the correlation between sleep time duration and periodontal status are shown in Table 6. No correlation was found between sleep time duration and depth of pocket or BOP in either the night-shift subjects (depth of pocket: p = 0.815, r = −0.470; BOP: p = 0.725, r = 0.710) or the non-night-shift subjects (depth of pocket: p = 0.753, r= −0.063; BOP: p = 0.136, r = 0.294).
 |
Table 6 Correlation Analysis Between Sleep Time Duration and Periodontal Status |
Relationship Between Sleep Time Duration and Salivary Melatonin, MDA, and TNF-α Levels
The correlation results for sleep time duration and salivary melatonin, MDA, and TNF-α levels are provided in Table 7. A correlation was found between sleep time duration and salivary melatonin in the night-shift subjects (p = 0.031; r = −0.416) and the non-night-shift subjects (p = 0.005; r = 0.524). There was no correlation between sleep time duration and salivary MDA or TNF-α levels.
 |
Table 7 Correlation Analysis for Sleep Time Duration and Salivary Melatonin, MDA, and TNF-α Levels |
Relationship Between Sleep Time Duration and Oral Inflammation and Periodontal Health
The regression analysis showed no relationship between sleep time duration and oral inflammation and periodontal health in either subject group. In the night-shift subjects, no effect of sleep time duration was observed on oral inflammation and periodontal health (r = 0.480; p = 0.320). There was also no effect of sleep time duration on depth of pocket (α = −0.066), BOP (α = 0.085), salivary melatonin (α = −0.009), MDA (α = 0.000), or TNF-α (α = 0.001) (Table 8).
 |
Table 8 The Relationship Between Sleep Time Duration and Oral Inflammation and Periodontal Health |
In the non-night-shift subjects, there was no effect of sleep time duration on oral inflammation and periodontal health (r = 0.593; p = 0.084). Duration of sleep also had no effect on depth of pocket (α = −0.137), BOP (α = −0.026), salivary melatonin (α = −0.003), MDA (α = 0.089), or TNF-α (α = 0.008) (Table 8).
Discussion
Working night shifts has a direct impact on people’s lives, especially on the body’s homeostasis and well-being. Night-shift workers can experience problems due to disruption of their biological circadian rhythms and sleep–wake cycle, which can result in physical and mental disorders. The primary impacts on night-shift workers are caused by long periods of light exposure, sleeping times, and sleep duration. These factors will disturb the regulation of the body, especially the release of melatonin.18,19
Long periods of light exposure affect the secretion of melatonin. Night-time exposure between midnight and 4:00 am (the peak of melatonin secretion) results in complete inhibition of secretion for the duration of full exposure. The effects of light exposure also depend on the intensity, duration, and spectral properties of the light, since intrinsic photosensitive retinal ganglion cells in the eye contain melanopsin, which is a photoreceptor that delivers light against dark signals in the retina. Melanopsin is very important for the functioning of the circadian system and for delivery to the suprachiasmatic nucleus (SCN). Even low-intensity light, such as that emitted by new technologies including LEDs, computer or television screens, cellphones, and tablets, can cause phase delays and slow melatonin secretion.4
Melatonin is released by the body into the saliva via the salivary gland around 24%–33%. As only the free melatonin in plasma enters the saliva, salivary melatonin levels reflect the proportion of free-circulating melatonin.8 Night-shift workers experience light exposure while working, which decreases the release of melatonin into the plasma, thus decreasing the workers’ sleepiness.20 Physiologically, humans experience sleepiness at night and wake up in the morning, which is in contrast to night-shift patterns, which require workers to be awake at night and fall asleep in the morning. Normal sleep duration is around 7–8 hours, but with changes in sleep time, the duration of sleep time will also change; this is because the function of the sleep cycle follows a physiological rhythm.21 This is confirmed in the findings of this study, which indicate that night-shift workers have a shorter sleep duration than non-night-shift workers. The effect on sleepiness during working may stimulate workers to eat at abnormal times. There are several reasons why night-shift workers eat during their shifts, and the most common of these is to remain alert. Night-shift workers experience sleep pressure and decreased alertness. When this condition arises, they may choose to eat and consume caffeine to help them stay alert.22 Eating during the night leads to an increased risk of obesity, which causes a predisposition to diabetes and increases dental caries, as confirmed in a previous study by Roestamadji et al.7
In this study, melatonin levels were determined by measuring salivary melatonin content. The salivary melatonin levels in night-shift workers were found to be lower than those in non-night-shift workers. This result is consistent with other findings that indicate that circulatory melatonin levels are lower in night-shift workers.4 The lower salivary melatonin level is caused by experiencing a change in time orientation due to changes in working and sleeping times. Such changes in time orientation can affect the secretion and regulation of melatonin,23 which plays a role in the regulation of body fat mass. If shift workers do not sleep at night for a long period of time, there will be a permanent reduction in their potential melatonin levels, which can result in a decrease in fat-mass regulation in the body and lead to obesity.24 These changes in fat-mass regulation, which tend to cause increased fat deposition, can affect the immune system, resulting in the production of pro-inflammatory cytokines. One of the pro-inflammatory cytokines produced in this way is TNF-α, which plays an important role in the activation of prostaglandins. The changes in TNF-α in night-shift workers can also be caused by sleep disorders. In addition, increased production of pro-inflammatory cytokines can be caused by the appearance of cortisol induced by stress-related conditions in shift workers.
In this study, we found higher salivary TNF-α levels in night-shift workers. This higher circulation of TNF-α and salivary TNF-α can cause a reduction in tooth attachment and alveolar bone resorption, as signs of periodontitis. This result is supported by Érica et al, who also show that shift workers have higher levels of salivary TNF-α than ordinary workers.25 Meanwhile, an increase in TNF-α, IL-1β, and IL-6 production serves as an indicator of inflammation in shift workers.26 In our results, the higher salivary TNF-α content is a sign of increased incidence of oral inflammation.
In addition, the confounding factor of periodontal damage can be caused by night-time eating behavior, which is influenced by the feeling of wanting to eat.27 Increased eating is caused by a decrease in melatonin that is compensated for by an increase in the hormone ghrelin, which functions as an appetite controller. As a result, if circadian rhythm disturbances occur continuously, causing eating activity at night will increase. This has a negative influence on oral health behaviors such as brushing teeth and sugar intake.28 Based on the results of the research, there are significant differences in BOP and depth of pocket. These factors are controlled by a mechanism for increasing the number of neutrophils as the main defense in inflammatory responses. A high increase in neutrophils that is offset by an increase in ROS in the periodontal tissue will trigger gingival tissue damage, which can cause BOP and periodontal pockets. The presence of BOP and higher depth-of-pocket values can increase the likelihood of periodontitis development.29
The current study found that night-shift workers had significantly higher salivary MDA levels than non-night-shift works. The increase of MDA may cause increased ROS and lead to increased TNF-α. Increased MDA causes damage to cell organelles and enzymes, which increases lipid peroxidation.30 An increase in salivary MDA can also fight the oral defense system, which interferes with intraoral balance. An increase in MDA in saliva can lead to the progressive development of pathogenic bacteria.
In our final analysis, we found no relationship between sleep duration and oral inflammation and periodontal health status in either night-shift workers or non-night-shift workers. There was, however, a correlation between sleep duration and salivary melatonin in both night-shift workers and non-night-shift workers. The increase of sleep time duration will have the effect of decreasing the salivary melatonin. As mentioned before, melatonin is released by the body into the saliva and blood. The salivary melatonin is released via the salivary gland of less than 33%. As only the free melatonin in plasma enters the saliva, salivary melatonin levels reflect the proportion of free-circulating melatonin.8 The reason why there is no relationship between sleep duration and oral inflammation and periodontal health status is illustrated in Figure 1. Night-shift workers have decreased circulatory melatonin and salivary melatonin due to their decreased sleep duration. This condition will impact upon salivary flow rate saliva. A previous study has shown that night-shift workers have decreased salivary flow rate saliva and increased dental caries.7 The incidence of dental caries is affected by abnormal eating times and oral hygiene maintenance behaviors, and this condition may lead to increased oral inflammation. Night-shift workers are also at higher risk of developing diabetes due to obesity. Obesity occurs due to increased BMI related to abnormal eating times and decreased circulation of melatonin that leads to increased metabolism. Previous research also confirms that night-shift workers have high BMI values.7 The increase in fat metabolism has the effect of increasing BMI31 as well as TNF-α production,32 which has a direct effect on periodontal status.33
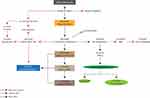 |
Figure 1 Possible mechanism of the relationship between sleep duration and oral inflammation and periodontal health status. |
The limitation of this study lies in the sample size. Future research should be performed on a larger sample and with other types of night-shift workers. The complexity of the sample is very important to help predict the possible mechanisms of the effects of night-shift working on general health issues such as obesity, diabetes, and oral health problems (eg, caries, salivary dysfunction, and periodontal health problems). With an understanding of these mechanisms, it is expected that preventive programs can be planned and provided for night-shift workers to improve their welfare.
Conclusions
The night-shift workers in our sample had lower salivary melatonin levels than the non-night-shift workers, and these levels had a correlation with sleep time duration. Overall, the night-shift workers also had higher levels of oral inflammation and periodontal health issue but there was no relationship between these factors and sleep time duration.
Disclosure
All the authors state that there are no conflicts of interest.
References
1. Fisk AS, Tam SKE, Brown LA, Vyazovskiy VV, Bannerman DM, Peirson SN. Light and cognition: roles for circadian rhythms, sleep, and arousal. Front Neurol. 2018;9(FEB):1–18.
2. Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, et al. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci. 2014;15(12):23448–23500. doi:10.3390/ijms151223448
3. Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie. 2019;23(3):147–156. doi:10.1007/s11818-019-00215-x
4. Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94–106. doi:10.1016/j.lfs.2017.02.008
5. Dakshayani KB, Subramanian P, Essa MM. Effect of melatonin on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats with reference to biochemical circadian rhythms. Toxicol Mech Methods. 2007;17(2):67–75. doi:10.1080/15376520500195798
6. Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11(8):589–599. doi:10.1038/nrn2868
7. Roestamadji RI, Nastiti NI, Surboyo MDC, Irmawati A. The risk of night shift workers to the glucose blood levels, saliva, and dental caries. Eur J Dent. 2019;13(3):323–329. doi:10.1055/s-0039-1697211
8. Cengiz Mİ, Cengiz S, Wang H-L. Melatonin and oral cavity. Int J Dent. 2012;2012:1–9. doi:10.1155/2012/491872
9. Najeeb S, Khurshid Z, Zohaib S, Zafar MS. Therapeutic potential of melatonin in oral medicine and periodontology. Kaohsiung J Med Sci. 2016;32(8):391–396. doi:10.1016/j.kjms.2016.06.005
10. Rastegar Moghaddam Mansouri M, Abbasian S, Khazaie M. Melatonin and exercise: their effects on malondialdehyde and lipid peroxidation. In: Melatonin - Molecular Biology, Clinical and Pharmaceutical Approaches. IntechOpen; 2018: 105–120. Available from: https://www.intechopen.com/books/melatonin-molecular-biology-clinical-and-pharmaceutical-approaches/melatonin-and-exercise-their-effects-on-malondialdehyde-and-lipid-peroxidation.
11. Mohammed BM, SJ. Alrubaye Y. The effect of melatonin administration on the oxidative status in children with autism. Int J Res Pharm Sci. 2019;10(4):2654–2660. doi:10.26452/ijrps.v10i4.1523
12. Oguz E, Yilmaz Z, Ozbilge H, et al. Effects of melatonin on the serum levels of pro-inflammatory cytokines and tissue injury after renal ischemia reperfusion in rats. Ren Fail. 2015;37(2):318–322. doi:10.3109/0886022X.2014.991263
13. Banda NR, Singh G, Markam V. Evaluation of total antioxidant level of saliva in modulation of caries occurrence and progression in children. J Indian Soc Pedod Prev Dent. 2016;34(3):227–232. doi:10.4103/0970-4388.186747
14. Lee W, Lim -S-S, Kim B, Won J-U, Roh J, Yoon J-H. Relationship between long working hours and periodontitis among the Korean workers. Sci Rep. 2017;7(1):7967. doi:10.1038/s41598-017-08034-6
15. Alqaderi H, Goodson JM, Agaku I. Association between sleep and severe periodontitis in a nationally representative adult US population. J Periodontol. 2020;91(6):767–774. doi:10.1002/JPER.19-0105
16. Shaju Jacob P. Measuring periodontitis in population studies: a literature review. Rev Odonto Cienc. 2011;26(4):346–354. doi:10.1590/S1980-65232011000400013
17. Holtfreter B, Albandar JM, Dietrich T, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: proposed standards from the joint EU/USA periodontal epidemiology working group. J Clin Periodontol. 2015;42(5):407–412. doi:10.1111/jcpe.12392
18. Costa G. Shift work and health: current problems and preventive actions. Saf Health Work. 2010;1(2):112–123. doi:10.5491/SHAW.2010.1.2.112
19. Razavi P, Devore EE, Bajaj A, et al. Shift work, chronotype, and melatonin rhythm in nurses. Cancer Epidemiol Biomarkers Prev. 2019;28(7):1177–1186. doi:10.1158/1055-9965.EPI-18-1018
20. Thottakam BMVJ, Webster NR, Cameron G, Galley HF. Melatonin in doctors and nurses working nightshifts—the MIDNIGHT trial. Br J Anaesth. 2019;122(3):e50–1. doi:10.1016/j.bja.2018.10.046
21. Chaput J-P, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep. 2018;10:421–430. doi:10.2147/NSS.S163071
22. Gupta CC, Coates AM, Dorrian J, Banks S. The factors influencing the eating behaviour of shiftworkers: what, when, where and why. Ind Health. 2019;57(4):419–453. doi:10.2486/indhealth.2018-0147
23. Tähkämö L, Partonen T, Pesonen AK. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int. 2019;36(2):151–170. doi:10.1080/07420528.2018.1527773
24. Kim TW, Jeong JH, Hong SC. The impact of sleep and circadian disturbance on hormones and metabolism. Int J Endocrinol. 2015;2015:1–9. doi:10.1155/2015/591729
25. Reinhardt ÉL, Fernandes PACM, Markus RP, Fischer FM. Night work effects on salivary cytokines TNF, IL-1β and IL-6. Chronobiol Int. 2019;36(1):11–26. doi:10.1080/07420528.2018.1515771
26. Cuesta M, Boudreau P, Dubeau-Laramée G, Cermakian N, Boivin DB. Simulated night shift disrupts circadian rhythms of immune functions in humans. J Immunol. 2016;196(6):2466–2475. doi:10.4049/jimmunol.1502422
27. Lowrey PL, Takahashi JS. Genetics of circadian rhythms in mammalian model organisms. J Invest Dermatol. 2011;175–230.
28. Ishizuka Y, Yoshino K, Takayanagi A, Sugihara N, Maki Y, Kamijyo H. Comparison of the oral health problems and behavior of male daytime-only and night-shift office workers: an internet survey. J Occup Health. 2016;58(2):155–162. doi:10.1539/joh.15-0146-OA
29. Mdala I, Olsen I, Haffajee AD, Socransky SS, Thoresen M, de Blasio BF. Comparing clinical attachment level and pocket depth for predicting periodontal disease progression in healthy sites of patients with chronic periodontitis using multi-state Markov models. J Clin Periodontol. 2014;41(9):837–845. doi:10.1111/jcpe.12278
30. Hegde MN, Shetty N, Shetty P. Salivary and serum malondialdehyde levels in type 2 diabetes mellitus with dental caries. Int J Dent Res. 2015;3(2):21–23. doi:10.14419/ijdr.v3i2.5112
31. Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10(3):207–215.
32. Alzamil H. Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J Obes. 2020;2020:1–5. doi:10.1155/2020/5076858
33. Dahiya P, Kamal R, Gupta R. Obesity, periodontal and general health: relationship and management. Indian J Endocrinol Metab. 2012;16(1):88–93. doi:10.4103/2230-8210.91200
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
