Back to Journals » Nature and Science of Sleep » Volume 12
Sleep-related disorders in patients with type 1 diabetes mellitus: current insights
Authors Perfect MM
Received 12 June 2018
Accepted for publication 21 January 2019
Published 11 February 2020 Volume 2020:12 Pages 101—123
DOI https://doi.org/10.2147/NSS.S152555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Steven A Shea
Michelle M Perfect
Department of Disability and Psychoeducational Studies, University of Arizona, Tucson, AZ, USA
Correspondence: Michelle M Perfect
Department of Disability and Psychoeducational Studies, University of Arizona, 1430 E. 2nd Street, Tucson, AZ 85721, USA
Tel +1 520 626 1128
Email [email protected]
Abstract: Type 1 diabetes mellitus (T1DM) is an autoimmune condition that results from destruction of beta cells in the pancreas. Several reviews have concluded that sleep contributes to poor glycemic control, diabetes management, and diabetes-related complications in individuals with T1DM and represents an untapped opportunity for intervention. However, at the current juncture, the American Diabetes Association’s Standards of Medical Care are devoid of recommendations about how to address sleep in the management of T1DM. This article summarizes reviews of sleep in youth and adults with T1DM and empirical studies that have examined various sleep parameters ranging from sleep disturbances (general, perceived sleep quality, sleepiness, awakenings, and sleep efficiency), sleep duration, sleep consistency, sleep-disordered breathing (SDB), and sleep architecture. The data show that many individuals with T1DM sleep less than recommendations; individuals with the poorest sleep have difficulties with diabetes management; and sleep deficiency including SDB often corresponds to several disease morbidities (neuropathy, nephropathy, etc). Mixed findings exist regarding direct associations of various sleep parameters and glycemic control. SDB appears to be just as prevalent, if not more, than other conditions that have been recommended for universal screening in individuals with T1DM. The article concludes with recommendations for collaborative research efforts to further elucidate the role of sleep in diabetes-related outcomes; investigations to test behavioral strategies to increase sleep quantity and consistency; and considerations for clinical care to address sleep.
Keywords: type 1 diabetes, sleep duration, quality, and consistency
Introduction
Type 1 diabetes mellitus (T1DM) is one of the most commonly diagnosed medical conditions in school-age youth, ranking third in the prevalence of pediatric conditions. About 2–3 youth per every 1,000 are currently diagnosed with T1DM.1 The pathogenesis of T1DM is autoimmune destruction of pancreatic islet β-cells. T1DM management typically includes exogenous administration of basal and bolus insulin via syringe, pen, or pump. Insulin doses are prescribed based on the individual’s carbohydrate intake, glucose levels, and needs. If the amount of insulin is insufficient to lower blood glucose concentrations, hyperglycemia resulting in polydipsia, polyuria, nocturia, and visual problems may occur. Sustained hyperglycemia may potentially lead to diabetic ketoacidosis (DKA), as acid accumulates in the blood stream due to the absence of insulin needed to convert glucose into energy for the cells.2 Conversely, excess insulin relative to carbohydrate intake can result in hypoglycemia.
The Diabetes Control and Complications Trial was a landmark study of over 1,000 individuals with T1DM, which demonstrated that intensive treatment with insulin was more effective in controlling diabetes and preventing morbidities relative to conventional treatment.3,4 However, maintaining strict glycemic control may lead to hypoglycemia, which if unaddressed may result in seizures, loss of consciousness, or mortality.3 Chronic glycemic dysregulation has been causally linked to several adverse outcomes, such as increased risk of cardiovascular disease, hypertension, and neuropathy.5,6 Another microvascular complication is retinopathy, which is caused by damage to retinal blood vessels, potentially leading to blindness. Poor management can also lead to nephropathy and end-stage kidney disease requiring dialysis or a renal transplant.6
Disease progression and management follow a developmental trajectory that reflects notable glucose dysregulation during adolescence. In addition to hormonal changes during puberty, adolescents struggle with self-care behaviors.7 Self-monitoring of blood glucose levels often creates distress attributable to the physical discomfort, increased burden over typical adolescent responsibilities, varying comfort and self-acceptance around peers, and the delicate balance of parental involvement and self-sufficiency.8 Adherence to self-management is often measured through self-report or parent-report inventories or based on the frequency of self-monitoring of blood glucose with a glucometer.
In contrast to T1DM, type 2 diabetes mellitus (T2DM) is not an autoimmune disease and develops as a result of metabolic dysfunction and inflammation. The glycemic dysregulation stems from insufficient production and inefficient cellular absorption of insulin.6 In 2017, the Standards of Medical Care in Diabetes (Standards), published annually, introduced recommendations to assess sleep quality, quantity, and sleep-disordered breathing (SDB), as part of the comprehensive evaluation to identify comorbidities in T2DM.9 The Standards referenced data substantiating sleep duration and SDB as causal factors in the etiology of T2DM to support this inclusion. Although there is burgeoning evidence supporting the role and influence of sleep in T1DM, the Standards have not adopted recommendations based on extant findings.
There may be important distinctions in how sleep influences disease outcomes in individuals with T1DM vs T2DM as a result of interactions between autonomic nervous system (ANS) activity and mediators of systemic inflammation. The ANS is composed of two primary branches – the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS). Short sleep duration and/or larger standard deviations of total sleep time (TST) have been linked with greater heart rate variability, suggesting compromised cardiac autonomic modulation,1,11 elevated systemic inflammatory responses,1 and disruptions in the hypothalamic–pituitary–adrenal (HPA) axis such as increased adrenocorticotropic hormone (ACTH) and cortisol.1,12 Experimental studies have demonstrated causal pathways between sleep restriction and inflammation,13 HPA axis disruption,14 and brain responses to food assessed via functional magnetic resonance imaging.15
SDB has been conceptualized as a cluster of respiratory abnormalities during sleep. Similar to the physiological impact of sleep loss, SDB has been associated with 1) cortisol elevations as a result of activation of the HPA; 2) increased SNS activity due to chronic intermittent hypoxia; and 3) elevated inflammatory markers such as tumor necrosis factor alpha, IL-6, and C-reactive protein.16 The sleep cycle comprises two sleep states: rapid eye movement (REM) and non-rapid eye movement (NREM). Three sleep stages (N1, N2, and N3) occur in NREM.1 Compared to REM and wake states, stage N3 (slow wave sleep [SWS]) is characterized by increased production of growth hormone17 and brain glucose utilization, reduced HPA activity,18 and imbalances in the proportion of SNS (increased) to PNS (decreased) activity.
The physiological pathways for how sleep affects glycemic regulation in T2DM have been established in experimental and meta-analytic studies.19–21 Specifically, deficient sleep contributes to reduced insulin release after meals, thereby maintaining glucose in the bloodstream.22 Additionally, insulin production is increased in an attempt to lower the elevated glucose levels attributable to increased cortisol circulating in the body following sleep loss. Concurrently, elevations in epinephrine due to increased SNS activity inhibit insulin release and promote glycogenolysis.23,24
The hormonal and physiological associations with sleep for T1DM are less clear; even with sufficient sleep or treated sleep disorders, in T1DM, the pancreas would not be able to respond to variations in sleep due to the absence of functional beta cells. Therefore, sleep debt, irregular sleep schedules, and untreated SDB may impact glycemic control via elevations in cortisol and an imbalance in PNS and SNS activity. Furthermore, the role of sleep in T1DM may also contribute to behaviors that interfere with optimal diabetes management. For instance, sleep restriction has been linked with executive functioning difficulties, memory impairment, and behavioral dysregulation;25–28 these are essential skills for effective diabetes management.29
This article aims to provide a comprehensive summary of empirical studies and previous reviews to identify trends in findings, summarize implications, and address gaps in research. Accordingly, the review describes empirical studies from peer-reviewed publications, consolidates findings from recent reviews of sleep in T1DM, and offers suggestions for future directions for sleep as a target in diabetes clinical care and research endeavors. The first objective was to conduct a systematic review of the literature to ascertain the prevalence and severity of various sleep parameters among individuals with T1DM. The second objective was to describe the findings that characterized differences in various sleep parameters between those with T1DM and those without. The third objective of this review was to elucidate the associations between sleep parameters and diabetes-related outcomes such as hyperglycemia, hypoglycemia, diabetes comorbidities, and diabetes management.
Article selection
The main goal of this article was to provide an up-to-date comprehensive overview of peer-reviewed publications containing empirical data that targeted a sleep parameter as a variable in the investigation (eg, sleep was an independent or dependent variable, or as a variable examined in relation to a diabetes-related outcome). The following sleep parameters were searched in tandem with T1D, T1DM, type 1 diabetes, or diabetes: sleep, sleep quality, sleep-disordered breathing, SDB, sleepiness, insomnia, sleep disturbance, or sleep architecture. The primary search engine used was PubMed. The search included articles through June 2018. Articles had to be peer-reviewed and published in English. Results of each paired search (eg, T1DM and sleep) were recorded in a spreadsheet. Duplicate articles that appeared in more than one search were removed. The abstract of each individual article was initially reviewed to confirm that the study measured or examined sleep in some capacity among individuals with T1DM. The second-level screening included reviewing the full article for abstracts that met inclusion criteria or for those articles for which eligibility could not be verified based upon the abstract review alone.
Articles were screened to identify studies that analyzed outcomes for individuals with T1DM separately from other groups (T2DM or those without diabetes). Retained articles needed to report data on at least one sleep metric among individuals with T1DM. The search process was replicated in the additional search engines of EMBASE and SCOPUS to determine additional unique articles. An a priori decision was made to include articles that were not yielded in the search, but were encountered during the writing process as searches often miss relevant references.3 Additionally, reference lists from the articles identified were also reviewed for any publications that had not been identified in the formal search. The final number of retained, empirical articles was 54; these appear in Table 1. An additional five review articles are also summarized in this article. A flowchart reflecting the results of the article selection process is shown in Figure 1.
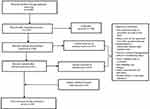 |
Figure 1 Flowchart highlighting inclusion of articles for manuscript. |
 |  |  |  |  |  |  |
Table 1 Summary of findings with sleep-related differences or associations in individuals with T1DM |
Diabetes-related outcomes included glucose levels via HbA1c or a continuous glucose monitor (CGM); insulin sensitivity or requirements (disposal rates or doses); co-morbidities such as nephropathy, neuropathy, retinopathy, cardiovascular disease, hypertension, and DKA; and diabetes management variables (number of fingersticks and diabetes care or management inventories).
Summary of sleep-related findings from studies of individuals with T1DM
The summary of findings is organized by sleep disturbances, sleep duration, sleep consistency, SDB, and sleep architecture. Table 1 presents the empirical articles reviewed in order of publication year (and month if possible); number of T1DM participants and controls if included or subgroups of those with T1DM; sleep assessments used; sleep parameters that were examined in relation to T1DM status, experiences, or a diabetes-disease outcome; and the primary findings. Findings were not highlighted if differences in the sleep parameters were examined based on non-diabetes factors (eg, sleepiness in those with SDB vs not) or if associations were not linked with a diabetes-related variable (eg, sleep and mood).
Sleep disturbances
Sleep disturbances have been conceptualized as general disruptions in sleep. Indicators that individuals have disturbed sleep include awakenings or arousals; poor sleep quality measured subjectively or as an objectively measured index of sleep efficiency (SE); insomnia symptoms of difficulty falling or staying asleep; or self- or caregiver-reported daytime sleepiness. With regard to Objective 1, sleep disturbances have been documented to be a prevalent phenomenon in individuals with T1DM across the lifespan, beginning in early childhood. In a preschool sample of 10 children with T1DM, 9 met the clinical cutoff value on a parent-reported measure of sleep disturbance.31 Another study noted that 29%–50% of the 24 parents of preschoolers with T1DM reported that at least once per week, their child had problems following the bedtime routine, falling asleep, calling parents back to the bedroom, and winding up in parents’ beds. In contrast, these parents reported less frequent concerns with prolonged (>10 minutes) nighttime awakenings.32 Actigraph (a wrist-sized device that estimates sleep based on a movement sensor) findings from a study of preschoolers with T1DM revealed that average SE was 82.5%, which is lower than the 85% threshold considered to be good sleep quality.31 With regard to Objective 2, the search did not identify studies that had examined if differences existed in sleep disturbances between those with and without diabetes in the preschool population.
Returning to Objective 1 in the school-age population, Jaser et al33 found that over two-thirds (67%) of 511 children with T1DM, aged 2–12 years, met the score cutoff on a parent-reported measure of disturbed sleep. A study that examined four items on a parent rating scale found that adolescents with T1DM experienced problems related to sleeping (15%), being tired (22%), sleeping more than peers (29%), or sleeping less than peers (18%).34 Perfect et al35 conducted a cross-sectional, case-controlled study that examined sleep using actigraphy, polysomnography (PSG), sleep diaries, and self- and parent-reported questionnaires in youth with T1DM (aged 10–16 years). The researchers compared sleep in youth with T1DM to sleep in a healthy control sample matched for sex, age, and body mass index (BMI). The matched control participants were obtained from the second examination of a large observational cohort study that had recruited from the same geographic region, used the same PSG equipment, and had the same scoring technician as the youth with T1DM.35,36 Responses to a sleep disturbance questionnaire completed by parents about the youth with T1DM revealed that 60% met the clinical cutoff.36 Meanwhile, the average actigraph-measured SE in youth with T1DM was 78.5%.35
With regard to Objective 2 in school-aged children, two PSG studies found disparate findings, with one revealing more awakenings in young children with T1DM than those without T1DM,38 whereas the other study did not find more awakenings or differences in SE.39 One study reported that compared to children and adolescents without diabetes, youth with T1DM had higher ratings on an overall measure of sleep disturbances, with particular difficulties related to sleeping too much, falling and staying asleep (eg, insomnia symptoms), and symptoms of restless leg syndrome and teeth grinding.4 In contrast, another study did not find higher rates of restless leg syndrome and insomnia symptoms between school-age youth with T1DM compared to their siblings.41 A recent study compared sleep concerns reported by those with T1DM to a convenience sample of those without diabetes (eg, friends and family members) at different age intervals (eg, children, adolescents, and adults). Compared to those without diabetes, children with T1DM actually showed fewer problems with sleeping too much and had lower scores on a subscale reflecting nighttime arousals. Adolescents with T1DM reported fewer respiratory problems during sleep and less daytime sleepiness.42 In the aforementioned case-controlled study,35 youth with T1DM did not report more sleepiness, trouble falling asleep, and early morning awakening than those without diabetes. However, direct comparisons were not possible as the diabetes and control samples used different assessment tools to capture those sleep disturbances.
Findings related to the occurrence of sleep disturbance in adults have been mixed (Objective 1). Using a few survey items, adults with T1DM were not characterized by sleep disturbances such as difficulties falling or staying asleep.43 In cross-sectional studies, rates of participants who were classified as reporting enough symptoms commensurate with sleep disturbances have ranged from 31% to 48%.44–47 In one of those studies, sleep disturbances were more prevalent in females than in males.47 With regard to daytime sleepiness, males and females had similar scores, with about 10% reporting difficulties in this area.47
A few studies have compared reports of sleep disturbances in adults with T1DM and those without T1DM (Objective 2). van Dijk et al44 did not find significant differences in scores on a measure of sleep disturbance, but they found that more adults with T1DM met the clinical cutoff for impairment relative to those without diabetes. Janovsky et al48 reported that a greater number of adult patients with T1DM met the cutoff on a measure of sleepiness than those without T1DM. In the aforementioned study that compared individuals with T1DM to a convenience sample from different age spans, adults who had T1DM evidenced more sleep-related complaints. Accordingly, although average scores on a sleep disturbance measure did not differ between the two groups, adults with T1DM reported higher rates of poor subjective sleep quality, delayed sleep onset, short sleep, and lower SE.42 Palladino et al49 found that self-reported sleep disturbances collected over two different time points did not differ between females with and without T1DM, but there was an interaction effect between males and diabetes status on sleep disturbance scores. Accordingly, males with T1DM reported significantly more disturbed sleep between time points, whereas males without T1DM noted improved sleep at follow-up.
With regard to Objective 3, several studies did not find associations between self-reported sleep disturbance, sleep quality, sleepiness, or SE with disease outcomes (glucose, comorbidities) in preschool children,32 children,42 adolescents,42,44 and adults.42,45,5 However, some data have supported that delays or disruptions in sleep may be linked to poor glycemic control, diabetes-related complications, and inconsistent diabetes management.39,41,51,52 For instance, Pillar et al39 found that a swift decline in glucose levels paralleled more PSG-recorded awakenings in children with T1DM. Supporting these findings, higher HbA1c, DKA, and more frequent hypoglycemic episodes were more likely to have been experienced by children with a higher severity of parent-reported sleep disturbances, but the number of fingersticks did not associate with sleep disturbance severity.33 In a cross-sectional study of 191 adolescents with T1DM, better sleep quality uniquely predicted better glycemic control (eg, lower HbA1c).51 Another study that utilized electronic daily self-reports of older adolescents with T1DM demonstrated that both sleep quality and variability in sleep quality synergistically predicted risk for elevated blood glucose levels and perceived challenges to checking their blood glucose levels. Regardless of youth's typical sleep quality, better sleep quality ratings on the preceding night positvely related to improved diabetes management the next day.52 Hazen et al34 found that parents’ ratings that their adolescent slept more than his or her same age peers significantly related to higher glucose levels, based on both HbA1c and CGM. Sleeping more than others of their age also related to poorer self-reported diabetes management. None of the sleep-related items correlated with the number of glucose meter readings. Although it is possible that hypersomnolence can negatively affect glycemic control, the sample consisted exclusively of adolescents who were in poor control (eg, HbA1c >7.5%). Furthermore, sleeping more than peers may still not reflect adequate or consistent sleep. Another study of adolescents and young adults found a significant correlation between sleep quality and HbA1c for males, but not for females.53 In a study focused on hypoglycemia, adolescents evidenced more motoric activity using an accelerometer during hypoglycemic episodes, suggesting that more restless sleep occurred when blood glucose levels were too low.37
With regard to Objective 3 in adults, one study found that sleepiness scores were higher for those with T1DM who had cardiovascular neuropathy relative to those who had not yet developed neuropathy.48 During an overnight sleep study of 18 adults who had T1DM, average glucose levels were positively associated with awakenings, and those with the most variability in glucose levels experienced the greatest number of awakenings.54 Likewise, a study with adults with T1DM found that more PSG-recorded arousals related to higher HbA1c levels.55
Although the objectives of this review focused on the frequency and impact of sleep disturbances in T1DM, aspects of nocturnal management of diabetes may also interfere with or facilitate restful sleep. For instance, parental ratings of sleep disturbances were lower when preschool children were on a fixed insulin administration schedule compared to those who received more intensive treatments consisting of blood glucose monitoring and variable insulin shots adjusted for glucose levels.32 A study that examined sleep disturbances in children, adolescents, and young adults found that sleep disturbances were higher in children who used a CGM compared to those who used a glucose meter. Additionally, children who used a CGM and/or those who wore a pump reported more insomnia symptoms than those who used a meter or those who delivered insulin via an injection, respectively.42 In contrast, Jaser and Ellis53 found fewer sleep disturbances among adolescents with T1DM who used a pump relative to those who did not. A mixed methods survey study with forced choice and open-ended responses completed by guardians of youth with T1DM, as well as by adults with T1DM, revealed that nearly one-fifth experienced nighttime awakenings to manage diabetes.56 Youth with T1DM were not included as part of that sample; thus, perspectives of youth’s own sleep were not captured.
In summary, scores on sleep disturbance measures have not consistently differentiated adults with and without diabetes. Nonetheless, adults with T1DM appear to exhibit higher rates of sleep disturbances when evaluating cutoff scores specified by the instruments that were used. Consequently, relative to those without diabetes, more individuals with T1DM may endorse more frequent and severe sleep problems. Moreover, factors such as poor sleep quality, daytime sleepiness, insomnia symptoms, or awakenings may contribute to worse glycemic control or difficulties engaging in diabetes self-management. In youth, there has been less support for a direct association between self-reported sleep disturbances and glycemic control. However, some of the youth studies and three of the adult studies found that disturbed sleep related to management aspects of diabetes. The preponderance of studies relied on self-reports of sleep disturbance, which may be appropriate given that in some respects, characteristics of sleep disturbances are subjective. Ambulatory technologies such as the CGM and actigraphy, or even home-based PSG, would provide objective assessments that would complement self-reported methods. The current methodologies that have been employed have not enabled researchers to ascertain if sleep disturbances are a causal contributor or a consequence of poor disease control. For example, although sleep disturbances could precede glycemic dysregulation or disease co-morbidities, significant pain associated with microvascular and macrovascular complications could disrupt sleep. Sophisticated longitudinal designs that incorporate analytic techniques, such as a dynamical systems approach,57 would elucidate how changes in diabetes disease outcomes and sleep disturbances affect the other over time.
Insufficient sleep duration
Several studies have examined the prevalence of inadequate sleep duration in individuals with diabetes from a cross-sectional perspective (Objective 1). Developmentally, a few studies with young children with T1DM have indicated early problems with sleep. Although Monaghan et al noted adequate average sleep duration per parent report for 24 children with T1DM aged 2–5 years, many did not meet the recommended TST, even with naps and overnight sleep periods combined.32 Using a wrist accelerometer, another study of 10 preschoolers found average TST to be only about 8 hours.31 In an epidemiological study of youth with T1DM, parents of preschool children reported an average of ~11 hours of sleep per night, whereas the average sleep duration of children between the ages of 5 and 12 years was 9.5 hours. These averages were still within the lower end of the recommended range for each age group, with 20% of the youth sleeping <9 hours.33 Among studies with adolescent samples, self-reported averages have consistently ranged below the recommended amount of sleep (7.4–8.6 hours/night).51,53,58,59
The method of assessing sleep duration may yield different amounts of sleep. Accordingly, two published studies of youth from the same cohort of adolescents with T1DM revealed that objectively measured mean TST was 6.96 hours,35 whereas the average self-reported sleep duration from sleep diaries was 8.76 hours across 5 days.36 Additionally, when asked about typical school night and non-school night sleep using a validated survey, these adolescents reported their sleep durations to be 8.34 and 9.43 hours, respectively.36 Similarly, Patel et al59 reported school night TST to be 7.26 hours and non-school night TST to be 6.26 hours using actigraphy, whereas TSTs computed from diaries for school nights and non-school nights were 7.75 and 8.75 hours, respectively. Several studies have examined the associations between TST and both glycemic control and diabetes management in youth (Objective 3). In the epidemiological study of participants with T1DM, parents’ reports of their child’s sleep duration were significantly related to parent-reported HbA1c, with those sleeping >9 hours per night having lower HbA1c.33 Although the findings were based on parent report and practical significance is unknown, a difference of 0.2% in HbA1c between the two sleep groups is consistent with the average change in HbA1c following adherence-promoting interventions.12 Further, in the adolescent sample of youth with T1DM from the case-controlled study, night-to-night TST measured via actigraphy did not predict CGM-recorded glucose levels the following day;35 however, the researchers did not report the overall correlation between actigraph-measured sleep duration and overall average glucose levels. Nonetheless, self-reported average TST significantly related to HbA1c in the same sample.36 Other studies with adolescents and young adults diagnosed with T1DM did not reveal a significant relation between self-reported sleep duration and HbA1c.51,53,58,59 With regard to management, sleep amount did not predict how much insulin the adolescents with T1DM required,51 but two studies found that youth who slept more tested their glucose levels more often,53,58 were more likely to use a pump,53 and were more likely to adjust their insulin when needed.58
With regard to Objective 1 in adults, Denic-Roberts et al47 reported that nearly half of their sample of adults with T1DM obtained less than the recommended sleep duration (7 hours). Meanwhile, another study found that 26% of the sample of adults with T1DM averaged <6 hours per night.6 With regard to Objective 2, one study did not find self-reported sleep duration to differ between adults with T1DM and those without.44 An observational study also did not find statistically significant differences between TST of adults with T1DM (n=18) and those without (n=9) using accelerometers and sleep diaries. However, participants with T1DM still reported 25 minutes less sleep and had larger within group variability, as expressed by a larger SD.54
Regardless of whether adults with T1DM sleep the same or worse than those without diabetes, evidence exists that inadequate sleep duration relates to diabetes outcomes (Objective 3). With regard to glycemic control, studies have found that adults with short sleep duration (6–6.5 hours) based on self-reported6 and objectively61 measured TST had significantly higher HbA1c than those with more sleep. Yet, when objectively measured sleep duration has been examined as a continuous value in other adult studies, the correlations with HbA1c have not been significant.5,54,62 There may be other factors that enhance or mitigate the contribution of sleep duration to disease outcomes. For instance, an epidemiological study found a moderating effect, such that shorter sleep was associated with lower HbA1c among participants with poor control, but sleep duration negatively related to glucose levels among those with good control. Moreover, those with longer self-reported sleep duration also appeared to have higher insulin need.47 It is important to keep in mind that due to the large sample size, the correlations were significant but indicated relatively small effects.
In summary, based on the extant data, similar to individuals without T1DM, as individuals with T1DM move through the developmental stages, the prevalence of inadequate sleep duration is likely to increase. To date, there have been mixed findings related to the contribution of sleep duration on glycemic control and diabetes management. However, the exclusive reliance on correlational research obfuscates the fact that there are a myriad of confounding factors that might affect the diabetes–sleep relationship. Few studies have examined the impact of inadequate sleep using controlled designs for individuals with T1DM. However, one experimental sleep restriction study in a laboratory setting showed that adults with T1DM who were limited to 4 hours of sleep exhibited lower glucose tolerance and insulin sensitivity compared to when they were provided the opportunity to obtain the recommended TST.63 Thus, very limited data are available that experimentally elucidate the mechanistic underpinnings and potential causal role of sleep duration in glycemic control and vice versa in individuals with T1DM. Experimental sleep manipulation studies using in-lab or at-home protocols have demonstrated that sleep restriction and extension cause changes in mood,64,65 behaviors,65,66 cognition,14,22,27,28,67,68 aspects of immune functioning,69,7 and metabolic control.71 Accordingly, prescribed sleep conditions as part of a multiday protocol such as in a residential program that mimics a camp-like environment would allow for control and precise monitoring of environmental and intraindividual factors that moderate the effects of sleep duration on metabolic health. Given the momentary fluctuations in glycemic control, microlongitudinal designs that utilize ambulatory technologies such as the CGM and actigraphy, or even home-based PSG, would provide evidence regarding unique temporal resolution of sleep, glucose, and daytime functioning in naturalistic settings. Moreover, a clinical trial72 aimed at increasing sleep duration has been developed to investigate the efficacy of a sleep extension intervention on diabetes-related outcomes. Such studies are needed to be able to test the effects of prescribing a lengthened sleep duration on the prognosis of T1DM.
Inconsistent sleep schedules
Although sleep disturbances and duration have been the focal point of most studies, the potential influence of sleep inconsistency or variability has more recently been examined in relation to glycemic control and other disease-related outcomes (eg, insulin dosage; Objective 3). A standard indicator of sleep consistency is the standard deviation (SD) of TST. However, a few studies have examined the difference between sleep duration on school/work nights and non-school/work nights. Bed and wake times, the differences in the latest and earliest bedtime, and the difference between school and non-school nights, have also been used to characterize sleep schedules. These indicators of sleep timing and variability are distinguished from another circadian rhythm metric referred to as “social jetlag”, which is derived by computing the difference between the halfway point between bedtime and awakening time on school/non-work nights and the midpoint of the sleep period on non-school/work nights. When examining social jetlag,51 a study did not find that adolescents with T1DM who experienced greater social jetlag had higher HbA1c, but these adolescents required more insulin to manage their diabetes.51 Another study of adolescents with T1DM using TST SD found that more pronounced variability significantly related to higher HbA1c.59 In adults, one study did find that social jetlag positively correlated with HbA1c.62 A different study with 41 adults with T1DM that characterized variability by examining the SD of both mid-sleep and sleep duration found that both these metrics predicted higher HbA1c and significantly associated with greater insulin need.5
The emphasis on sleep variability is nascent and has been overshadowed by the focus on sleep duration in studies with individuals with T1DM. The complexity of the sleep–wake cycle has not been fully realized in research with T1DM. Sleep misalignment can be captured through several metrics of circadian rhythm beyond that of differences in timing and social jetlag. For instance, more recent studies have considered daily variations, such as the sleep regularity index and the composite phase delay in adults samples.73,74 Similarly, global metrics of glucose levels as opposed to day-to-day fluctuations in glycemic control may not capture the immediate and cumulative effects of having glucose levels out of the target range such as with chronic hyperglycemia or intermittent hypoglycemia.
Sleep-disordered breathing
Data have supported not only the potentially higher prevalence of SDB in individuals with T1DM but also the myriad of morbidities associated with SDB in this population. Only three studies have examined SDB in youth with T1DM (Objectives 1 and 2). A prospective, case-controlled study reported the rate of SDB to be 35% (14/40) using an apnea–hypopnea index (AHI) ≥1.5.35 The rates of SDB were comparable between those with and without T1DM in these three studies.35,75,76 However, in two of the pediatric studies, individuals with T1DM had more apneic35,75 and central apneic events35 than those without T1DM.
With regard to Objective 1 in adults, prevalence rates from an epidemiological study utilizing self-report at a single time point found that 23% of the 222 adults endorsed items suggestive of high SDB risk.47 Using PSG, researchers have reported the rates of individuals who exhibited various AHIs. Using an AHI of 5, multiple studies have found high rates (45%–63%) of SDB.77,78 Using an AHI cutoff of 10, studies reported the prevalence of SDB to be 27%–46%.77,79 Using a cutoff of 15, the prevalence rates have ranged from 8% to 40%.78,8,81 Using an AHI > or ≥ 30, studies have found rates from 6% to 25%.78–8 These studies were based on samples ranging between 10 and 58 individuals. Therefore, it is difficult to compare the findings to studies that have been conducted in those without diabetes or those with T2DM to determine if the prevalence of SDB is equivalent or greater than those samples.82 Nonetheless, the use of PSG provides an objective, “gold” standard metric that has revealed that roughly half of the patients with T1DM may have at least mild SDB. In regard to Objective 3, all three pediatric studies linked SDB with poor glycemic control. For instance, in comparison to youth with well-controlled diabetes, youth with poor control had more respiratory difficulties, such as respiratory arousals,76 higher AHIs,76 more apneas,75 and more central apneas.75 In one of those pediatric studies,35 when comparing glycemic control based on SDB status, although HbA1c did not differ, CGM-recorded glucose levels were significantly higher (>40 mg/dL difference) and individuals with T1DM who had SDB were more hyperglycemic relative to those without. In adults, untreated SDB may contribute to long-term microvascular and macrovascular complications. Some studies found nephropathy, neuropathy, hypertension, and heart complications to be more common among adults with T1DM who had SDB compared to adults with T1DM who did not show evidence of SDB.79,81,83 One study did not find differences in the number of individuals with diabetic retinopathy based on their SDB status (AHI >15).81
Altogether, individuals with T1DM appear to be at high risk for at least mild SDB. Moreover, those with SDB evidence significantly worse glycemic control or experience more morbidities that arise from complications of having T1DM. Although it is not possible to experimentally induce SDB as a way to demonstrate that SDB causes poor disease control, a more important question is whether treatment of SDB will reduce the risk or existence of comorbidities. Accordingly, a meta-analytic study found evidence that treatment of SDB has led to improvements of metabolic control.84 More recently, factors such as the duration of treatment, compliance with treatment, and severity of SDB have been postulated to influence whether continuous positive airway pressure (CPAP) has a secondary benefit of improving glucose regulation.82,85 However, CPAP as a treatment concomitant with current T1DM standard of care practices is unknown. Thus, randomized controlled trials (RCTs) are needed to examine the effect of CPAP and surgical interventions (adenoidectomy and tonsillectomy) on alleviating SDB and improving diabetes control.
Sleep architecture
Regarding Objective 2, one study in adolescents found that individuals with T1DM spent a greater percentage of time in stage N2 than those without diabetes.35 The study also found that those with T1DM spent less time in stage N3.35 Similarly, one adult study found more stage N2 in those with T1DM compared to those without.86 However, a few studies in children and adults have not shown differences in the percentage of time in NREM stages38,39,76 compared to those without T1DM, nor have any of the studies noted a difference in REM35,38,39,54,76,86 based on diabetes status.
With regard to relations with glycemic control (Objective 3), two studies did not find sleep architectural differences in youth who had experienced hypoglycemia during the sleep period,38,87 whereas another study found that children who had experienced hypoglycemia spent more time in SWS compared to youth with T1DM who did not experience hypoglycemia.39 A single night lab-based PSG did not reveal significant differences in the proportion of time spent in each sleep stage between youth with T1DM classified as in good control compared to those classified as poor control based on their CGM recordings during the sleep study. However, the authors noted that their previous research had demonstrated that when classified by HbA1c (>9 vs <9), youth with higher glucose spent significantly more time in lighter stages of sleep.76 Perfect et al35 reported that percentage of time in stages N2 (more) and N3 (less) both related to higher HbA1c and stage N2 significantly related to weekly average CGM levels in adolescents with T1DM. A greater proportion of time spent in SWS was associated with lower Hb1Ac in a small study with adults as well. Moreover, another study found that individuals experienced significantly less hypoglycemia as measured by CGM during stage N3.88 Furthermore, when examining the correspondence between CGM recordings and sleep architecture via EEG patterns among adults with T1DM, researchers found strong associations between glucose levels and the variability of those levels with glucose-EEG power coherence in the alpha and delta bands. The direction of the relationship supports the possibility that brain activity and glucose may have greater influence on each other when individuals with T1DM are in better control.89 Notably, no studies that met inclusion criteria in this review found an association between glycemic control and REM in either children35,38,39,76,87 or adults.88
Summary of reviews of T1DM and sleep
Three comprehensive reviews have been published summarizing the empirical findings pertaining to sleep among individuals with T1DM.9–92 A 2016 meta-analysis that involved an international collaboration of T1DM researchers spearheaded by the first author of that publication examined the differences in sleep quality, daytime sleepiness, sleep duration, SDB, and sleep architecture in youth and adults with T1DM compared to those without as well as those sleep parameters in relation to glycemic control.9 Reutrakul et al noted that at the time of their meta-analysis, studies examining sleep disturbance in children did not utilize a consistent measurement to aggregate the findings. With regard to sleep disturbances in adults, PSG-measured SE and rates of poor sleep quality did not differ between adults with T1DM and controls and were not related to HbA1c. However, adults with T1DM had significantly worse scores on sleep quality measures than those without, and HbA1c levels were significantly higher among those with poor sleep quality, relative to those with good sleep quality.
When examining sleep duration, the authors concluded that TST assessed via PSG was significantly shorter in children with T1DM relative to those without. Youth who slept the recommended number of hours per night did not differ in glycemic control compared to those who slept less than the recommended number of hours. Comparison of those in good vs poor control did not reveal differences in TST, although there was a trend for adolescents in good control to sleep longer. With regard to findings involving objective measurements of sleep duration in adults, differences in self-reported TST did not exist between adults with and without T1DM, and there was not a correspondence between those in good vs poor control and short vs adequate sleep. In contrast, those classified as having short sleep based on self-reported TST had significantly worse glycemic control, and those with poor control slept significantly less than those in good control.
In this meta-analysis, there were not enough pediaric studies to report SDB prevalence rates or examine AHI in relation to HbA1c. Similar to the current study, the authors noted that more than half of the adult participants with T1DM had at least mild SDB, with about one-sixth having moderate or severe SDB. The difference in HbA1c between those with and without SDB was not significant, although the difference in HbA1c in adults with more severe SDB approached significance. Moreover, those with poor glycemic control had more severe SDB than those in good control. Finally, there were not enough studies in either children or adults to report sleep architectural differences. However, there was a trend for adults with poor glycemic control to spend more time in lighter stages of sleep and less time in stage N3.9
In another review, Farabi91 summarized the findings related to sleep disturbances and quality, sleep duration, SDB, and architecture. The author explained that the structure of sleep appears to differ in individuals with T1DM. Additionally, various difficulties with sleep onset, sleep maintenance, sleep duration, and depth of sleep have been noted. Moreover, SDB appears to be more prevalent among individuals with T1DM, and those with SDB may be at higher risk for diabetes complications. Nonetheless, the author noted that most studies in T1DM have been cross-sectional and did not explain how or why sleep duration and glucose may be related.
Perez et al92 provided a more recent review of sleep duration, variability, and sleep architecture in youth with T1DM. The authors drew similar conclusions in that youth with T1DM appear to have insufficient and inconsistent sleep. Furthermore, sleep problems characterized by inadequate, inconsistent, and light sleep have been linked with poorer glycemic control. The authors also noted that there may be greater differences between the number of hours slept on non-school/work nights compared to school–work nights in individuals with T1DM than those without. Such a notion might not only reflect greater sleep inconsistency in this population but also suggest that average sleep duration aggregated across all days in a week may not capture that gap.92
Two additional meta-analytic studies focused on retinopathy in adults with T1DM. They each only identified one study, but these studies were not the same. Both concluded that there was a positive association between respiratory problems during sleep and retinopathy in adults with T1DM.93,94
Discussion
About 60 studies with varying sample sizes have now been published across the lifespan investigating the role of sleep in T1DM. Rates of sleep disorders and problems across studies were consistently 15% or higher. The current rates for Hashimoto’s disease and celiac disease range from 1% to 15% and up to 30%, respectively.6 The Standards for T1DM recommend routine screening for these two conditions given the high co-occurrence. Unlike T2DM patients, the Standards do not mention sleep as part of their practice parameters for T1DM. This gap is most likely due to the varying ways in which sleep has been assessed, smaller sample sizes, and absence of longitudinal, case-controlled, or experimental studies in individuals with T1DM. Nonetheless, as more studies are published, the significance of these findings may warrant inclusion of sleep recommendations in future iterations of these guidelines.
Implications for the practice setting
Given the prevalence, evaluation of sleep concerns and screening for disorders may facilitate diabetes management and glycemic control efforts. With regard to perceived sleep disturbances in children, practitioners can incorporate one of the most commonly used questionnaires such as the Children’s Sleep Habit Questionnaire95 (parent report measure) or the Pittsburgh Sleep Quality Index96,97 (older adolescents and adults). A newer measure that has not yet been utilized in youth with T1DM but can be administered to children and adolescents is the Children’s Report of Sleep Patterns.98–1 Furthermore, some CGM companies have been incorporating technological applications that integrate data from accelerometers that generate sleep metrics. Although these commercially available devices have not undergone the validation process relative to research-grade devices, they do have the potential to objectively depict sleep patterns and disruptions in restful sleep. Monitoring sleep on a daily basis is particularly relevant given that burgeoning evidence has supported that sleep variability or inconsistency may be related to negative outcomes.
Despite the risk for SDB, screening for SDB is not part of routine practice and both clinicians and patients lack awareness regarding the potential impact of SDB on disease outcomes.1 Several screening approaches have been proposed in youth and adults, with varying predictive validity for SDB. For instance, the STOP-BANG1,1 (or A modified version1) is an acronym for snoring, tired, blood pressure, BMI, age >50 years (or academic problems for children), neck circumference >95th percentile, and male gender. Studies have shown that positive indicators of three or more represent moderate risk for SDB, with SDB being more likely with an increasing number of criteria met.1,1,1 A positive screen with a STOP-BANG may warrant a referral for a PSG. It is important to consider that this approach has not been validated in individuals with T1DM, and thus, it is not known if additional risk factors include poor glycemic control or comorbidities. In adults, the Berlin Questionnaire that was used in several studies included in this review has shown some promising predictive validity but is still problematic as it may yield false negatives.1,1
Directions for future research
Multiple study designs have been employed, yet notably absent have been longitudinal, experimental, or RCTs. The rigor of research on sleep in individuals with T1DM might increase if researchers formed a network to collaborate on a multisite, epidemiological study to characterize the phenotypical aspects of sleep in this population and determine the clinical relevance of different sleep parameters for disease outcomes. Studies tracking sleep in participants’ natural environments provide ecologically valid contexts; however, another approach could be a crossover study that includes experimentally manipulated sleep conditions (short, healthy sleep opportunity, perhaps alter sleep times to assess effects of variability) as part of a multiday protocol such as in residential summer program akin to an overnight camp setting. This would enable researchers to collect biomedical and psychosocial data, document environmental conditions, and precisely monitor physical activity, diet intake, and mood. Additionally, at the time of this review, no peer-reviewed publications exist that have systematically and comprehensively studied the treatment of sleep disorders or problems in individuals with T1DM. One study detailed the components of the intervention to extend sleep, with final results still pending.17 Two other prospective studies are being developed or underway to facilitate youth obtaining adequate sleep and establishing healthy sleep schedules.1,1
Limitations
This review did not address the potential effects of sleep insufficiency on daytime functioning, such as internalizing symptoms, externalizing symptoms, academic achievement, neurobehavioral performance, and quality of life. A few studies have established or proposed the associations of sleep and neurobehavioral outcomes in individuals with T1DM.35,36,4 Sensitive, longitudinal ambulatory cognitive assessments and ecological momentary assessments of mood and well-being would document the day-to-day influences of sleep and their interaction with glycemic control and intervention effects. To further support the integration of evaluation for sleep problems into clinical care, future research is needed to address critical issues such as establishing recommendations for universal screening practices; selecting tools that have the most clinical utility in identifying those with a sleep disorder that are cost effective, sensitive, and not burdensome; pinpointing the best timing to monitor and treat different aspects of sleep over the course of diabetes clinical care; and establishing evidence-based intervention components that are effective in reducing sleep disturbances, lengthening sleep, regularizing sleep schedules, and treating SDB.
Conclusion
The prevalence of sleep problems appears to be higher among individuals with T1DM. Although research has yielded mixed results, several sleep parameters are associated with diabetes-related complications. On a final note, given that managing T1DM requires considerable resources and time for families, notably absent from the majority of studies was a cross-cultural or diversity perspective. Consequently, a multicultural framework with family systems thinking including examination of the socioeconomic influences on sleep patterns in this population would help to contextualize the role of sleep in the disease trajectory and potentially enhance interventions targeting sleep to maximize outcomes.
Acknowledgments
This review was supported, in part, by a grant from the National Institutes of Health grant #5R01DK110528. The author would like to acknowledge the assistance of Dr Sara Frye, Caroline Champagne, Kenneth Bottrill, and An Le.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Pettitt DJ, Talton J, Dabelea D, et al. Prevalence of diabetes in U.S. youth in 2009: the search for diabetes in youth study. Diabetes Care. 2014;37(2):402–408.
2. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes. Diabetes Care. 2018;41(Suppl 1):S55–S64.
3. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986.
4. Nathan DM; DCCT/EDIC Research Group. The Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16.
5. Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group. Update on cardiovascular outcomes at 30 years of the Diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):39–43.
6. American Diabetes Association. 3. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S28–S37.
7. Tfayli H, Arslanian S. The challenge of adolescence: hormonal changes and sensitivity to insulin. Diabetes Voice. 2007;52:28–30.
8. Perfect MM, Jaramillo E. Relations between resiliency, diabetes-related quality of life, and disease markers to school-related outcomes in adolescents with diabetes. Sch Psychol Q. 2012;27(1):29–40.
9. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities. Sec. 3. in standards of medical care in diabetes 2017. Diabetes Care. 2017;40(Suppl. 1):S25–S32.
10. Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311(1):
11. Rodríguez-Colón SM, He F, Bixler EO, et al. Sleep variability and cardiac autonomic modulation in adolescents – Penn State Child Cohort (PSCC) study. Sleep Med. 2015;16(1):67–72.
12. Räikkönen K, Matthews KA, Pesonen AK, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab. 2010;95(5):2254–2261.
13. Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152.
14. Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99(5):651–656.
15. Simon SL, Field J, Miller LE, Difrancesco M, Beebe DW. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PLoS One. 2015;10(2):e0115434.
16. Tahrani AA, Ali A. Obstructive sleep apnoea and type 2 diabetes. Eur Endocrinol. 2014;10(1):43–50.
17. Serrano RM, Navascués I, Ordóñez A, et al. Nocturnal growth hormone surges in type 1 diabetes mellitus are both sleep- and glycemia-dependent: assessment under continuous sleep monitoring. Diabetes Res Clin Pract. 1990;10(1):1–8.
18. Pallayova M, Donic V, Gresova S, Peregrim I, Tomori Z. Do differences in sleep architecture exist between persons with type 2 diabetes and nondiabetic controls? J Diabetes Sci Technol. 2010;4(2):344–352.
19. Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537.
20. Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Re Endocrinol. 2009;5(5):253–261.
21. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420.
22. Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–2133.
23. Nedeltcheva AV, Imperial JG, Jacqueline G, Penev PD. Effects of sleep restriction on glucose control and insulin secretion during diet-induced weight loss. Obesity (Silver Spring). 2012;20(7):1379–1386.
24. Petit JM, Burlet-Godinot S, Magistretti PJ, Allaman I. Glycogen metabolism and the homeostatic regulation of sleep. Metab Brain Dis. 2015;30(1):263–279.
25. Beebe DW, Rose D, Amin R. Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J Adolesc Health. 2010;47(5):523–525.
26. Jiang F, Vandyke RD, Zhang J, Li F, Gozal D, Shen X. Effect of chronic sleep restriction on sleepiness and working memory in adolescents and young adults. J Clin Exp Neuropsychol. 2011;33(8):892–900.
27. Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, McLaughlin EN. Manipulating sleep duration alters emotional functioning and cognitive performance in children. J Pediatr Psychol. 2013;38(10):1058–1069.
28. Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Development. 2003;74(2):444–455.
29. McNally K, Rohan J, Pendley JS, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33(6):1159–1162.
30. Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331(7524):1064–1065.
31. Jaser SS, Lord JH, Simmons JH, Malow BA. Brief report: sleep disturbances in young children with type 1 diabetes. Diabetes Res Clin Pract. 2016;120:232–234.
32. Monaghan M, Herbert LJ, Cogen FR, Streisand R. Sleep behaviors and parent functioning in young children with type 1 diabetes. Child Health Care. 2012;41(3):246–259.
33. Jaser SS, Foster NC, Nelson BA, et al. Sleep in children with type 1 diabetes and their parents in the T1D exchange. Sleep Med. 2017;39:108–115.
34. Hazen RA, Fehr KK, Fidler A, Cousino MK, Macleish SA, Gubitosi-Klug R. Sleep disruption in adolescents with type 1 diabetes mellitus: relationships with adherence and diabetes control. Diabetes Manag. 2015;5(4):257–265.
35. Perfect MM, Patel PG, Scott RE, et al. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep. 2012;35(1):81–88.
36. Perfect MM. The relations of sleep and quality of life to school performance in youth with type 1 diabetes. J Appl Sch Psychol. 2014;30(1):7–28.
37. Radan I, Rajer E, Uršič Bratina N, Neubauer D, Kržišnik C, Battelino T. Motor activity during asymptomatic nocturnal hypoglycemia in adolescents with type 1 diabetes mellitus. Acta Diabetol. 2004;41(2):33–37.
38. Matyka KA, Crawford C, Wiggs L, Dunger DB, Stores G. Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: relationship to nocturnal hypoglycemia. J Pediatr. 2000;137(2):233–238.
39. Pillar G, Schuscheim G, Weiss R, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142(2):163–168.
40. Caruso NC, Radovanovic B, Kennedy JD, et al. Sleep, executive functioning and behaviour in children and adolescents with type 1 diabetes. Sleep Med. 2014;15(12):1490–1499.
41. Happe S, Treptau N, Ziegler R, Harms E. Restless legs syndrome and sleep problems in children and adolescents with insulin-dependent diabetes mellitus type 1. Neuropediatrics. 2005;36(02):98–103.
42. Adler A, Gavan MY, Tauman R, Phillip M, Shalitin S. Do children, adolescents, and young adults with type 1 diabetes have increased prevalence of sleep disorders? Pediatr Diabetes. 2017;18(6):450–458.
43. Olsson L, Ahlbom A, Grill V, Midthjell K, Carlsson S. Sleep disturbances and low psychological well-being are associated with an increased risk of autoimmune diabetes in adults. Results from the Nord-Trøndelag Health Study. Diabetes Res Clin Pract. 2012;98(2):302–311.
44. van Dijk M, Donga E, van Dijk JG, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54(8):1967–1976.
45. Nefs G, Donga E, van Someren E, Bot M, Speight J, Pouwer F. Subjective sleep impairment in adults with type 1 or type 2 diabetes: results from diabetes MILES–The Netherlands. Diabetes Res Clin Pract. 2015;109(3):466–475.
46. Menting J, Nikolaus S, van der Veld WM, Goedendorp MM, Tack CJ, Knoop H. Severe fatigue in type 1 diabetes: exploring its course, predictors and relationship with HbA1c in a prospective study. Diabetes Res Clin Pract. 2016;121:127–134.
47. Denic-Roberts H, Costacou T, Orchard TJ. Subjective sleep disturbances and glycemic control in adults with long-standing type 1 diabetes: The Pittsburgh’s Epidemiology of Diabetes Complications study. Diabetes Res Clin Pract. 2016;119:1–12.
48. Janovsky CC, Rolim LC, Sá JR, et al. Cardiovascular autonomic neuropathy contributes to sleep apnea in young and lean type 1 diabetes mellitus patients. Front Endocrinol (Lausanne). 2014;5(1):119.
49. Palladino DK, Helgeson VS, Reynolds KA, Becker DJ, Siminerio LM, Escobar O. Emerging adults with type 1 diabetes: a comparison to peers without diabetes. J Pediatr Psychol. 2013;38(5):506–517.
50. Chontong S, Saetung S, Reutrakul S. Higher sleep variability is associated with poorer glycaemic control in patients with type 1 diabetes. J Sleep Res. 2016;25(4):438–444.
51. von Schnurbein J, Boettcher C, Brandt S, et al. Sleep and glycemic control in adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19(1):143–149.
52. Turner SL, Queen TL, Butner J, Wiebe D, Berg CA. Variations in daily sleep quality and type 1 diabetes management in late adolescents. J Pediatr Psychol. 2016;41(6):661–669.
53. Jaser SS, Ellis D. Sleep in adolescents and young adults with type 1 diabetes: associations with diabetes management and glycemic control. Health Psychol Behav Med. 2016;4(1):49–55.
54. Barone MT, Wey D, Schorr F, et al. Sleep and glycemic control in type 1 diabetes. Arch Endocrinol Metab. 2015;59(1):71–78.
55. Farabi SS, Carley DW, Akasheh RT, Quinn L. Tumor necrosis factor alpha increases following sleep in young adults with type 1 diabetes. Acta Diabetol. 2016;53(6):1049–1051.
56. Barnard K, James J, Kerr D, Adolfsson P, Runion A, Serbedzija G. Impact of chronic sleep disturbance for people living with type 1 diabetes. J Diabetes Sci Technol. 2016;10(3):762–767.
57. Winnick JB, Berg CA, Wiebe DJ, Schaefer BA, Lei PW, Butner JE. Metabolic control and academic achievement over time among adolescents with type 1 diabetes. School Psychol Quart. 2017;32(1):105–117.
58. McDonough RJ, Clements MA, DeLurgio SA, Patton SR. Sleep duration and its impact on adherence in adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18(4):262–270.
59. Patel NJ, Savin KL, Kahanda SN, et al. Sleep habits in adolescents with type 1 diabetes: variability in sleep duration linked with glycemic control. Pediatr Diabetes. 2018;19(6):1100–1106.
60. Matejko B, Kiec-Wilk B, Szopa M, Trznadel Morawska I, Malecki MT, Klupa T. Are late-night eating habits and sleep duration associated with glycemic control in adult type 1 diabetes patients treated with insulin pumps? J Diabetes Investig. 2015;6(4):460–464.
61. Borel AL, Pépin JL, Nasse L, Baguet JP, Netter S, Benhamou PY. Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes Care. 2013;36(10):2902–2908.
62. Larcher S, Gauchez AS, Lablanche S, Pépin JL, Benhamou PY, Borel AL. Impact of sleep behavior on glycemic control in type 1 diabetes: the role of social jetlag. Eur J Endocrinol. 2016;175(5):411–419.
63. Donga E, van Dijk M, van Dijk JG, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33(7):1573–1577.
64. Gruber R, Cassoff J, Frenette S, Wiebe S, Carrier J. Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics. 2012;130(5):e1155–e1161.
65. Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: a randomized controlled trial. J Child Psychol Psychiatry. 2014;55(3):273–283.
66. Fallone G, Acebo C, Seifer R, Carskadon MA. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep. 2005;28(12):1561–1567.
67. Lucassen EA, Piaggi P, Dsurney J, et al. Sleep extension improves neurocognitive functions in chronically sleep-deprived obese individuals. PLoS One. 2014;9(1):e84832.
68. de Bruin EJ, van Run C, Staaks J, Meijer AM. Effects of sleep manipulation on cognitive functioning of adolescents: a systematic review. Sleep Med Rev. 2017;32:45–57.
69. Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10(5):643–653.
70. Oztürk L, Pelin Z, Karadeniz D, Kaynak H, Çakar L, Gözükirmizi E. Effects of 48 hours sleep deprivation on human immune profile. Sleep Res Online. 1999;2(4):107–111.
71. Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43.
72. Perfect MM, Beebe D, Levine-Donnerstein D, Frye SS, Bluez GP, Quan SF. The development of a clinically relevant sleep modification protocol for youth with type 1 diabetes. Clin Pract Pediatr Psychol. 2016;4(2):227–240.
73. Bei B, Wiley JF, Trinder J, Manber R. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:108–124and.
74. Phillips AJK, Clerx WM, O’Brien CS, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017;7(1):3216.
75. Villa MP, Multari G, Montesano M, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia. 2000;43(6):696–702.
76. Kostkova M, Durdik P, Ciljakova M, et al. Short-term metabolic control and sleep in children and adolescents with type 1 diabetes mellitus. J Diabetes Complications. 2018;32(6):580–585.
77. Banghoej AM, Nerild HH, Kristensen PL, et al. Obstructive sleep apnoea is frequent in patients with type 1 diabetes. J Diabetes Complications. 2017;31(1):156–161.
78. Vale J, Manuel P, Oliveira E, et al. Obstructive sleep apnea and diabetes mellitus. Rev Port Pneumol (2006). 2015;21(2):55–60.
79. Manin G, Pons A, Baltzinger P, et al. Obstructive sleep apnoea in people with type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabet Med. 2015;32(1):90–96.
80. Borel AL, Benhamou PY, Baguet JP, et al. High prevalence of obstructive sleep apnoea syndrome in a type 1 diabetic adult population: a pilot study. Diabet Med. 2010;27(11):1328–1329.
81. Schober AK, Neurath MF, Harsch IA. Prevalence of sleep apnoea in diabetic patients. Clin Respir J. 2011;5(3):165–172.
82. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504.
83. Mondini S, Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Ann Neurol. 1985;17(4):391–395.
84. Iftikhar IH, Khan MF, das A, Magalang UJ. Meta-analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Ann Am Thorac Soc. 2013;10(2):115–120.
85. Chasens ER, Strollo PJ. Treatment of obstructive sleep apnea on insulin resistance: not an “Anti-Sugar Pill”. Ann Am Thorac Soc. 2013;10(2):150–151.
86. Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31(6):1183–1188.
87. Porter PA, Byrne G, Stick S, Jones TW. Nocturnal hypoglycaemia and sleep disturbances in young teenagers with insulin dependent diabetes mellitus. Arch Dis Child. 1996;75(2):120–123.
88. Feupe SF, Frias PF, Mednick SC, McDevitt EA, Heintzman ND. Nocturnal continuous glucose and sleep stage data in adults with type 1 diabetes in real-world conditions. J Diabetes Sci Technol. 2013;7(5):1337–1345.
89. Farabi SS, Carley DW, Quinn L. EEG power and glucose fluctuations are coupled during sleep in young adults with type 1 diabetes. Clin Neurophysiol. 2016;127(8):2739–2746.
90. Reutrakul S, Thakkinstian A, Anothaisintawee T, et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med. 2016;23:26–45.
91. Farabi SS. Type 1 diabetes and sleep. Diabetes Spectr. 2016;29(1):10–13.
92. Perez KM, Hamburger ER, Lyttle M, et al. Sleep in type 1 diabetes: implications for glycemic control and diabetes management. Curr Diab Rep. 2018;18(2):5.
93. Leong WB, Jadhakhan F, Taheri S, Chen YF, Adab P, Thomas GN. Effect of obstructive sleep apnoea on diabetic retinopathy and maculopathy: a systematic review and meta-analysis. Diabet Med. 2016;33(2):158–168.
94. Zhu Z, Zhang F, Liu Y, et al. Relationship of obstructive sleep apnoea with diabetic retinopathy: a meta-analysis. Biomed Research International. Biomed Res Int. 2017.
95. Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1–9.
96. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
97. de la Vega R, Tomé-Pires C, Solé E, et al. The Pittsburgh sleep quality index: validity and factor structure in young people. Psychol Assess. 2015;27(4):e22–e27.
98. Meltzer LJ, Brimeyer C, Russell K, et al. The children’s report of sleep patterns: validity and reliability of the sleep hygiene index and sleep disturbance scale in adolescents. Sleep Med. 2014;15(12):1500–1507.
99. Meltzer LJ, Avis KT, Biggs S, Reynolds AC, Crabtree VM, Bevans KB. The children’s report of sleep patterns (CRSP): a self-report measure of sleep for school-aged children. J Clin Sleep Med. 2013;9(3):235–245.
100. Meltzer LJ, Biggs S, Reynolds A, Avis KT, Crabtree VM, Bevans KB. The Children’s Report of Sleep Patterns–Sleepiness Scale: a self-report measure for school-aged children. Sleep Med. 2012;13(4):385–389.
101. Ioja S, Chasens ER, Ng J, Strollo PJ, Korytkowski MT. Obstructive sleep apnea in adults with type 1 and type 2 diabetes: perspectives from a quality improvement initiative in a university-based diabetes center. BMJ Open Diabetes Res Care. 2017;5(1):e000433.
102. Chung F, Abdullah HR, Liao P. Stop-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–638.
103. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, Stop-Bang, stop, and Epworth Sleepiness Scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70.
104. Combs D, Goodwin JL, Quan SF, Morgan WJ, Parthasarathy S. Modified Stop-Bang tool for stratifying obstructive sleep apnea risk in adolescent children. PLoS One. 2015;10(11):e0142242.
105. Nagappa M, Liao P, Wong J, et al. Validation of the Stop-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10(12):e0143697.
106. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491.
107. Perfect MM, Beebe DW, Levine-Donnerstein D, Frye SS, Bluez GP, Quan SF. The development of a clinically relevant sleep modification protocol for youth with type 1 diabetes. Clin Pract Pediatr Psychol. 2016;4(2):227–240.
108. Perfect MM, Frye SS. Considering the Z-factor in type 1 diabetes. Oral presentation at: International Pediatric Sleep Association Congress; April, 2018; Paris, France.
109. Bergner EM, Williams R, Hamburger ER, et al. Sleep in teens with type 1 diabetes: perspectives from adolescents and their caregivers. Diabetes Educ. 2018;44(6):541–548.
110. Borel AL, Benhamou PY, Baguet JP, et al. Short sleep duration is associated with a blood pressure nondipping pattern in type 1 diabetes: the DIAPASOM study. Diabetes Care. 2009;32(9):1713–1715.
111. Blanz BJ, Rensch-Riemann BS, Fritz-Sigmund DI, Schmidt MH. IDDM is a risk factor for adolescent psychiatric disorders. Diabetes Care. 1993;16(12):1579–1587.
112. Sturrock NDC, Moriarty KT. An assessment of perceived wellbeing in a diabetic population. Pract Diab Int. 1995;12(6):260–262.
113. Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27(12):2942–2947.
114. Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care. 2010;33(11):e142.
115. Miculis CP, de Campos W, da Silva Boguszweski MC. Correlation between glycemic control and physical activity level in adolescents and children with type 1 diabetes. J Phys Act Health. 2015;12(2):232–237.
116. Bot M, Pouwer F, De Jonge P, Tack CJ, Geelhoed-Duijvestijn PH, Snoek FJ. Differential associations between depressive symptoms and glycaemic control in outpatients with diabetes. Diabet Med. 2013;30(3):e115–e122.
117. Bächle C, Lange K, Stahl-Pehe A, Castillo K, Holl RW, Giani G, Rosenbauer J. Associations between HbA1c and depressive symptoms in young adults with early-onset type 1 diabetes. Psychoneuroendocrinology. 2015;1(55):48–58.
118. Bischoff AN, Reiersen AM, Buttlaire A, Al-Lozi A, Doty T, Marshall BA, Hershey T. Selective cognitive and psychiatric manifestations in Wolfram Syndrome. Orphanet J Rare Dis. 2015;10(1):66.
119. Tang Z, Wang J, Zhang H, et al. Associations between Diabetes and Quality of Life among Breast Cancer Survivors. PLoS One. 2016;11(6):e0157791.
120. Hood KK, Rohan JM, Peterson CM, Drotar D. Interventions with adherence-promoting components in pediatric type 1 diabetes: meta-analysis of their impact on glycemic control. Diabet Care. 2010;33:1658–64.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
