Back to Archived Journals » ChronoPhysiology and Therapy » Volume 5
Sleep electroencephalography as a biomarker in depression
Authors Steiger A, Pawlowski M, Kimura M
Received 21 November 2014
Accepted for publication 30 December 2014
Published 29 April 2015 Volume 2015:5 Pages 15—25
DOI https://doi.org/10.2147/CPT.S41760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Marc Hébert
Video presented by Axel Steiger and Marcel Pawlowski.
Views: 704
Axel Steiger, Marcel Pawlowski, Mayumi Kimura
Max Planck Institute of Psychiatry, Munich, Germany
Abstract: The sleep electroencephalogram (EEG) provides biomarkers of depression, which may help with diagnosis, prediction of therapy response, and prognosis in the treatment of depression. In patients with depression, characteristic sleep EEG changes include impaired sleep continuity, disinhibition of rapid-eye-movement (REM) sleep, and impaired non-REM sleep. Most antidepressants suppress REM sleep in depressed patients, healthy volunteers, and in animal models. REM suppression appears to be an important, but not an absolute requirement, for antidepressive effects of a substance. Enhanced REM density, a measure for frequency of REM, characterizes high-risk probands for affective disorders. REM-sleep changes were also found in animal models of depression. Sleep-EEG variables were shown to predict the response to treatment with antidepressants. Furthermore, certain clusters of sleep EEG variables predicted the course of the disorder for several years. Some of the predicted sleep EEG markers appear to be related to hypothalamic–pituitary–adrenal system activity.
Keywords: biomarkers, depression, sleep EEG, antidepressants, prediction, animal models
Introduction
Impaired sleep is a risk factor and a symptom of depression as well. Objective sleep is investigated by polysomnography, also termed a sleep electroencephalogram (EEG). The sleep EEG appears to provide biomarkers of depression and of the response to antidepressants. This review provides an overview of the state of the art in this area of research.
In the 1970s, the interest of psychiatrists in sleep EEG increased for two major reasons. On the one hand, rapid eye movement (REM) latency was suggested to be an indicator of depression.1 On the other hand, it was observed that most antidepressant drugs suppressed REM sleep.2 In the 1970s, it was therefore suggested that REM latency could be used to distinguish between certain subtypes of depression. Furthermore, the hypothesis was submitted that REM suppression would be the mechanism of action of antidepressants.3 Since that time, much more complex results from further research have developed.
Mammalian sleep consists of REM and non-REM sleep periods. Non-REM and REM sleep periods alternate in a cyclic fashion in healthy young humans subjects. The criteria developed by Rechtschaffen and Kales differentiate between four stages of non-REM sleep;4 a more recent classification consists of three stages only.5 Sleep stage 1 or N1 is a transition from drowsiness to light sleep.4,5 It is characterized by a slowing of EEG activity compared to the wake state. Sleep spindles and K-complexes occur during sleep stage 2, otherwise known as N2. Slow wave sleep (SWS) contains synchronized slow waves and consists of sleep stages 3 and 4 or N3 and N4, respectively.4,5 REM sleep is characterized by a faster EEG activity and subtle rapid eye movements and atonia of skeletal muscles. In animals, particularly in most laboratory animals that are used in experiments, sleep EEG resembles human sleep.6 In contrast to human sleep, however, animal sleep is often polyphasic, with episodic sleep–wake repetition. Non-REM sleep in animals is often not classified into several stages. The depth of non-REM sleep is interpreted according to the power of SWS.7 Since detection of eye movements in small animals is difficult, in these species, REM sleep (synonymously known as paradoxical sleep) is defined by a combination of a wake-like EEG pattern and electromyogram indicating muscle atonia.8 Computerized quantitative EEG analysis is applied in humans and animals to investigate the microstructure of sleep. It includes spectral analysis of EEG power across several frequency bands as beta, sigma, alpha, theta, and delta.
Young healthy human subjects enter N1 soon after going to bed, which is followed subsequently by N2 and SWS. After a duration of ~90 minutes in the first non-REM period, the first REM period occurs. During the first non-REM period, the largest amounts of SWS and slow wave activity (SWA), EEG delta power are found. In healthy subjects, the first REM period is relatively short. The duration of the REM periods increases throughout the night. According to this pattern, the first half of the night is dominated by SWS, whereas N2 non-REM sleep and REM sleep preponderate during the second half of the night (Figure 1). Four to five sleep cycles consisting of one episode of non-REM sleep and one episode of REM sleep occur during the night in most subjects. In laboratory rodents, however, non-REM sleep episodes last on average for a few minutes, during which they are often interrupted by brief periods of wakefulness or REM sleep.9,10 In nocturnal rats and mice, the resting phase occurs during the light period. Similar to humans, non-REM sleep preponderates first, followed by REM sleep, due to homeostatic control of non-REM sleep, a characteristic of both human and animal sleep.11
  | Figure 1 Hypnogram in young and old healthy volunteers, and patients with depression. |
Sleep EEG in patients with depression
In patients with depression, impaired sleep is a key symptom. Eighty percent of depression patients report insomnia, whereas 15%–35% complain of hypersomnia.12,13 The characteristic sleep EEG changes (Figure 1) in depressed patients consist of: 1) impaired sleep continuity (increase of sleep latency, elevated number of intermittent awakenings, early morning awakening); 2) disinhibited REM sleep: shortened REM latency, or sleep onset REM period (SOREMP; REM latency 0–20 minutes), prolonged first REM period, and elevated REM density (a measure of the amount of REM), particularly during the first REM period; and 3) changes of non-REM sleep (decreases of SWS, SWA, and N2; in younger patients, SWS and SWA shift from the first to the second non-REM period).14,15
Sleep EEG is influenced by age and sex in normal subjects and also in patients with depression. Already during the third decade of life, a decrease in SWS and SWA can be noted in both depressed and normal subjects. Menopause is a major turning point in sleep quality of women. A continuous decline in sleep quality during aging is found in male subjects. Age and illness act synergistically on sleep in patients with depression. Studies on age-related sleep EEG changes in depressed patients and in healthy subjects showed that REM latency is affected by age, and that no group differences occur until the middle of the third decade of life.16,17 REM density, however, does not vary with age and is elevated in all investigated age groups in patients. SWS decreases without differences between depressed patients and healthy subjects at any particular age.16,17
A bimodal distribution in REM latency was found in patients with depression.18 In this study, REM latencies between 20–40 minutes were rare, and most patients showed either very short SOREMPs 0–10 minutes after sleep onset, or latencies in the range between 40–60 minutes. SOREMPs were frequently found in older patients and in patients with psychotic depression.19,20 In depressed patients with either insomnia or hypersomnia, REM sleep disinhibition occurred.21
When drug-free depressed patients were studied during several consecutive days, the sleep EEG changes varied between days.22 During a period of 3 weeks, the sleep EEG of drug-free patients with depression showed continuous changes in REM latency, sleep continuity, and non-REM sleep as characteristic for depression, whereas the psychopathology improved.23 Between acute depression and a state of remission, no improvement in sleep EEG variables was found in patients with depression.24,25 In one of these studies, N4 decreased after remission in comparison to baseline.25 Similar results were reported in depressed adolescents.26 They showed reduced sleep efficiency, shorter REM latency, and elevated REM density in comparison to healthy subjects. After remission, no changes in sleep variables were found.26 These findings support the hypothesis that persisting sleep EEG changes in remitted patients may represent a biological scar.
Some studies reported an inverse correlation between severity of depression according to the Hamilton Depression Score and REM latency.1,27,28 In contrast, Feinberg et al found no relationship between sleep EEG and the severity of depression.29 In depressed children and adolescents, no sleep EEG changes were found in comparison to age-matched healthy controls in most studies.30–32 In contrast, other studies reported similar changes in depressed adults, children, and adolescents.26,33,34
Kupfer and Foster submitted the hypothesis that a shortened REM latency is a specific marker of depression.1 This view was challenged by observations that in other psychiatric disorders, similar changes occur, in particular in mania, schizophrenia, schizoaffective disorder, panic disorder, eating disorders, obsessive compulsive disorder, and in the case of sexual impotence.35–41 The reports of persisting sleep EEG changes in remitted patients point to a comorbidity of depression or anamnesis of depression as an explanation for shortened REM latency in these disorders.24,25 The latter view is supported by two studies.42,43 These authors compared healthy volunteers with three groups of patients with depression, anorexia nervosa, or bulimia. The latter two groups were never depressed. REM density was elevated in the patients with depression.42 The comparison of patients with depression, patients with panic disorder who were never depressed, and healthy subjects showed differences restricted to the first sleep cycle. SWS was reduced and REM time and REM density were increased in the patients with depression during this interval, whereas this sleep cycle was shortened in panic disorder. REM latency was shortened in comparison to healthy volunteers in both groups of patients.43 Increasing abnormality of REM sleep variables during subsequent episodes of depression was found in a long-term study.44 REM latency and decreased SWS appear not to differ between first and recurrent episodes of depression.45 However, increased phasic REM sleep and reduced sleep efficiency were reported in patients with recurrent unipolar depression.46 Further studies suggest a relationship between sleep EEG changes before treatment and outcome of treatment. These studies reported an association between shortened REM latency before treatment and response to antidepressant treatment.47–49 The predictive value for response to psychotherapy was reported for a set of disturbed sleep EEG variables (sleep efficiency, REM latency, and REM density).50,51 In patients who did not respond to psychotherapy, REM density before treatment was elevated, and an increased REM density after psychotherapy was a robust correlate of remission.52,53
Risk genes for depression and sleep EEG
The P2RX7 gene was identified as a susceptibility locus for affective disorders. A non-synonymous coding single nucleotide polymorphism in this gene (rs2230912), the Gln460Arg variant, is associated with major depression.54 To investigate whether carriers of the risk variant of the P2RX7 gene show sleep EEG changes, young healthy volunteers with low risk of depression underwent polysomnography. Homozygous (A/A) subjects were compared with heterozygous (A/G) carriers of the risk variant. Sleep EEG differed significantly between groups. In the A/G genotype, sleep latency was prolonged; sleep period time was shortened; the number of entries from N2 into shallow N1 and wakefulness were elevated during the first sleep cycle; frequencies in the lower spindle range were elevated, particularly in parietal regions; during non-REM sleep, peak frequencies of all sleep spindles were lower; and particularly in parietal derivations, beta frequencies during N2 were enhanced. Obviously, healthy volunteers with a potential risk for depression related to their P2RX7 genotype differ in the sleep EEG from the non-carriers. In the P2RX7 genotype, instability of N2 and changes in sleep spindles and in beta frequencies were observed.55
Sleep EEG in high-risk probands for affective disorders
The Munich Vulnerability Study On Affective Disorders applied a prospective high-risk design in order to identify premorbid vulnerability factors for affective disorders.56 In this study, high-risk probands were included. They had a high genetic load for affective disorders because of their positive family history. The comparison group was composed of healthy probands with a negative family history for affective disorders. At the index investigation, REM density was enhanced, and the time spent in SWS during the first non-REM period was reduced in high-risk probands.56 This finding remained stable for about 4years, until a follow-up examination.57 The cholinomimetic agent RS86 was used to perform the cholinergic REM sleep induction test in a subgroup of high-risk probands. After cholinergic stimulation, REM latency was reduced in the high-risk probands, whereas no difference was found at baseline between these probands and the normal controls.58 This observation points to a threshold cholinergic dysfunction in the high-risk probands. Their response pattern in the REM sleep induction test predicted the onset of the first episode of depression.59 Twenty subjects of the sample of 82 high-risk probands enrolled in the study developed an affective disorder during the follow-up period. REM density was increased during the total night’s sleep, and during the first REM period, in comparison to normal controls as measured by the premorbid sleep EEG in these affected high-risk probands.60 These findings show that enhanced REM density meets the requirements for biological vulnerability markers for affective disorders.
Sleep EEG changes after antidepressants in patients and healthy volunteers
Most antidepressants suppress REM sleep. In humans, REM suppression was observed after tricyclics (except for trimipramine) (Figure 2), iprindole, reversible and short-acting reversible monoaminooxidase inhibitors, tetracyclics, selective serotonin-reuptake inhibitors (SSRIs), selective noradrenaline-reuptake inhibitors and selective serotonin and noradrenaline-reuptake inhibitors.2,61–74 REM sleep is not affected by only a few antidepressants, specifically trimipramine, bupropion, the noradrenergic and specific serotoninergic antidepressant mirtazapine, and the serotonin-reuptake enhancer tianeptine.64,75–80 REM suppression includes elevated REM latency, reduced REM time, and decreased REM density. Withdrawal of REM-suppressing antidepressants is followed by REM rebound. During REM rebound, REM latency is shortened, and REM time and REM density are elevated. All these variables exceed baseline values. In healthy volunteers who were treated with antidepressants for 2 weeks, the REM rebound persisted 1 week after cessation.81
  | Figure 2 Changes in hypnograms during treatment of depression with trimipramine or imipramine. Imipramine, but not trimipramine, suppresses REM sleep. |
The ability to suppress REM sleep differs between various antidepressants. Total abolishment of REM sleep is found after clomipramine and the irreversible monoaminooxidase inhibitors phenelzine and tranylcypromine.66,82 A nearly total REM suppression was found after a single dose of the combined SSRI and serotonin 5-HT1A receptor agonist vilazodone in healthy volunteers.83 In addition to these substances, the dosage and the plasma concentrations of antidepressants influence the extent of changes in REM sleep.82
The REM-suppressing effect of antidepressants is shared by other substances, especially barbiturates, amphetamines, and alcohol. However, subchronic administration of these substances is followed by relatively fast adaptation. In contrast, REM suppression after antidepressants persists for longer periods.84 A weak adaptation was found, however, after administration of tricyclic antidepressants.85,86 The reversible monoaminooxidase inhibitors prompt a REM suppression for several months.66,67 Small amounts of REM sleep reoccurred after 3 to 6 months’ treatment with phenelzine in three depressed patients.87 Vogel et al submitted the hypothesis that REM suppression is a mode of action characteristic of antidepressants.3 This view is supported by the observation of these authors that selective REM sleep deprivation by awakenings for 3 weeks has antidepressant effects, whereas selective non-REM sleep deprivation had no effect.3 In another study, no effect of selective REM sleep suppression after testing for 11 days was found.88 The hypothesis submitted by Vogel et al3 is also challenged by the antidepressive effect of substances that do not suppress REM sleep, such as trimipramine, mirtazapine, and bupropion. REM sleep even increased during treatment of depression with these drugs.64,75,79,89 REM suppression appears to indicate the antidepressive action of the substance, but it is not an absolute determinant. The importance of REM suppression as prerequisite for antidepressive effect of a substance is supported by a study comparing the effects of the stereoisomers of oxaprotiline, R(−)oxaprotiline, and S(+)oxaprotiline. S(+)oxaprotiline, but not R(−)oxaprotiline, suppresses REM sleep in depressed patients. The antidepressive effects of S(+)oxaprotiline were superior to R(−)oxaprotiline.90
Although most antidepressants exert similar effects on REM sleep, differences exist in their influence on non-REM sleep and on sleep continuity. SWS increases after most tricyclics.2 In contrast, SWS decreases after clomipramine and imipramine.64,82 No change in SWS was observed after desipramine, nortriptyline, and amitriptyline in patients with depression.85.86,91 SSRIs increase intermittent wakefulness and impair sleep continuity, but do not exert major effects on SWS.92,93 Also, the noradrenaline-reuptake inhibitor reboxetine decreases sleep efficiency and increases intermittent wakefulness andN2.73 After administration of vilazodone, in addition to nearly total REM suppression, SWS and SWA increased in the first and the last third of the night in healthy volunteers.83 During treatment of depression with the selective serotonin and noradrenaline-reuptake inhibitor duloxetine, N3 increased.74 By the second day of treatment of patients with depression with mirtazapine, total sleep time and sleep efficiency increased, and the time spent awake decreased. These changes persisted after 4 weeks. At this time, SWS, low delta, theta, and alpha activity were elevated.79 A correlation was found between the increase in REM latency after 2 days of treatment with amitriptyline, and the clinical outcome after 4 weeks.94 Similar findings were reported for imipramine, but not for clomipramine.64,95
Biomarkers as predictors of therapy response and relapse
The effects of the SSRI paroxetine and the serotonin-reuptake enhancer tianeptine on sleep EEG were investigated in a trial80 with a focus on a treatment response. Sleep EEG was recorded at days 7 and 42 of active treatment. Independently of medication in male responders to treatment a decline in the higher sigma frequency range (14–16 Hz) during non-REM sleep was observed. In contrast, in both male and female non-responders, no marked changes in this frequency range were found. A decrease in the time spent in REM sleep and an increase in intermittent wakefulness occurred after paroxetine, but not after tianeptine. REM density after 1 week of treatment was a predictor of treatment response in the total sample of patients. An inverse correlation of the changes in REM density and the changes in the Hamilton Depression Score was found in the paroxetine group (Figure 3), but not in the tianeptine group. These findings underline that it is important to take sex differences into account in studies of neurobiological drug effects.80
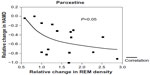  | Figure 3 Correlation between the relative change in HAMD (difference HAMD at D7 – HAMD at D42) in the course of treatment with paroxetine, in relation to the relative change in REM density. REM density increases with the decline in HAMD. |
Regarding quantitative electroencephalographic measures, there are three methods for measuring resting state EEG, which have repeatedly been shown to predict antidepressant treatment outcome in the course of early treatment: 1) cordance,96 2) the Antidepressant Treatment Response Index, and 3) low-resolution brain electromagnetic tomography.97 Until now, only cordance has been applied within sleep EEG for the purpose of treatment prediction. Cordance is calculated from a full scalp electrode array and integrates information from absolute and relative power measures of EEG spectra in a single measurement. Cordance has been proven to show moderate associations with underlying cerebral perfusion.98,99 A considerable body of research during resting state EEG supports the assertion that an early reduction in prefrontal EEG cordance can serve as a predictor of the response to antidepressant medication and that this is particularly the case for theta frequency ranges.100–106 Cordance derived from sleep EEG was examined after 1 week of treatment with antidepressants.107 An association of first week theta cordance with the HAM-D score at the last treatment of 4 weeks was found. This sleep EEG related approach differs from the resting state EEG measurements. The latter method requires examinations at two time points (baseline and first week) in order to calculate the changes of cordance. In the sleep EEG-derived approach, one time point is sufficient to determine correlations between prefrontal theta cordance at the first week and the HAM-D after 4 weeks of treatment. This method allows researchers to separate responders and non-responders to antidepressant treatment with a positive predictive value of 83%, which is higher than in the resting state (72%).101
In an exploratory follow-up study,108 sleep EEG of patients with depression who had participated in an earlier trial with trimipramine over 6 weeks was investigated. Their sleep EEG was examined in order to determine whether 1) the previous number of depressive episodes, and 2) the prospective long-term outcome as reflected by further episodes, were associated with sleep EEG variables. The previous long-term course of depression was related with sleep EEG variables during the acute episode. The lower the sleep continuity (eg, total sleep time, sleep efficiency index, time spent awake, and the number of awakenings), the higher the number of previous episodes. This association disappeared at the end of drug treatment. Furthermore, an association between a lower amount of SWS, particularly during the first third of the sleep period, enhanced REM density, and the number of previous episodes was found. In addition, an association between the prospective long-term course and sleep EEG was demonstrated. Reduced time spent time in SWS and elevated REM density at the end of treatment were related to an increased recurrence rate of depressive episodes during the follow-up period. These sleep EEG variables were related to changes in the hypothalamic–pituitary–adrenal (HPA) system, as reflected by abnormal dexamethasone/ corticotropin-releasing hormone (CRH) test results. Obviously, patients with an unfavorable long-term course of depression show increasingly disturbed sleep regulation; these changes appear to predict the treatment response during the acute episode of illness, and also predict relapses during the follow-up. These predictive sleep EEG biomarkers of the long-term course of depression seem to be related to HPA activity. The more sleep EEG markers were aberrant, the more the HPA system was disturbed.108 The view that HPA overactivity contributes to sleep EEG aberrances in depressed patients is supported by these findings.109
The role of the HPA system in sleep EEG changes in depression
Elevated nocturnal cortisol and adrenocorticotropic hormone concentrations are state markers of acute depressive episodes.25,110 Administration of the key hormone of the HPA system, CRH, induced more shallow sleep in rats, rabbits, and mice.9,111–113 Similarly, after pulsatile injection of CRH to healthy volunteers, sleep–endocrine changes similar to those in depressed patients were induced. In particular, in young male healthy subjects, decreases in SWS and growth hormone occurred, and in young healthy women, decrease inN3, increased wakefulness, and more REM sleep during the first third of the night were found.114,115 Subchronic treatment with the synthetic glucocorticoid receptor agonist methylprednisolone in female patients with multiple sclerosis prompted sleep EEG changes resembling those in patients with depression (shortened REM latency, enhanced REM density, shift of SWS, and SWA from the first to the second non-REM period).116 Already in kindergarten-aged children, an association between unfavorable sleep EEG pattern, elevated HPA activity, and more difficult behavior in the psychosocial dimension was reported.117 In this study, sleep EEG was recorded, and saliva samples were collected after awakening. Furthermore, saliva was sampled before, during, and after psychological challenge in order to examine HPA activity after stress. Poor sleepers among the children showed higher cortisol morning levels than good sleepers. After stress, elevated cortisol was associated with an increased number of intermittent awakenings and increases in N1 and N2 of non-REM sleep. Furthermore, psychological difficulties such as over-anxiousness, impulsivity, and social inhibition were found to be associated with reduced sleep efficiency.117
Two different dose ranges of the CRH-1 receptor antagonist R121919 were given to depressed patients.29 In a subgroup of these patients, sleep EEG was recorded before treatment, at the end of the first week, and at the end of the fourth week of active medication.
One week and after 4 weeks of active treatment, the time spent in SWS increased when compared to baseline. During the same time period, the number of awakenings and REM density decreased. In a separate evaluation of these findings that was performed for both dose ranges, significant effects in the lower dose range were found, whereas in the higher dosage group, REM density decreased and SWS increased significantly between baseline and week 4. This observation supports the hypothesis that CRH is involved in the pathophysiology of sleep EEG changes during depression. In addition, the finding suggests that CRH-1 receptor antagonism helps to normalize sleep of patients with depression.118
Sleep phenotypes in animal models of depression: from the perspective of altered stress-response systems
Earlier studies on animal models of depressive disorders have already demonstrated significant changes in animal sleep patterns. Most of these study models were generated from rats or mice, which were genetically stress-vulnerable strains or selectively bred depending on their negative stress-coping behavior in response to aversive environments.10,39,119 Validated not only by behavioral phenotypes, the models often have a disposition to alternations in HPA-axis activity. Learned helplessness is an example that accompanies elevated plasma levels of corticosterone, REM sleep enhancement, and also resembles the findings in patients with depression, as mentioned in the section on Sleep EEG in patients with depression.120–122 Enhanced REM sleep has been acknowledged to predict a model for depression. Indeed, increased REM sleep is a prominent sleep phenotype in two different types of depression models: prenatally stressed rats and Wister-Kyoto rats.123,124 Such REM sleep changes displayed by the animal models can correspond to REM sleep disinhibition observed in depressed human patients, while insomniac phenomena such as prolonged wakefulness or reduced SWS are not very visible in the animal models.120,125,126 Similarly, the distinct stress reactivity mouse model in reference to the HPA axis that shows high stress reactivity, spends time in more REM sleep than the other lines with intermediate or low stress reactivity.127,128 The amount of non-REM sleep is indistinguishable between these three separate breeding lines selected for high (HR), intermediate (IR), or low (LR) corticosterone increases in response to stressors. However, the high stress reactivity line shows lower SWA during non-REM sleep, whereas the theta power in the LR line is lower than the others across all vigilance states.
Considering the key role of HPA overactivity in depression, the effects of central CRH on sleep–wake behavior were investigated using brain-site specific CRH-overexpressing mice.129 The conditional CRH-overexpressing (CRH-COE) lines have normal HPA-axis activity; although the mice have more CRH in a particular part of their brain, peripheral levels of stress hormones are intact.130 Interestingly, central nervous system-specific and forebrain-specific CRH-COE mice exhibit enhanced REM sleep, whereas non-REM sleep and SWA are not markedly affected.131 From this study, REM sleep disinhibition could be suggested to occur depending on a local hypersecretion of CRH, independently from the whole HPA overstimulation. Further, the CRH-COE mice seemed to be more sensitive than control littermates to a muscarinic agent in relation to the appearance of REM sleep.132 Since the Flinders Sensitive rat line also show increased responses to an anti-cholinergic agent, some animal models of depression with elevated REM sleep may resemble depressed human patients in terms of hypercholinergic reactivity.122,133–135 Again, these observations emphasize that elevated REM sleep is a credible marker for the animal models of depression.
In addition, chronic stress is often used to establish models accounting for stress vulnerability/resilience and mechanisms of depression.136,137 With exposure to inescapable chronic mild stress, a mouse model that has received early life stress (long maternal separation, otherwise known as LMS) shows different sleep responses.138 Compared to its control group (brief maternal separation), the power of EEG spectra during both non-REM and REM sleep was significantly lower in the LMS group during recovery after chronic stress. Although the amount of sleep, especially REM sleep, does not differ under baseline condition between these two groups, LMS, which is a sort of chronic stress, leads to increased vulnerability and is detected in sleep EEG. Taken together, spectrum changes in EEG activity during sleep are useful parameters to validate models for mood disturbances.
Conclusion
Sleep EEG variables appear to provide biomarkers for the diagnosis, drug treatment, and prognosis of depression. Sleep EEG variables are among the biomarkers that should be included in the classification of mood disorders. Even healthy volunteers with a risk gene of depression show subtle sleep EEG changes. In healthy subjects at high risk for affective disorders, elevated REM density was found. In patients with depression disturbed sleep continuity, REM sleep disinhibition and impaired non-REM sleep are characteristic findings. Sleep-EEG variables like REM latency, certain clusters of sleep variables, and variables derived from cordance during sleep were shown to predict responses to treatments with certain antidepressants, or the course of the illness, for several years. Until today, sleep EEG variables have not been applied for general use in clinical practice in order to select the most effective drug for individual patients. More efforts are necessary in this area of research. Nowadays, the applied methods in sleep EEG recording are often complicated and too difficult for practical use. Therefore, more simple methods should be developed to allow wide-spread use. Most, but not all antidepressants suppress REM sleep in humans and in animal models, whereas REM suppression is not mandatory for a substance to act as an antidepressant. Therefore, another important research task is the identification of sleep EEG variables that are indicative of antidepressive effects.
Disclosure
The authors declare no conflicts of interest in this work.
References
Kupfer DJ, Foster FG. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet. 1972;2:684–686. | |
Chen CN. Sleep, depression and antidepressants. Br J Psychiatry. 1979;135:385–402. | |
Vogel GW, Thurmond A, Gibbons P, Sloan K, Walker M. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32:765–777. | |
Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: US Department of Health, Education and Welfare, Neurological Information Network; 1968. | |
Iber C, Anoli-Israel S, Chesson A, Quan SF; American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. | |
Allison T, Van Twyver H. The evolution of sleep. Nat Hist. 1970;79: 56–65. | |
Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. | |
Jouvet M, Michel F, Courjon J. Sur un stade d’activité électrique cérebrale rapide a cours du sommeil physiologique [On a stage of rapid cerebral electrical activity in the course of physiological sleep]. C R Seances Soc Biol Fil. 1959;153:1024–1028. French. | |
Romanowski C, Fenzl F, Flachskamm C, Deussing JM, Kimura F. CRH-R1 is involved in effects of CRH on NREM, but not REM, sleep suppression. Sleep Biol Rhythms. 2007;5(Suppl 1):A53. | |
Jakubcakova V, Flachskamm C, Landgraf R, Kimura M. Sleep phenotyping in a mouse model of extreme trait anxiety. PLoS One. 2012;7: e40625. | |
Deboer T. Behavioral and electrophysiological correlates of sleep and sleep homeostasis. In: Current Topics in Behavioral Neurosciences. Geyer MA, Ellenbroek BA, Marsden CA, Barnes TRE, editors. Berlin: Springer; 2013:1–24. | |
Hawkins DR, Taub JM, Van de Castle RL. Extended sleep (hypersomnia) in young depressed patients. Am J Psychiatry. 1985;142:905–910. | |
Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand. 2007;115(Suppl 433):104–115. | |
Reynolds CF 3rd, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10:199–215. | |
Benca RM, Okawa M, Uchiyama M, et al. Sleep and mood disorders. Sleep Med Rev. 1997;1:45–56. | |
Lauer C, Riemann D, Wiegand M, Berger M. From early to late adulthood. Changes in EEG sleep of depressed patients and healthy volunteers. Biol Psychiatry. 1991;29:979–993. | |
Riemann D, Hohagen F, Lauer C, Berger M. Longterm evolution of sleep in depression. In: Smirne S, Franceschi M, Ferini-Strambi L, editors. Sleep and Aging. Paris: Masson; 1991:195–204. | |
Schulz H, Lund R, Cording C, Dirlich G. Bimodal distribution of REM sleep latencies in depression. Biol Psychiatry. 1979;14:595–600. | |
Ansseau M, Kupfer DJ, Reynolds CF 3rd, McEachran AB. REM latency distribution in major depression: clinical characteristics associated with sleep onset REM periods. Biol Psychiatry. 1984: 1651–1666. | |
Kupfer DJ, Reynolds CF 3rd, Grochocinski VJ, Ulrich RF, McEachran A. Aspects of short REM latency in affective states: a revisit. Psychiatry Res. 1986;17:49–59. | |
Gillin JC, Sitaram N, Wehr T, et al. Sleep and affective illness. In: Post RM, Ballenger JC, editors. Neurobiology of Mood Disorders. Baltimore: Williams and Wilins; 1984;157–189. | |
Schulz H, Lund R, Doerr P. The measurement of change in sleep during depression and remission. Arch Psychiatr Nervenkr. 1978;225: 233–245. | |
Coble PA, Kupfer DJ, Spiker DG, Neil JF, McPartland RJ. EEG sleep in primary depression. A longitudinal placebo study. J Affect Disord. 1979;1:131–138. | |
Rush AJ, Erman MK, Giles DE, et al. Polysomnographic findings in recently drug-free and clinically remitted depressed patients. Arch Gen Psychiatry. 1986;43:878–884. | |
Steiger A, von Bardeleben U, Herth T, Holsboer F. Sleep EEG and nocturnal secretion of cortisol and growth hormone in male patients with endogenous depression before treatment and after recovery. J Affect Disord. 1989;16:189–195. | |
Rao U, Poland RE. Electroencephalographic sleep and hypothalamic-pituitary-adrenal changes from episode to recovery in depressed adolescents. J Child Adolesc Psychopharmacol. 2008;18:607–613. | |
Kupfer DJ, Foster FG, Reich L, Thompson SK, Weiss B. EEG sleep changes as predictors in depression. Am J Psychiatry. 1976;133: 622–626. | |
Spiker DG, Coble P, Cofsky J, Foster FG, Kupfer DJ. EEG sleep and severity of depression. Biol Psychiatry. 1978;13:485–488. | |
Feinberg M, Gillin JC, Carroll BJ, Greden JF, Zis AP. EEG studies of sleep in the diagnosis of depression. Biol Psychiatry. 1982;17: 305–316. | |
Taub JM, Hawkins DR, Van de Castle RL. Electrographic analysis of the sleep cycle in young depressed patients. Biol Psychol. 1978;7: 203–214. | |
Puig-Antich J, Goetz R, Hanlon C, et al. Sleep architecture and REM sleep measures in prepubertal children with major depression: a controlled study. Arch Gen Psychiatry. 1982;39:932–939. | |
Goetz RR, Puig-Antich J, Ryan N, et al. Electroencephalographic sleep of adolescents with major depression and normal controls. Arch Gen Psychiatry. 1987;44:61–68. | |
Kupfer DJ, Coble P, Kane J, Petti T, Conners CK. Imipramine and EEG sleep in children with depressive symptoms. Psychopharmacology (Berl). 1979;60:117–123. | |
Lahmeyer HW, Poznanski EO, Bellur SN. EEG sleep in depressed adolescents. Am J Psychiatry. 1983;140:1150–1153. | |
Hudson JI, Lipinski JF, Frankenburg FR, Grochocinski VJ, Kupfer DJ. Electroencephalographic sleep in mania. Arch Gen Psychiatry. 1988; 45:267–273. | |
Zarcone VP Jr, Benson KL, Berger PA. Abnormal rapid eye movement latencies in schizophrenia. Arch Gen Psychiatry. 1987;44:45–48. | |
Reich L, Weiss BL, Coble P, McPartland R, Kupfer DJ. Sleep disturbance in schizophrenia. A revisit. Arch Gen Psychiatry. 1975;32: 51–55. | |
Uhde TW, Roy-Byrne P, Gillin JC, et al. The sleep of patients with panic disorder: a preliminary report. Psychiatry Res. 1984;12:251–259. | |
Katz JL, Kuperberg A, Pollack CP, Walsh BT, Zumoff B, Weiner H. Is there a relationship between eating disorder and affective disorder? New evidence from sleep recordings. Am J Psychiatry. 1984;141: 753–759. | |
Insel TR, Gillin C, Moore A, Wallace BM, Loewenstein RJ, Murphy DL. The sleep of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1982;39:1372–1377. | |
Schmidt HS, Nofzinger EA. Short REM latency in impotence without depression. Biol Psychiatry. 1988;24:25–32. | |
Lauer CJ, Krieg JC, Riemann D, Zulley J, Berger M. A polysomnographic study in young psychiatric inpatients: major depression, anorexia nervosa, bulimia nervosa. J Affect Disord. 1990;18:235–245. | |
Lauer CJ, Krieg JC, Garcia-Borreguero D, Ozdaglar A, Holsboer F. Panic disorder and major depression: a comparative electroencephalogramic sleep study. Psychiatry Res. 1992;44:41–54. | |
Kupfer DJ, Ehlers CL, Frank E, Grochocinski VJ, McEachran AB. EEG sleep profiles and recurrent depression. Biol Psychiatry. 1991;30: 641–655. | |
Thase ME, Kupfer DJ, Buysse DJ, et al. Electroencephalographic sleep profiles in single-episode and recurrent unipolar forms of major depression: I. Comparison during acute depressive states. Biol Psychiatry. 1995;38:506–515. | |
Jindal RD, Thase ME, Fasiczka AL, et al. Electroencephalographic sleep profiles in single-episode and recurrent unipolar forms of major depression: II. Comparison during remission. Biol Psychiatry. 2002;51: 230–236. | |
Svendsen K, Christensen PG. Duration of REM sleep latency as predictor of effect of antidepressant therapy. A preliminary report. Acta Psychiatr Scand. 1981;64:238–243. | |
Rush AJ, Giles DE, Jarrett RB, et al. Reduced REM latency predicts response to tricyclic medication in depressed outpatients. Biol Psychiatry. 1989;26:61–72. | |
Heiligenstein JH, Faries DE, Rush AJ, et al. Latency to rapid eye movement sleep as a predictor of treatment response to fluoxetine and placebo in nonpsychotic depressed outpatients. Psychiatry Res. 1994;52: 327–339. | |
Thase ME, Simons AD, Reynolds CF 3rd. Abnormal electroencephalographic sleep profiles in major depression: association with response to cognitive behavior therapy. Arch Gen Psychiatry. 1996;53:99–108. | |
Thase ME, Kupfer DJ, Fasiczka AJ, Buysse DJ, Simons AD, Frank E. Identifying an abnormal electroencephalographic sleep profile to characterize major depressive disorder. Biol Psychiatry. 1997;41: 964–973. | |
Buysse DJ, Tu XM, Cherry CR, et al. Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and nonremitters. Biol Psychiatry. 1999;45:205–213. | |
Buysse DJ, Hall M, Begley A, et al. Sleep and treatment response in depression: new findings using power spectral analysis. Psychiatry Res. 2001;103:51–67. | |
Lucae S, Salyakina D, Barden N, et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. | |
Adamczyk M, Friess E, Uhr M, Steiger A. Sleep-EEG differences in healthy volunteers related to Gln460arg polymorphism of P2RX7 gene. Biol Psychiatry. 2014;75(Suppl):71. | |
Lauer CJ, Schreiber W, Holsboer F, Krieg JC. In quest of identifying vulnerability markers for psychiatric disorders by all-night polysomnography. Arch Gen Psychiatry. 1995;52:145–153. | |
Modell S, Ising M, Holsboer F, Lauer CJ. The Munich Vulnerability Study on Affective Disorders: stability of polysomnographic findings over time. Biol Psychiatry. 2002;52:430–437. | |
Schreiber W, Lauer CJ, Krumrey K, Holsboer F, Krieg JC. Cholinergic REM sleep induction test in subjects at high risk for psychiatric disorders. Biol Psychiatry. 1992;32:79–90. | |
Lauer CJ, Modell S, Schreiber W, Krieg JC, Holsboer F. Prediction of the development of a first major depressive episode with a rapid eye movement sleep induction test using the cholinergic agonist RS 86. J Clin Psychopharmacol. 2004;24:356–357. | |
Modell S, Ising M, Holsboer F, Lauer CJ. The Munich vulnerability study on affective disorders: premorbid polysomnographic profile of affected high-risk probands. Biol Psychiatry. 2005;58:694–699. | |
Dunleavy DL, Brezinova V, Oswald I, MacLean AW, Tinker M. Changes during weeks in effects of tricyclic drugs on the human sleeping brain. Br J Psychiatry. 1972;120:663–672. | |
Dunleavy DL, Oswald I. Phenelzine, mood response, and sleep. Arch Gen Psychiatry. 1973;28:353–356. | |
Passouant P, Cadilhac J, Billiard M, Besset A. La suppression du sommeil paradoxal par la clomipramine. Thérapie. 1973;28:379–392. French. | |
Sonntag A, Rothe B, Guldner J, Yassouridis A, Holsboer F, Steiger A. Trimipramine and imipramine exert different effects on the sleep EEG and on nocturnal hormone secretion during treatment of major depression. Depression. 1996;4:1–13. | |
Cramer H, Ohlmeier D. Ein fall von tranylcypromin undtTrifluoperazin - (jatrosom)-sucht: psychopathologische, schlafphysiologische und biochemische Untersuchungen. Arch Psychiatr Nervenkr. 1967;210: 182–197. German. | |
Akindele MO, Evans JI, Oswald I. Monoamine oxidase inhibitors, sleep and mood. Electroencephalogr Clin Neurophysiol. 1970;29:47–56. | |
Wyatt RJ, Fram DH, Kupfer DJ, Snyder F. Total prolonged drug-induced REM sleep suppression in anxious depressed patients. Arch Gen Psychiatry. 1971;24:145–155. | |
Cohen RM, Pickar D, Garnett D, Lipper S, Gillin JC, Murphy DL. REM sleep suppression induced by selective monoamine oxidase inhibitors. Psychopharmacology (Berl). 1982;78:137–140. | |
Landolt HP, Raimo EB, Schnierow BJ, Kelsoe JR, Rapaport MH, Gillin JC. Sleep and sleep electroencephalogram in depressed patients treated with phenelzine. Arch Gen Psychiatry. 2001;58:268–276. | |
Steiger A, Benkert O, Holsboer F. Effects of long-term treatment with the MAO-A inhibitor moclobemide on sleep EEG and nocturnal hormonal secretion in normal men. Neuropsychobiology. 1994;30:101–105. | |
Shipley JE, Kupfer DJ, Dealy RS, et al. Differential effects of amitriptyline and of zimelidine on the sleep electroencephalogram of depressed patients. Clin Pharmacol Ther. 1984;36:251–259. | |
von Bardeleben U, Steiger A, Gerken A, Holsboer F. Effects of fluoxetine upon pharmacoendocrine and sleep-EEG parameters in normal controls. Int Clin Psychopharmacol. 1989;4 (Suppl 1):1–5. | |
Künzel HE, Murck H, Held K, Ziegenbein M, Steiger A. Reboxetine induces similar sleep EEG changes like SSRI`s in patients with depression. Pharmacopsychiatry. 2004;37:193–195. | |
Kluge M, Schüssler P, Steiger A. Duloxetine increases stage 3 sleep and suppresses rapid eye movement (REM) sleep in patients with major depression. Eur Neuropsychopharmacol. 2007;17:527–531. | |
Nofzinger EA, Reynolds CF 3rd, Thase ME, et al. REM sleep enhancement by bupropion in depressed men. Am J Psychiatry. 1995;152: 274–276. | |
Ruigt GSF, Kemp B, Groenhout CM, Kamphuisen HA. Effect of the antidepressant Org 3770 on human sleep. Eur J Clin Pharmacol. 1990;38:551–554. | |
Aslan S, Isik E, Cosar B. The effects of mirtazapine on sleep: a placebo controlled, double-blind study in young healthy volunteers. Sleep. 2002;25:677–679. | |
Winokur A, DeMartinis NA3rd, McNally DP, Gary EM, Cormier JL, Gary KA. Comparative effects of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. J Clin Psychiatry. 2003;64:1224–1229. | |
Schmid DA, Wichniak A, Uhr M, et al. Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin and leptin and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacology. 2006;31:832–844. | |
Murck H, Nickel T, Künzel H, et al. State markers of depression in sleep EEG: dependency on drug and gender in patients treated with tianeptine or paroxetine. Neuropsychopharmacology. 2003;28: 348–358. | |
Steiger A, von Bardeleben U, Guldner J, Lauer C, Rothe B, Holsboer F. The sleep EEG and nocturnal hormonal secretion. Studies on changes during the course of depression and on effects of CNS-active drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:125–137. | |
Steiger A. Effects of clomipramine on sleep-EEG and nocturnal penile tumescence (NPT), a long-term study in a healthy man. J Clin Psychopharmacol. 1988;8:349–354. | |
Murck H, Frieboes RM, Antonijevic IA, Steiger A. Distinct temporal pattern of the effects of the combined serotonin-reuptake inhibitor and 5-HT1A agonist EMD 68843 on the sleep EEG in healthy men. Psychopharmacology (Berl). 2001;155:187–192. | |
Vogel GW. Evidence for REM sleep deprivation as the mechanism of action of antidepressant drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:343–349. | |
Gillin JC, Wyatt RJ, Fram D, Snyder F. The relationship between changes in REM sleep and clinical improvement in depressed patients treated with amitriptyline. Psychopharmacology (Berl). 1978;59: 267–272. | |
Shipley JE, Kupfer DJ, Griffin SJ, et al. Comparison of effects of desipramine and amitriptyline on EEG sleep of depressed patients. Psychopharmacology (Berl). 1985;85:14–22. | |
Landolt HP, de Boer LP. Effect of chronic phenelzine treatment on REM sleep: report of three patients. Neuropsychopharmacology. 2001;25(Suppl 5):S63–S67. | |
Grözinger M, Kogel P, Röschke J. Effects of REM sleep awakenings and related wakening paradigms on the ultradian sleep cycle and the symptoms in depression. J Psychiatr Res. 2002;36:299–308. | |
Wiegand M, Berger M, Zulley J, von Zerssen D. The effect of trimipramine on sleep in patients with major depressive disorder. Pharmacopsychiatry. 1986;19:198–199. | |
Steiger A, Gerken A, Benkert O, Holsboer F. Differential effects of the enantiomers R(-) and S(+) oxaprotiline on major endogenous depression, the sleep EEG and neuroendocrine secretion: studies on depressed patients and normal controls. Eur Neuropsychopharmacol. 1993;3:117–126. | |
Kupfer DJ, Spiker DG, Rossi A, Coble PA, Shaw D, Ulrich R. Nortriptyline and EEG sleep in depressed patients. Biol Psychiatry. 1982;17:535–546. | |
Saletu B, Frey R, Krupka M, Anderer P, Grunberger J, See WR. Sleep laboratory studies on the single-dose effects of serotonin reuptake inhibitors paroxetine and fluoxetine on human sleep and awakening qualities. Sleep. 1991;14:439–447. | |
Sharpley AL, Williamson DJ, Attenburrow ME, Pearson G, Sargent P, Cowen PJ. The effects of paroxetine and nefazodone on sleep: a placebo controlled trial. Psychopharmacology (Berl). 1996;126: 50–54. | |
Kupfer DJ. REM latency: a psychobiologic marker for primary depressive disease. Biol Psychiatry. 1976;11:159–174. | |
Riemann D, Berger M. The effects of total sleep deprivation and subsequent treatment with clomipramine on depressive symptoms and sleep electroencephalography in patients with a major depressive disorder. Acta Psychiatr Scand. 1990;81:24–31. | |
Cook IA, Hunter AM, Gilmer WS, et al. Quantitative electroencephalogram biomarkers for predicting likelihood and speed of achieving sustained remission in major depression: a report from the biomarkers for rapid identification of treatment effectiveness in major depression (BRITE-MD) trial. J Clin Psychiatry. 2013;74:51–56. | |
Mulert C, Juckel G, Brunnmeier M, et al. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–225. | |
Leuchter AF, Cook IA, Lufkin RB, et al. Cordance: a new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage. 1994;1:208–219. | |
Leuchter AF, Uijtdehaage SH, Cook IA, O’Hara R, Mandelkern M. Relationship between brain electrical activity and cortical perfusion in normal subjects. Psychiatry Res. 1999;90:125–140. | |
Bares M, Brunovsky M, Kopecek M, et al. Early reduction in prefrontal theta QEEG cordance value predicts response to venlafaxine treatment in patients with resistant depressive disorder. Eur Psychiatry. 2008;23: 350–355. | |
Cook IA, Leuchter AF, Morgan M, et al. Early changes in prefrontal activity characterize clinical responders to antidepressants. Neuropsychopharmacology. 2002;27:120–131. | |
Bares M, Brunovsky M, Novak T, et al. The change of prefrontal QEEG theta cordance as a predictor of response to bupropion treatment in patients who had failed to respond to previous antidepressant treatments. Eur Neuropsychopharmacol. 2010;20:459–466. | |
Cook IA, Leuchter AF, Morgan ML, Stubbeman W, Siegman B, Abrams M. Changes in prefrontal activity characterize clinical response in SSRI nonresponders: a pilot study. J Psychiatr Res. 2005;39:461–466. | |
Cook IA, Hunter AM, Abrams M, Siegman B, Leuchter AF. Midline and right frontal brain function as a physiologic biomarker of remission in major depression. Psychiatry Res. 2009;174:152–157. | |
Kopecek M, Sos P, Brunovsky M, Bares M, Stopkova P, Krajca V. Can prefrontal theta cordance differentiate between depression recovery and dissimulation? Neuro Endocrinol Lett. 2007;28:524–526. | |
Kopecek M, Tislerova B, Sos P, et al. QEEG changes during switch from depression to hypomania/mania: a case report. Neuro Endocrinol Lett. 2008;29:295–302. | |
Pawlowski M, Dresler M, Holsboer F, Steiger A. Cordance as a biomarker in sleep EEG for depression: responders versus nonresponders: a naturalistic study after antidepressant medication. Eur Neuropsychopharmacol. 2011;21:S360–S361. | |
Hatzinger M, Hemmeter UM, Brand S, Ising M, Holsboer-Trachsler E. Electroencephalographic sleep profiles in treatment course and long-term outcome of major depression: association with DEX/CRH-test response. J Psychiatr Res. 2004;38:453–465. | |
Steiger A. Neurochemical regulation of sleep. J Psychiatr Res. 2007;41:537–552. | |
Linkowski P, Mendlewicz J, Kerkhofs M, et al. 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab. 1987;65:141–152. | |
Ehlers CL, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–474. | |
Opp M, Obál F Jr, Krueger JM. Corticotropin-releasing factor attenuates interleukin 1-induced sleep and fever in rabbits. Am J Physiol. 1989;257:R528–R535. | |
Sanford LD, Yang L, Wellman LL, Dong E, Tang X. Mouse strain differences in the effects of corticotropin releasing hormone (CRH) on sleep and wakefulness. Brain Res. 2008;1190:94–104. | |
Holsboer F, von Bardeleben U, Steiger A. Effects of intravenous corticotropin-releasing hormone upon sleep-related growth hormone surge and sleep EEG in man. Neuroendocrinology. 1988;48:32–38. | |
Schüssler P, Kluge M, Dresler M, Yassouridis A, Steiger A. Sleep-impairing effect of intravenous corticotropin-releasing hormone on sleep EEG in young healthy women. Exp Clin Endocrinol Diabetes. 2009;117:664. | |
Antonijevic IA, Steiger A. Depression-like changes of the sleep-EEG during high dose corticosteroid treatment in patients with multiple sclerosis. Psychoneuroendocrinology. 2003;28:780–795. | |
Hatzinger M, Brand S, Perren S, et al. Electroencephalographic sleep profiles and hypothalamic-pituitary-adrenocortical (HPA) activity in kindergarten children: Early indication of poor sleep quality associated with increased cortisol secretion. J Psychiatr Res. 2008;42: 532–543. | |
Held K, Künzel H, Ising M, et al. Treatment with the CRH1-receptor antagonist R121919 improves sleep EEG in patients with depression. J Psychiatr Res. 2004;38:129–136. | |
Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. | |
Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol Behav. 1991;49:257–262. | |
El Yacoubi M, Bouali S, Popa D, et al. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 2003;100:6227–6232. | |
Popa D, El Yacoubi M, Vaugeois JM, Hamon M, Adrien J. Homeostatic regulation of sleep in a genetic model of depression in the mouse: effects of muscarinic and 5-HT1A receptor activation. Neuropsychopharmacology. 2006;31:1637–1646. | |
Dugovic C, Maccari S, Weibel L, Turek FW, Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J Neurosci. 1999;19:8656–8664. | |
Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. Neuroreport. 2000;11:627–631. | |
Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–252. | |
Grønli J, Murison R, Bjørvatn B, Sørensen E, Portas CM, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res. 2004;150:139–147. | |
Touma C, Fenzl T, Ruschel J et al. Rhythmicity in mice selected for extremes in stress reactivity: Behavioural, endocrine and sleep changes resembling endophenotypes of major depression. PLoS One. 2009;4:e4325. | |
Fenzl T, Touma C, Romanowski CP, et al. Sleep disturbances in highly stress reactive mice: modeling endophenotypes of major depression. BMC Neurosci. 2011;12:29. | |
Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 2010;61:81–109. | |
Lu A, Steiner MA, Whittle N, et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008;13:1028–1042. | |
Kimura M, Müller-Preuss P, Lu A, et al. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry. 2010;15:154–165. | |
Curzi ML, Flachskamm C, Deussing JM, Kimura M. Enhanced REM sleep and cholinergic hyperactivity in forebrain-specific CRH-overexpressing mice. J Sleep Res. 2012;21(Suppl 1):192. | |
Shiromani PJ, Overstreet D, Levy D, Goodrich CA, Campbell SS, Gillin JC. Increased REM sleep in rats selectively bred for cholinergic hyperactivity. Neuropsychopharmacology.1988;1:127–133. | |
Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. | |
Berger M, Höchli D, Zulley J, Lauer C, von Zerssen D. Cholinomimetic drug RS 86, REM sleep, and depression. Lancet. 1985;1:1385–1386. | |
Grønli J, Fiske E, Murison R, et al. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav Brain Res. 2007;181:42–51. | |
Novati A, Roman V, Cetin T, et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31: 1579–1585. | |
Mrdalj J, Pallesen S, Milde AM, et al. Early and later life stress alter brain activity and sleep in rats. PLoS One. 2013;8:e69923. | |
Steiger A. Neuroendocrinology of sleep disorders. In: D’haenen D, den Boer JA, Westenberg H, Willner P, editors. Textbook of Biological Psychiatry. London: John Wiley and Sons, Ltd; 2002:1229–1246. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
