Back to Journals » Cancer Management and Research » Volume 12
SLAMF1 Promotes Methotrexate Resistance via Activating Autophagy in Choriocarcinoma Cells
Authors Shi D, Zhang Y, Tian Y
Received 24 August 2020
Accepted for publication 14 December 2020
Published 30 December 2020 Volume 2020:12 Pages 13427—13436
DOI https://doi.org/10.2147/CMAR.S278012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Dazun Shi, Yu Zhang, Yan Tian
Department of Gynecology and Obstetrics, Xiangya Hospital, Central South University, Changsha, Hunan Province, People’s Republic of China
Correspondence: Yan Tian
Department of Gynecology and Obstetrics, Xiangya Hospital, Central South University, Changsha 410008, People’s Republic of China
Email [email protected]
Objective: The acquisition of chemoresistance to methotrexate (MTX) still remains one of the major challenges for choriocarcinoma treatment. Herein, we aimed to evaluate the potential role of Signaling Lymphocytic Activation Molecule Family Member 1 (SLAMF1) as a possible regulator of chemoresistance to MTX in choriocarcinoma.
Material and Methods: MTX-resistant JEG3 and JAR sublines (JEG3/MTX, JAR/MTX) were used to study SLAMF1 function. CCK8 assay and soft agar assay were conducted to measure the cell viability and clonogenesis of choriocarcinoma cells, respectively; MDC incorporation assay was conducted for the quantification of intracellular autophagy; BrdU labeling was used to assess the proliferative potential of choriocarcinoma cells; SLAMF1 protein expression was analyzed by Western blotting.
Results: Upregulation of SLAMF1 expression was observed in MTX-resistant JEG3/MTX and JAR/MTX sublines compared to their parental JEG3 and JAR cell lines, respectively. Knockdown of SLAMF1 markedly attenuated cell viability and soft agar clonogenesis after incubation with MTX in JEG3/MTX and JAR/MTX cells. In contrast, constitutive expression of SLAMF1 rescued cell survival soft agar clonogenesis in JEG3 and JAR cells treated with MTX. Moreover, autophagy is apparently activated in MTX-resistant JEG3/MTX and JAR/MTX sublines compared to their parental cell lines. Autophagy inhibitor 3-methyladenine and bafilomycin A1 enhanced MTX-induced cytotoxicity in MTX-resistant JEG3 and JAR sublines. Further, SLAMF1 might activate autophagy-related mechanism to promote resistance to MTX in choriocarcinoma cells. Depletion of SLAMF1 suppressed autophagy and induced apoptosis in MTX-treated JEG3/MTX and JAR/MTX cells.
Conclusion: SLAMF1 might promote MTX resistance via activating protective autophagy in choriocarcinoma cell lines. Targeting SLAMF1 might be a useful therapeutic strategy to sensitize choriocarcinoma cells to MTX-based regimens.
Keywords: choriocarcinoma, methotrexate, drug resistance, SLAMF1, autophagy
Introduction
Choriocarcinoma is a very rare malignancy occurred in the placenta of pregnant women. The main biochemical marker, used in treatment and prognosis for choriocarcinoma is human chorionic gonadotropin (hCG). Choriocarcinoma is often characterized by its fast-growing and aggressive nature, which ultimately leads to patient death.1 Methotrexate (MTX) has been successfully used for the treatment of choriocarcinoma for decades.2 However, chemoresistance to MTX still represents a major challenge for choriocarcinoma treatment. A considerable fraction (20~30%) of low-risk choriocarcinoma may fail to achieve complete remission after MTX treatment.3,4 Moreover, about 10~20% patients with high-risk choriocarcinoma may render an incomplete remission to MTX-containing regimens.5,6 Although chemoresistance to MTX in choriocarcinoma has been well recognized, its regulatory mechanisms are not fully understood. A number of molecular mechanisms responsible for MTX resistance are documented, including drug efflux via ATP-binding-cassette (ABC) transporters,7,8 upregulation of dihydrofolate reductase (DHFR) gene,9 inhibition of apoptosis,10 involvement of interferon signaling,11 and enrichment of cancer stem cell fraction.12 Recently, our quantitative proteomics analysis identified multiple genes/pathways potentially associated with chemoresistance to MTX in JEG3 choriocarcinoma cells, which still awaits functional validation.13
Signaling Lymphocytic Activation Molecule Family Member 1 (SLAMF1, CD150) belongs to signaling lymphocyte activation molecule (SLAM) family of cell-surface receptors in hematopoietic cells.14 In hematologic malignancies, SLAMF1 expression is mainly expressed in cutaneous T-cell lymphomas, Hodgkin’s lymphoma, chronic lymphocytic leukemia, and multiple myeloma.15–17 In chronic lymphocytic leukemia and Hodgkin’s lymphoma, SLAMF1 plays an critical role in malignant cell fate decision and formation of tumor microenvironment.18,19 Recently, SLAMF1 has been shown to exert a crucial role in autophagy in chronic lymphocytic leukemia cells.20 However, the function of SLAMF1 in the regulation of MTX resistance still remains largely unknown. In our previous proteomics study, highly elevated expression of SLAMF1 was observed in MTX-resistant JEG3/MTX subline compared to parental JEG3,13 which sparked our interest to investigate its function in the chemoresistance to MTX in choriocarcinoma.
Materials and Methods
Reagents and Cell Lines
Primary antibodies used in this study were provided by the following sources: SLAMF1 (PA5-96046), Invitrogen (Carlsbad, CA, USA); β-actin (#58169), LC3B (#2775), p62 (#8025), and cleaved Caspase-3 (#9664), Cell Signaling (Danvers, MA, USA). MTX, Monodansylcadaverine (MDC), Bafilomycin A1, 5-bromo-2ʹ-deoxyuridine (BrdU), and 3-Methyladenine (3-MA) were provided by Sigma-Aldrich (St. Louis, MO, USA). Human choriocarcinoma cell lines JEG3 and JAR were provided by ATCC culture collection (Manassas, VA, USA). MTX-resistant JEG3/MTX and JAR/MTX sublines were established as we described previously.13 The use of these choriocarcinoma cell lines in this study was approved by the institutional research ethics committee in Xiangya Hospital, Central South University. These cell lines were routinely grown in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Lentiviral plasmids expressing non-targeting scramble or shSLAMF1 were provided by Genecopoeia Inc. (Rockville, MD, USA). Lentiviral plasmids LV105 expressing empty vector or SLAMF1 open reading frames (ORFs) were also provided by Genecopoeia Inc. The production of recombined lentivirus was performed according to the manufacturer’s instruction.13
Soft Agar Clonogenesis Assay
Soft agar clonogenesis assay was used to examine the clonogenesis potential of choriocarcinoma cells as we described previously.21 After incubation with or without MTX for 10~12 days, the colonies (>50 cells) were counted.
Cell Viability Analysis
Cell viability was evaluated with CCK-8 assay as described previously.13 Briefly, choriocarcinoma cells were seeded in a 96-well plate in triplicate (3×103 cells/well). Cell viability was measured at 48 hours after incubation with MTX. IC50 values were calculated in SPSS software as we described previously.13
BrdU Incorporation Assay
BrdU incorporation assay was conducted using Cell Proliferation Assay Kit (Cell Signaling, Boston, MA) as we described previously.13 Briefly, choriocarcinoma cells were plated (3×103 cells/well) into a 96-well plate overnight. After MTX incubation for 48 hours, choriocarcinoma cells were incubated with BrdU (10 μM, 2 hours). Incorporated BrdU was measured at OD450 using a Multiskan MK3 microplate reader.
Protein Extraction and Western Blotting
Western blotting was conducted as we described previously.13 Briefly, Cells were washed in cold PBS and lysed in a radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM Phenylmethanesulfonyl fluoride (PMSF), 1 mM Ethylenediamine tetra-acetic acid (EDTA), 5 mg/mL aprotinin, 5 mg/mL leupeptin, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS). The cell extract with 25 μg proteins was loaded on a 10% or 15% SDS-polyacrylamide gel and then transferred to polyvinylidene fluoride (PVDF) membranes. The membrane was incubated for 1 h in Tris-buffered saline (TBS) containing 5% skim milk. After blotting with primary antibody, the protein expression was revealed with the appropriate horseradish peroxidase–labeled secondary antibody, which was detected using ECL chemiluminescence. Mouse anti-human β-Actin was used as loading control.
MDC Incorporation Assay
MDC incorporation assay was conducted to measure the level of intracellular autophagy as described previously.22 Choriocarcinoma cells were incubated with 50 μM MDC for 10 min. The incorporated MDC was detected (excitation 380 nm; emission 525nm) in F97Pro fluorospectrometer (Lengguang Technology, Shanghai, China).
Transmission Electron Microscopy
To demonstrate the autophagic morphology in choriocarcinoma cells, we performed transmission electron microscopy as described previously.22 JEG3 and JEG3/MTX cells were fixed with ice-cold glutaraldehyde (2% in 0.1 M cacodylate buffer, pH 7.4) for 30 min. After fixation, the samples were postfixed in 1% OsO4 in the same buffer for 1 h and then subjected to the electron microscopic analysis. Representative areas were chosen for ultrathin sectioning and viewed with a Philips CM120 transmission electron microscope.
Immunofluorescence Staining
JEG3 and JEG3/MTX cells were seeded on the coverslips and grown overnight. Cells were fixed in 4% paraformaldehyde, permeated with 0.25% Triton X-100, blocked with 3% normal goat serum, stained with LC3 antibody overnight, and labeled with goat anti-rabbit IgG conjugated with tetramethylrhodamine (TRITC) (Zymed Laboratories, CA, USA). The cells were counterstained with sealant containing 40.6-diamidino-2-phenylindole (DAPI) (Meilunbio, Dalian, China) and examined under an Olympus BX40 fluorescence microscope (Olympus, Japan).
Statistical Analysis
SPSS 16.0 was used to conduct the statistical analysis. Data were expressed as mean ± standard deviation. Student’s t test was conducted to compare the means of two groups. One-way ANOVA analysis was used to compare the means of three or more groups. P < 0.05 was considered statistically significant for all the statistical tests.
Results
SLAMF1 Expression is Increased in MTX-Resistant Cell Models
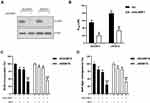
In our previous proteomics study,13 we identified multiple genes/pathways potentially involved in MTX resistance in JEG3:JEG3/MTX cell models. SLAMF1 was shown to be the top protein highly enriched in JEG3/MTX subline (P<0.05; Figure 1A and B). Consistently, Western blotting analysis revealed an increase in SLAMF1 expression in MTX-resistant sublines compared to parental JEG3 and JAR cells, respectively (Figure 1C).
Knockdown of SLAMF1 Sensitized MTX-Resistant Choriocarcinoma Cells to MTX Treatment
To examine the effect of SLAMF1 on the drug response of JEG3/MTX and JAR/MTX cells to MTX, we depleted SLAMF1 expression through shRNA-mediated knockdown. The expression of SLAMF1 was mostly reduced by shRNA targeting SLAMF1 compared to scramble (Scr) control (Figure 2A). Knockdown of SLAMF1 enhanced MTX chemosensitivity, as IC50 value was lower in shSLAMF1 group than in Scr control in MTX-resistant JEG3 and JAR sublines (Figure 2B). In addition, the effect of SLAMF1 on cellular proliferative potential was examined by BrdU incorporation assay in JEG3/MTX and JAR/MTX cells. The shSLAMF1 group was found less BrdU incorporation compared with Scr group after incubation with MTX (10 µM) for 48 hours (Figure 2C). Consistently, knockdown of SLAMF1 reduced soft agar clonogenesis in JEG3/MTX and JAR/MTX cells after MTX treatment (10 µM), as compared with Scr control (Figure 2D).
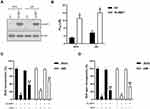
Over-Expression of SLAMF1 Promotes Resistance to MTX in Choriocarcinoma Cells
We next overexpressed SLAMF1 in order to examine its function in JEG3 and JAR cells. SLAMF1 expression was apparently increased in this SLAMF1 group compared to empty vector (EV) control (Figure 3A). Overexpression of SLAMF1 enhanced chemoresistance to MTX, as IC50 value was higher in SLAMF1 group than in EV control in JEG3 and JAR cells (Figure 3B). In addition, the effect of SLAMF1 on cellular proliferative potential was examined by BrdU incorporation assay in JEG3 and JAR cells. The SLAMF1 group was found more BrdU incorporation compared with EV group after incubation with MTX (3 µM) for 48 hours (Figure 3C). Consistently, overexpression of SLAMF1 increased soft agar clonogenesis in JEG3 and JAR cells after MTX treatment (3 µM), as compared with EV control (Figure 3D).
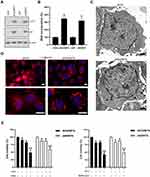
Autophagy Might Be Involved in MTX Resistance in Choriocarcinoma Cells
SLAMF1 is a crucial regulator of autophagy in chronic lymphocytic leukemia cells,20 which might implicate autophagy as possible mechanism of chemoresistance to MTX in choriocarcinoma. As shown in Figure 4A, increased protein levels of autophagy-related genes LC3-II and p62 were observed in MTX-resistant sublines compared to parental choriocarcinoma cells, respectively. Consistently, More MDC incorporation was noted in MTX-resistant sublines than that in parental choriocarcinoma cells (Figure 4B). Electron microscopic observation on the ultrastructural features revealed enormous autophagic vacuoles in JEG3/MTX cells compared to JEG3 (Figure 4C). Consistently, punctate LC3 immunostaining was seen in JEG3/MTX, which represents typical autophagy morphology (Figure 4D). Cell viability in MTX-resistant sublines was greatly reduced in MTX plus 3-MA group or MTX plus bafilomycin, group, as compared with either drug alone (Figure 4E). These results suggested that autophagy might serve as a prosurvival mechanism in MTX-resistant choriocarcinoma cells.
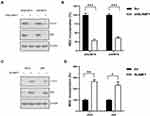
SLAMF1 Regulates Autophagy in Choriocarcinoma Cell Lines
We next examined the effect of SLAMF1 on autophagy level in choriocarcinoma cells. Knockdown of SLAMF1 attenuated the expression of autophagy-related genes (LC3-II and p62) and inhibited MDC incorporation compared to Scr control in JEG3/MTX and JAR/MTX cells (Figure 5A and B). In contrast, over-expression of SLAMF1 induced the expression of autophagy-related genes (LC3-II and p62) and induced MDC incorporation compared to empty vector (EV) control in JAR and JEG3 cells (Figure 5C and D).
Depletion of SLAMF1 Induced Apoptosis in JEG3/MTX and JAR/MTX Cells After MTX Treatment
We depleted SLAMF1 expression in MTX-resistant choriocarcinoma cells in order to elucidate the role of autophagy on cellular apoptosis. Western blotting showed that SLAMF1 depletion could considerably reduce LC3-II and p62 levels in JEG3/MTX (Figure 6). Meanwhile, the level of apoptosis-related cleaved caspase-3 was significantly increased after MTX treatment in shSLAMF1 group compared to Scr control (Figure 6).
Discussion
The acquisition of chemoresistance to methotrexate (MTX) still remains one of the major challenges for choriocarcinoma treatment. Exploring the mechanism of MTX resistance would help to develop therapeutic strategy to combat MTX-resistant choriocarcinoma. The signaling lymphocytic activation molecule (SLAM) family has been shown to regulate inflammation and immune response.23 Recently, SLAM genes have been implicated in drug response regulation in cancerous cells. Fouquet et al showed that SLAMF3 inhibits liver cancer growth and might predict resistance to sorafenib.24 Bologna et al showed that loss of SLAMF1 was associated with cellular response to fludarabine and ABT-737 in chronic lymphocytic leukemia cells.20 Recently, our proteomic profiling identified SLAMF1 as a potential regulator of MTX resistance in JEG3/JEG3/MTX cell models.13 Knockdown of SLAMF1 markedly attenuated cell viability and soft agar clonogenesis after incubation with MTX in JEG3/MTX and JAR/MTX cells. In contrast, overexpression of SLAMF1 rescued cell survival soft agar clonogenesis in JEG3 and JAR cells treated with MTX. Therefore, our findings might connect the novel function of the SLAMF1 with the modulation of drug resistance in choriocarcinoma.
Autophagy is an important cellular mechanism that recycles cytoplasmic components into lysosome for bulk degradation.25,26 Physiologically, autophagy pathway is dramatically induced in order to survive from nutrient starvation and stress.27,28 Autophagy could also be induced by cancer therapy, which contributes to cancer cell survival and the eventual recurrence of the disease.29,30 Autophagy machinery could be activated in several cell models in response to MTX treatment. Xu et al showed that autophagy induction contributes to the resistance to methotrexate treatment in rheumatoid arthritis fibroblast-like synovial cells.31 Shen et al revealed a survival mechanism of the switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals in methotrexate-resistant choriocarcinoma cells.32 Similarly, our findings showed that autophagy-related genes (LC3-II, p62) were apparently increased in MTX-resistant choriocarcinoma cells compared to parental cell lines. Further, autophagy inhibitor 3-MA and Bafilomycin could enhance MTX-induced cytotoxicity in MTX-resistant choriocarcinoma cells. These results suggested that autophagy-related mechanisms might play an important role in MTX resistance in choriocarcinoma cells.
Recently, SLAMF1 has been shown to be a critical regulator of autophagy induced by fludarabine or ABT-737 in chronic lymphocytic leukemia cells, which might connect SLAMF1-regulated autophagy with drug response in cancer cells.20 However, the role of SLAMF1 in regulating MTX resistance in choriocarcinoma cells still remains unknown. Herein, we showed that manipulation of SLAMF1 expression could modulate autophagy in choriocarcinoma cell models, suggesting SLAMF1 might be a critical regulator of autophagy in choriocarcinoma cells. Further, autophagy regulated by SLAMF1 might be an important prosurvival mechanism, as knockdown of SLAMF1 suppressed autophagy and induced apoptosis in JEG3/MTX and JAR/MTX cells treated with MTX. Ma et al showed that SLAMF1 could recruit a Beclin-1/Vps34/UVRAG complex under oxidative stress condition, which leads to activation of the membrane NADPH oxidase 2 complexes.33 Therefore, SLAMF1 might regulate autophagy-related pathway to activate antioxidant enzymes, which would be crucial in mediating chemoresistance to MTX in choriocarcinoma cells.
In conclusion, our findings suggest that SLAMF1 might promote MTX resistance via activating protective autophagy in choriocarcinoma cell lines. Targeting SLAMF1 might be a useful therapeutic strategy to sensitize choriocarcinoma cells to MTX-based regimens.
Acknowledgments
This work was funded by the Natural Science Foundation of Hunan Province, China (2019JJ40492).
Disclosure
The authors have no competing interests to declare.
References
1. Cheung AN, Zhang HJ, Xue WC, Siu MK. Pathogenesis of choriocarcinoma: clinical, genetic and stem cell perspectives. Future Oncol. 2009;5(2):217–231. doi:10.2217/14796694.5.2.217
2. May T, Goldstein DP, Berkowitz RS. Current chemotherapeutic management of patients with gestational trophoblastic neoplasia. Chemother Res Practice. 2011;2011:806256. doi:10.1155/2011/806256
3. Matsui H, Suzuka K, Yamazawa K, et al. Relapse rate of patients with low-risk gestational trophoblastic tumor initially treated with single-agent chemotherapy. Gynecol Oncol. 2005;96(3):616–620. doi:10.1016/j.ygyno.2004.11.011
4. Maestá I, Nitecki R, Horowitz NS, Goldstein DP. Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: the New England Trophoblastic Disease Center experience. Gynecol Oncol. 2018;148(1):161–167. doi:10.1016/j.ygyno.2017.10.028
5. Bolis G, Bonazzi C, Landoni F, et al. EMA/CO regimen in high-risk gestational trophoblastic tumor (GTT). Gynecol Oncol. 1988;31(3):439–444. doi:10.1016/S0090-8258(88)80029-5
6. Bower M, Newlands ES, Holden L, et al. EMA/CO for high-risk gestational trophoblastic tumors: results from a cohort of 272 patients. J Clin Oncol. 1997;15(7):2636–2643. doi:10.1200/JCO.1997.15.7.2636
7. Wu J, Wang D. CLIC1 induces drug resistance in human choriocarcinoma through Positive regulation of MRP1. Oncol Res. 2017;25(6):863–871. doi:10.3727/096504016X14772315906527
8. Ding R, Shi J, Pabon K, Scotto KW. Xanthines down-regulate the drug transporter ABCG2 and reverse multidrug resistance. Mol Pharmacol. 2012;81(3):328–337. doi:10.1124/mol.111.075556
9. Han B, Xiang Y, Wang Y, Wang Z, Zhang H, Huang S. Dihydrofolate reductase transcript level is not suitable for methotrexate-resistance prediction in choriocarcinoma cell line. Int J Gynecol Cancer. 2010;20(7):1259–1263. doi:10.1111/IGC.0b013e3181f05128
10. Wang X, Zong L, Wang W, Yang J, Xiang Y. CD105 overexpression mediates drug-resistance in choriocarcinoma cells through BMP9/Smad pathway. J Cancer. 2020;11(2):272–283. doi:10.7150/jca.34965
11. Elias KM, Harvey RA, Hasselblatt KT, Seckl MJ, Berkowitz RS. Type I interferons modulate methotrexate resistance in gestational trophoblastic neoplasia. Am J Reprod Immunol. 2017;77(6):6. doi:10.1111/aji.12666
12. Cai J, Peng T, Wang J, et al. Isolation, Culture and Identification of Choriocarcinoma Stem-Like Cells from the Human Choriocarcinoma Cell-Line JEG-3. Cell Physiol Biochem. 2016;39(4):1421–1432. doi:10.1159/000447845
13. Jun F, Peng Z, Zhang Y, Shi D. Quantitative proteomic analysis identifies novel regulators of methotrexate resistance in choriocarcinoma. Gynecol Oncol. 2020;157(1):268–279. doi:10.1016/j.ygyno.2020.01.013
14. Dragovich MA, Mor A. The SLAM family receptors: potential therapeutic targets for inflammatory and autoimmune diseases. Autoimmun Rev. 2018;17(7):674–682. doi:10.1016/j.autrev.2018.01.018
15. Gordiienko I, Shlapatska L, Kovalevska L, Sidorenko SP. SLAMF1/CD150 in hematologic malignancies: silent marker or active player? Clin Immunol. 2019;204:14–22. doi:10.1016/j.clim.2018.10.015
16. Yurchenko MY, Kovalevska LM, Shlapatska LM, Berdova GG, Clark EA, Sidorenko SP. CD150 regulates JNK1/2 activation in normal and Hodgkin’s lymphoma B cells. Immunol Cell Biol. 2010;88(5):565–574. doi:10.1038/icb.2010.14
17. Gordiienko I, Shlapatska L, Kholodniuk V, et al. The interplay of CD150 and CD180 receptor pathways contribute to the pathobiology of chronic lymphocytic leukemia B cells by selective inhibition of Akt and MAPK signaling. PLoS One. 2017;12(10):e0185940. doi:10.1371/journal.pone.0185940
18. Gordienko IM, Shlapatska LM, Kovalevska LM, Sidorenko SP. Differential expression of CD150/SLAMF1 in normal and malignant B cells on the different stages of maturation. Exp Oncol. 2016;38(2):101–107. doi:10.31768/2312-8852.2016.38(2):101-107
19. Rigolin GM, Saccenti E, Melandri A, et al. In chronic lymphocytic leukaemia, SLAMF1 deregulation is associated with genomic complexity and independently predicts a worse outcome. Br J Haematol. 2020. doi:10.1111/bjh.16865
20. Bologna C, Buonincontri R, Serra S, et al. SLAMF1 regulation of chemotaxis and autophagy determines CLL patient response. J Clin Invest. 2016;126(1):181–194. doi:10.1172/JCI83013
21. Jun F, Peng Z, Zhang Y, Shi D. Quantitative Proteomic Profiling Identifies SOX8 as Novel Regulator of Drug Resistance in Gestational Trophoblastic Neoplasia. Front Oncol. 2020;10:557. doi:10.3389/fonc.2020.00557
22. Fu J, Shao C-J, Chen F-R, Ng H-K, Chen Z-P. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro Oncol. 2010;12(4):328–340. doi:10.1093/neuonc/nop005
23. Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol. 2010;2(3):a002469. doi:10.1101/cshperspect.a002469
24. Fouquet G, Debuysscher V, Ouled-Haddou H, et al. Hepatocyte SLAMF3 reduced specifically the multidrugs resistance protein MRP-1 and increases HCC cells sensitization to anti-cancer drugs. Oncotarget. 2016;7(22):32493–32503. doi:10.18632/oncotarget.8679
25. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi:10.1002/path.2697
26. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi:10.1016/j.cell.2011.10.026
27. Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188(188):53–67. doi:10.1016/j.lfs.2017.08.029
28. Puente C, Hendrickson RC, Jiang X. Nutrient-regulated Phosphorylation of ATG13 Inhibits Starvation-induced Autophagy. J Biol Chem. 2016;291(11):6026–6035. doi:10.1074/jbc.M115.689646
29. Sui X, Chen R, Wang Z, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4(10):e838. doi:10.1038/cddis.2013.350
30. Li X, Zhou Y, Li Y, et al. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed Pharmacother. 2019;119:109415. doi:10.1016/j.biopha.2019.109415
31. Xu K, Cai Y-S, Lu S-M, et al. Autophagy induction contributes to the resistance to methotrexate treatment in rheumatoid arthritis fibroblast-like synovial cells through high mobility group box chromosomal protein 1. Arthritis Res Ther. 2015;17(1):374. doi:10.1186/s13075-015-0892-y
32. Shen Y, Yang J, Zhao J, Xiao C, Xu C, Xiang Y. The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: A survival mechanism in methotrexate-resistant choriocarcinoma cells. Exp Cell Res. 2015;334(2):207–218. doi:10.1016/j.yexcr.2015.04.010
33. Ma C, Wang N, Detre C, Wang G, O’Keeffe M, Terhorst C. Receptor signaling lymphocyte-activation molecule family 1 (Slamf1) regulates membrane fusion and NADPH oxidase 2 (NOX2) activity by recruiting a Beclin-1/Vps34/ultraviolet radiation resistance-associated gene (UVRAG) complex. J Biol Chem. 2012;287(22):18359–18365. doi:10.1074/jbc.M112.367060
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.