Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 11
Sirt1 protects neural stem cells from apoptosis by decreasing acetylation of histone 3K9
Authors Jian C, Zou C, Xu N, Chen G, Zou D
Received 11 May 2018
Accepted for publication 5 July 2018
Published 7 September 2018 Volume 2018:11 Pages 39—41
DOI https://doi.org/10.2147/SCCAA.S173852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Chongdong Jian,1,* Cuihua Zou,1,* Ning Xu,2 Guoying Chen,2 Donghua Zou2
1Youjiang Medical University for Nationalities, Baise, Guangxi 533000, People’s Republic of China; 2Department of Neurology, The Fifth Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, People’s Republic of China
*These authors contributed equally to this work
Objective: To explore the role and mechanism of Sirt1 in protecting neural stem cells (NSCs) from apoptosis.
Materials and methods: Transfection was used to overexpress Sirt1 in rat NSCs. The effect of Sirt1 overexpression on camptothecin-induced apoptosis of NSCs was evaluated. Western blotting was used to examine the expression of Sirt1, cleaved caspase-3, and acetylated histone 3K9.
Results: Overexpression of Sirt1 in NSCs decreased the cleavage of caspase-3 and acetylation of histone 3K9.
Conclusion: Sirt1 may protect NSCs from apoptosis by decreasing the acetylation of histone 3 on K9.
Keywords: neural stem cells, apoptosis, Sirt1, caspase-3, acetylated histone 3K9
Introduction
Our previous study found that proliferation of endogenous neural stem cells (NSCs) was temporarily enhanced in the p25 transgenic mouse model; however, the survival of NSCs was impaired.1 NSCs undergo apoptosis following temporary enhanced proliferation. Therefore, intervening strategies that can enhance the survival of NSCs may be beneficial for the treatment of neural injuries or degenerative diseases.
Originally identified in yeast, sirtuins are nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases that play an important role in longevity.2 There are seven sirtuins in mammals. Sirt1 is involved in many biological functions, such as oxidative stress and inflammatory responses, glucose and lipid metabolism, autophagy, cell mitosis, apoptosis, cell cycle regulation, cell proliferation, cell senescence, and metabolism.3,4 In addition, Sirt1 plays important roles in the occurrence and development of neurons, the maintenance of normal neuronal functions, and the protection of neurons.5,6 However, its role in protecting NSCs from apoptosis has not been elucidated. In this study, we overexpressed Sirt1 in rat NSCs and induced cellular injury with camptothecin to determine the role and mechanism of Sirt1 in protecting NSCs during apoptosis.
Materials and methods
NSC culture, transfection, and Western blotting were performed as previously published.1 The rat NSC line was obtained from the Fred Gage Laboratory7 (The Salk Institute, La Jolla, CA, USA). Briefly, 1 µL of human simplex virus (MIT viral core facility, Cambridge, MA, USA) expressing flag or flag-Sirt1 was added to the medium to transfect NSCs. Transfected NSCs were transferred to 6-well plates. Different concentrations of camptothecin (0, 0.5, 2, and 10 µM) were added to the medium for 16 hours and cells were collected for Western blot analysis. Total proteins were extracted from cultured NSCs using lysis buffer. The primary antibodies used in this study were anti-cleaved caspase-3 (1:500, Cell Signaling Technology; Denvers, MA, USA), anti-acetyl-Histone3K9 (1:500, Cell Signaling Technology), anti-flag (1:1,000, Sigma-Aldrich Co., St Louis, MO, USA), and anti-α-tubulin (1:2,000, Cell Signaling Technology). Protein bands were captured and analyzed using Quantity One software for OD values.
Results
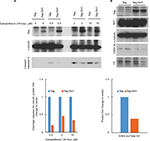
Overexpression of flag-Sirt1 in rat NSCs decreased the level of cleaved caspase-3 protein when the cells were treated with camptothecin (Figure 1A). Similarly, the levels of acetylated histone 3K9 were reduced following camptothecin treatment (Figure 1B).
Discussion
Sirt1 plays important roles in many pathophysiological processes by deacetylating various substrates. Studies have reported that Sirt1 can inhibit apoptosis through various pathways in tissues. For example, Sirt1 reduced endoplasmic reticulum stress and apoptosis of brown adipocytes in vivo and in vitro by inhibiting Smad3/ATF4 signaling.8 Activation of Sirt1 promotes recovery of mitochondrial proteins and functions by increasing mitochondrial biogenesis and by reducing apoptosis following intracerebral hemorrhage via the PGC-1α mitochondrial pathway.9 Sirt1 may attenuate endoplasmic reticulum stress-induced cardiomyocyte apoptosis via PERK/eIF2α, ATF6/CHOP, and IRE1α/JNK-mediated pathways.10 Additionally, Sirt1 inhibits apoptosis by deacetylating p53.11
There are limited reports on whether Sirt1 can protect NSCs from apoptosis. We found that overexpressing Sirt1 in rat NSCs resulted in reduced levels of cleaved caspase-3 and reduced apoptosis in response to camptothecin, suggesting that Sirt1 protects NSCs from apoptosis. In addition, the expression of acetylated histone 3K9 in NSCs was reduced. Acetylation of histone 3K9 is frequently associated with DNA damage. Sirt1 can remove acetyl groups from histone lysine residues, resulting in deacetylation of histone H3 on K9.12 Thus, our data suggest that Sirt1 could reduce DNA damage that triggers apoptosis.
Conclusion
In conclusion, Sirt1 may protect NSCs from apoptosis by decreasing the acetylation of histone 3 on K9.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant number: 81860244), Guangxi Natural Science Foundation (grant number: 2016GXNSFCA380012), the Basic Ability Enhancement Program for Young and Middle-age Teachers of Guangxi (grant number: 2017KY0516), the Project of Nanning Scientific Research and Technology Development Plan (grant number: 20163142), the Scientific Research Project of Guangxi Health and Family Planning Commission (grant number: Z20170001), and the China Scholarship Council (201708455059).
Disclosure
The authors report no conflicts of interest in this work.
References
Zou D, Zhou Y, Liu L, et al. Transient enhancement of proliferation of neural progenitors and impairment of their long-term survival in p25 transgenic mice. Oncotarget. 2016;7(26):39148. | ||
Klar AJ, Fogel S, Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979;93(1):37. | ||
Sosnowska B, Mazidi M, Penson P, Gluba-Brzózka A, Rysz J, Banach M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis. 2017;265:275–282. | ||
Yi YW, Kang HJ, Kim HJ, Kong Y, Brown ML, Bae I. Targeting mutant p53 by a SIRT1 activator YK-3-237 inhibits the proliferation of triple-negative breast cancer cells. Oncotarget. 2013;4(7):984–994. | ||
Liu CM, Wang RY, Saijilafu JZX, Jiao ZX, Zhang BY, Zhou FQ. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev. 2013;27(13):1473–1483. | ||
Sugino T, Maruyama M, Tanno M, Kuno A, Houkin K, Horio Y. Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells. FEBS Lett. 2010;584(13):2821–2826. | ||
Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31(3):560–573. | ||
Liu Z, Gu H, Lu G, et al. Reducing Smad3/ATF4 was essential for Sirt1 inhibiting ER stress-induced apoptosis in mice brown adipose tissue. Oncotarget. 2016;8:9267. | ||
Zeng K, Feng QG, Lin BT, Ma DH, Liu CM. Effects of microRNA-211 on proliferation and apoptosis of lens epithelial cells by targeting SIRT1 gene in diabetic cataract mice. Biosci Rep. 2017;37(4):BSR20170695. | ||
Guo R, Liu W, Liu B, Zhang B, Li W, Xu Y. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism. Int J Cardiol. 2015;191:36. | ||
Jang J, Huh YJ, Cho HJ, et al. SIRT1 Enhances the Survival of Human Embryonic Stem Cells by Promoting DNA Repair. Stem Cell Reports. 2017;9(2):629–641. | ||
Khobta A, Anderhub S, Kitsera N, Epe B. Gene silencing induced by oxidative DNA base damage: association with local decrease of histone H4 acetylation in the promoter region. Nucleic Acids Res. 2010;38(13):4285–4295. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.