Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 12
Single Nucleotide Polymorphisms (SNPs) of COL1A1 and COL11A1 in Class II Skeletal Malocclusion of Ethnic Javanese Patient
Authors Ardani IGAW, Aulanni'am , Diyatri I
Received 30 January 2020
Accepted for publication 31 March 2020
Published 24 April 2020 Volume 2020:12 Pages 173—179
DOI https://doi.org/10.2147/CCIDE.S247729
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
I Gusti Aju Wahju Ardani,1 Aulanni’am,2 Indeswati Diyatri3
1Orthodontics Department, Faculty of Dental Medicine, Airlangga University, Surabaya, Indonesia; 2Biochemistry Department, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; 3Oral Biology, Faculty of Dental Medicine, Airlangga University, Surabaya, Indonesia
Correspondence: I Gusti Aju Wahju Ardani
Orthodontics Department, Faculty of Dental Medicine, Airlangga University, Surabaya, Indonesia
Tel +623 15030255
Email [email protected]
Background: The prevalence of malocclusion cases in the orthodontic specialist clinic in Airlangga University’s Dental Hospital in Surabaya, Indonesia, in 2014– 2016 is fairly high, as 55.34% of the occurrences were identified as class II skeletal malocclusion. This type of skeletal malocclusion, which is usually recognized in adults, occurs as a result of variation during growth and development. Lately, there have been many reports on gene polymorphisms of COL1A1 and COL11A1, which are assumed to be associated with class II skeletal malocclusion in Caucasians.
Purpose: This study aims to analyze the relationship between single nucleotide polymorphisms (SNPs) of COL1A1 and COL11A1 with class II skeletal malocclusion in Javanese ethnic group patients with mandibular micrognathism.
Materials and Methods: The diagnosis of class II skeletal malocclusion was established using the lateral cephalometric radiographs (ANB angle ≥ 4°) (n=50). DNA was extracted from the patient’s peripheral blood. After that, PCR, electrophoresis, and DNA sequencing were conducted on the extracted DNA based on COL1A1 and COL11A1 primers.
Results: The SNPs in COL1A1 are c.20980G/A in 27 patients and c.20980G>A in 8 patients, whereas SNPs in COL11A1 are both c.134373C/A and c.134555C/T in 8 patients and both c.[134373A>C] and c.134582G>A in 10 patients. All samples show the deletion (c.[134227delA]) in COL11A1.
Conclusion: SNPs in COL1A1 and COL11A1 have been found in class II skeletal malocclusion of Javanese ethnic group patients. Seventy percent of SNPs in COL1A1 occur in rs.2249492, whereas 36% of newly discovered SNPs appear in COL11A1. All samples also have deletion in COL11A1.
Keywords: COL1A1, COL11A1, class II malocclusion, mandibular micrognathism, ethnic Javanese
Introduction
Class II skeletal malocclusion is defined as a distal relation of mandible to the maxilla and has both sagittal anomaly and vertical anomaly characteristics.1 Class II skeletal malocclusion with anteroposterior skeletal anomaly, which is shown by ANB angle 6.42 ± 1.70°, presents maxillary and mandibular disharmony to the cranial base.2
Moreover, there are two divisions of class II skeletal malocclusion: division one and division two. Dental characteristics of division one class II skeletal malocclusion are maxillary protrusion, a deep bite, and a large overbite, whereas division two is characterized by a deep bite with maxillary central incisors proclination and lateral incisors retroclination or retroclination of all maxillary incisors. However, most patients with class II skeletal malocclusion in Airlangga University Dental Hospital (AUDH) have special characteristics which make them different from the typical class II skeletal malocclusions in Caucasians. Most patients have not only a deep bite, but also protrusion of both maxilla and mandible, therefore the overjet is not too large. Protrusion on maxilla only are rarely found. The results of previous studies showed that class II skeletal malocclusion in AUDH increased ANB which, for the most part, was not caused by the increased SNA (82.34 ± 3.49°). It was due to the decreased SNB (75.91 ± 3.77°). Based on McNamara analysis, class II skeletal malocclusion cases with a short mandible (107.87 ± 8.55 mm) was found more often than the exceeded maxillary length.3,4 This result is consistent with class II skeletal malocclusion characteristics in the Brazilian population, whose mandibular length is 107.87 ± 8.54 mm.5 However, studies on other ethnic groups show different results, such as in French Canadian, Italian, and Iraqi populations.6–8
Meanwhile, the patterns and direction of skeletal growth can be identified by examining the profile photo, cephalometric radiograph, and cast model. However, class II skeletal malocclusion cases are mostly diagnosed once patients have reached adulthood. The late diagnosis of skeletal malocclusion limits treatment options to camouflage orthodontic treatment or orthognathic surgery. On the other hand, an earlier diagnosis enables any growth modification treatment to be performed on children or teenagers, which provides a shorter treatment duration and relatively more stable result compared to those diagnosed later as adults. Dental and skeletal malocclusion that left untreated can lead to temporomandibular disorder (TMD) such as TMJ arthritis, which can affect oral health related to quality of life (OHRQoL) in adolescents.9
Nowadays, many studies found that polymorphism/gene variation also can be the cause of malocclusion.10–12 Previous studies stated that the development of malocclusion was affected by human evolution and some genetic factors, including the muscle fibers plasticity and heterogeneity of both jaw skeletal morphology and masticatory muscle, which can be inherited.13
The existence of genetic variations in skeletal malocclusion can be investigated through the detection of single nucleotide polymorphisms (SNPs). Therefore, this study raised the question of whether genetics can cause variations in class II skeletal malocclusions in the Javanese ethnic group population, which belongs to the Deutero – Malay race who have special characteristics of class II skeletal malocclusion with short mandibles. It also determined whether the SNPs of COL1A1 and COL11A1, which is found in Caucasian’s class II skeletal malocclusion, are also found in the Javanese population. This study aims to analyze the relationship between the SNPs of COL1A1 and COL11A1 with class II skeletal malocclusion in Javanese ethnic group patients with mandibular micrognathism.
Materials and Methods
This research was conducted at the Airlangga University Dental Hospital in Surabaya, Indonesia, from April 2015 to April 2016. This study was approved by the Ethical Clearance Committee of the Faculty of Dental Medicine, Airlangga University with the reference number 059/HRECC.FODM/V/2018. The inclusion sample criteria of this study were Javanese men and women, aged between 15–35 years old, who had never had any previous orthodontic treatment and all permanent teeth had erupted except for the third molar. Fifty patients fulfilled the inclusion criteria and agreed to become samples in this study. All participants, and a parent/legal guardian of participants under the age of 18 years provided written informed consent, and that this study was conducted in accordance with the Declaration of Helsinki.
Class II skeletal malocclusion was determined using the digital cephalometric tracing (OrthoVision - 2017, South Korea). Anatomical landmarks (such as: N, S, A, B, Ba, Gn, Co) were determined manually using the cephalometric analysis software (Figure 1). DNA extraction was done using QiaAmp Blood Mini Kit (Qiagen), as instructed by standard blood-DNA extraction protocol. DNA amplification was completed using the PCR technique with a pair of primers. Primers COL1A1_F: 5ʹ-GCCTCCCAGAACATCACCTA-3ʹ and COL1A1_R: 5ʹ-TGGGATGGAGGGAGTTTACA-3ʹ were used for COL1A1, both with a target of 456 bp. Primers COL11A1_F: 5ʹ-TGTGTGCCTGTATTCCCAGA-3ʹ and COL11A1_R: 5ʹ-GAGGAC CCTGCATGAGAA-3ʹ were used for COL11A1, both with a target of 415 bp. GoTaq Green Master Mix (Promega) was utilized as PCR Mix. A total of 35 cycles of PCR (Thermocycler SensoQuest GmbH) were performed, starting with Pre-Denaturation (94°C) for 4 minutes, Denaturation (94°C) for 30 seconds, Annealing (55°C) for 30 seconds, Extension (72°C) for 1 minute and Final Extension (72°C) for 7 minutes. Positive results would be shown at the location of 456 bp for COL1A1 and 415 bp for COL11A1.
The PCR product then underwent an electrophoresis using 2% agarose gel (BioRad) and was analyzed using Bio-Rad Gel Doc EZ Imager. Sequencing was performed by Sanger sequencing method (Genetics Science, Jakarta, Indonesia). Then, the data were again analyzed using BLAST to find any homology and BioEdit-Win software version 7.2.3 to find their alignment.
 |
Figure 1 The digital cephalometric tracing using OrthoVision-2017. It showed the anatomical landmarks (such as: N, S, A, B, Ba, Gn, Co) for cephalometric analysis. |
Results
The DNA extraction from peripheral blood was completed from a total of 50 patients with class II skeletal malocclusion with ANB ≥ 4°; Co–Gn ≤ 125 o (see Table 1). DNA extraction results were then confirmed using 1% agarose electrophoresis and amplified using PCR and 2% agarose electrophoresis, which led to the formation of a single band of amplicons at 456 bp and 415 bp (see Figure 2). The difference in the DNA sequences in COL1A1 and COL11A1 can be seen in Figure 3. DNA parallelism shows homology in COL1A1 (NCBI RefSeq: NG_007400.1) at a position between 20,744–21,154 and changes in c.20980A/G. COL11A1 also shows homology with NCBI RefSeq: NG_008033.1 at a position between 134,221–134,583 (see Figure 4). The alignment results of COL1A1 and COL11A1 sequences can be seen in Figure 5.
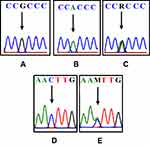 |
Figure 3 The differences in the DNA sequence of COL1A1 and COL11A1. The arrows indicate G (A); A (B); double peak c.20980A/G=R (C); C (D); double peak c.134373C/A=M (E). |
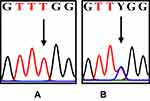 |
Figure 4 The differences in the DNA sequence of COL11A1. The arrows indicate T (A); double peak c.134555C/T=Y (B). |
 |
Table 1 Cephalometric Analysis of Javanese Ethnic Patients with Class II Skeletal Malocclusion (n=50) |
Discussion
This study confirmed that there are SNPs in COL1A1 and COL11A1 in class II skeletal malocclusion in Javanese ethnic group patients with mandibular micrognathism. These genes are related to temporomandibular joint (TMJ) growth and development, as it is important in determining mandibular length and height. Condylar growth, as a part of TMJ, is a result of endochondral ossification, which occurs at the deep surface of human condylar cartilage (MCC).14 MCC arises from the edge of bone membrane after (secondary) bone formation. Periosteum at condyle appears to be converted into perichondrium after the formation of condylar cartilage. Secondary cartilage has several definitions: one of its broadest definitions is that secondary cartilage arises later in embryonic development and is not influenced by the primary cartilage framework.15,16 There are four zones of adult mandibular condyles: (1) collagen I-expressing fibrous tissue at the articular surface that faces the disc, (2) proliferative cells in the precondroblastic zone that express collagen I, (3) chondroblastic zone that expresses collagen II, proteoglycans aggrecan, decorin, chondroitin sulfate PG, and keratin sulfate PG, and (4) hypertrophic zone adjacent to the bone expressing collagen X. The fibrous cell layer is described as the fibrocartilage of articular surface.14,15
The articular surface of mandibular condyle is the periosteum fibrous layer, whereas the underlying proliferative zone is the cambium layer. Like the menisci of the knee joint, articular discs are biconcave fibrocartilages attached to the capsule and condyle which distribute the load. Fibrocartilage consists of 30% chondrocytes with no hyaline pericellular matrix and 70% fibroblasts. The main components of extracellular (ECM) discs are collagen I, with a small amount of collagen II, a trace amount of collagen III as well as non-fibrillar collagen (VI, IX, XII). Collagen fibers are arranged to form a ring at the edge of the disc and run mediolaterally to the center of the disc. Fibers in the intermediate zone align in the anteroposterior direction. This alignment is important for optimum tensile strength of various tissues.15,17,18
Moreover, the development of condylar articular cartilage displays a special multilevel organization, which consists of a thin, shallow zone of flat and tightly bound cells, the polymorphic (pm)/ancestral (pr) layer, where chondroprogenitors actively multiply and produce chondrocytes with suitable growth. The lower zones contain flattened/mature and hypertrophic chondrocytes, where cells undergo the endochondral bone formation.15 Unlike long bones, which contain secondary ossification centers at each apical end, the condylar cartilage acts both as an articular cartilage and as a growth site of mandible. Consequently, it is characterized by the expression of multigene products including proteoglycans, proteoglycan-hyaluronan combination, tenascin-C, and type II and type X collagens (Col-II and Col-X). Each of them has a different purpose, such as markers of immature and hypertrophic chondrocytes, protein signaling, transcription factors and extracellular matrix.20 Similar to the superficial and polymorphic/chondroprogenitor layers in mice, condylar chondrocytes express type I collagen on the surface of mature cartilage condyle coated with fibrous/superficial tissue. These fibrocartilaginous condylar characteristics can also be found in other species, such as rats and human beings.14,15
COL11A1 is normally found in cartilage, which is responsible for the formation of cartilage structures in the early stages of skeleton development.19 The presence of its gene polymorphism in patients with class II skeletal malocclusion with mandibular micrognathia may indicate its relation to the failure of skeletal morphogenesis. This is in accordance with the previous study from Li et al, which stated that there is a relationship between human chondrodysplasia and mutations in the COL11A1 gene.20
PCR process is considered successful if a band is formed in agarose gel that conforms to the previously designed primer. In this study, a band is formed at 456 bp in COL1A1 and at 415 bp in COL11A1 in all patients, which indicates that the PCR process is successful on the corresponding genes. The electropherogram graphs show the existence of double peaks in the COL1A1 gene sequences. This is in accordance with the reports by Karki et al and Kurniawati et al, which revealed that the double peak indicates the presence of SNPs. SNPs in the COL1A1 gene sequence is found in 54% of the samples, while SNPs in the COL11A1 gene sequence is found in 36% of the samples.21,22 SNPs can cause variations in human phenotypes. In future studies, it can also be used to identify its association with complex diseases.23
According to da Fontoura, one of the gene candidates from class II skeletal malocclusion is COL1A1.12 As stated by Shibata et al, COL1A1 is expressed in the mandibular condylar cartilage, where the center of mandibular growth takes place. In COL1A1, SNPs are found at c.20980A/G, which is consistent with rs2249492.16 In Caucasians, rs2249492 has an increased risk of class III skeletal malocclusion, whereas it is found in 70% of class II skeletal malocclusion cases in Javanese patients with mandibular micrognathia.12 This SNP affects the synthesis of matrix metalloproteinase 2 (MMP2) enzymes, which cause an abnormal facial appearance, short stature and osteogenesis disorders.24 According to Baumert, SNP rs224942 in muscles shows decreased muscle strength. In this study, rs224942G>A or rs224942G/A (NCBI RefSeq: NG_007400.1) (see Figure 2A–C) is found; therefore there is a probability of a relationship between class II skeletal malocclusion and muscle tension.25
Conclusion
Based on this study, it is found that in the COL11A1 gene sequence, SNPs at c.134373A>C or c.134373C/A occur in 36% of the samples. Moreover, SNPs at both c.134373M and c.134555Y are seen in the same patients, which is 16% of the samples. In addition, there is a difference in DNA sequences found in this study compared to those at GeneBank, which is deletion at c.[134227delA]. This deletion is in NCBI RefSeq: NG_008033.1. This is suspected to be a characteristic allele of the Javanese ethnic group.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Proffit WR, Fields HW, Larson BE, Sarver DM. Contemporary Orthodontics.
2. Graber LW, Vig KWL, Vanarsdall RL, Huang GJ. Orthodontics: Current Principles and Techniques.
3. Jacobson A, Jacobson RL. Radiographic Cephalometry: From Basic to 3D Imaging.
4. Ardani I, Sanjaya ML, Sjamsudin J. Cephalometric characteristic of skeletal class II malocclusion in Javanese population at Universitas Airlangga Dental Hospital. Contemp Clin Dent. 2018;9(2):S342. doi:10.4103/ccd.ccd_465_18
5. Freitas MR, Lima DV, Freitas KMS, Janson G, Henriques JFC. Cephalometric evaluation of Class II malocclusion treatment with cervical headgear and mandibular fixed appliances. Eur J Orthod. 2008;30:477–482. doi:10.1093/ejo/cjn039
6. Sharma BP, Xin C. Comparative cephalometric analysis of angle class II division 1 malocclusion between Chinese male and female subjects. Orthod J Nepal. 2014;4:1–3. doi:10.3126/ojn.v4i1.11306
7. Wu JJ, Weis MA, Kim LS, Carter BG, Eyre DR. Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue. J Biol Chem. 2009;284(9):5539–5545. doi:10.1074/jbc.M806369200
8. Ali AA. Mcnamara’s cephalometric analysis for Iraqi population in Mosul city. Int J Enhanc Res Sci Technol Eng. 2014;3:287–99.
9. Isola G, Perillo L, Migliorati M, et al. The impact of temporomandibular joint arthritis on functional disability and global health in patients with juvenile idiopathic arthritis. Eur J Orthod. 2019;41(2):117–124. doi:10.1093/ejo/cjy034
10. Weaver CA, Miller SF, CSG DF, et al. Candidate gene analyses of 3-dimensional dentoalveolar phenotypes in subjects with malocclusion. Am J Orthod Dentofacial Orthop. 2017;151(3):539–558. doi:10.1016/j.ajodo.2016.08.027
11. Uribe LM, Miller SF. Genetics of the dentofacial variation in human malocclusion. Orthod Craniofac Res. 2015;18(1):91–99. doi:10.1111/ocr.12083
12. da Fontoura CS, Miller SF, Wehby GL, et al. Candidate gene analyses of skeletal variation in malocclusion. J Dent Res. 2015;94(7):913–920. doi:10.1177/0022034515581643
13. Isola G, Anastasi GP, Matarese G, et al. Functional and molecular outcomes of the human masticatory muscles. Oral Dis. 2018;24(8):1428–1441. doi:10.1111/odi.12806
14. Hinton RJ. Genes that regulate morphogenesis and growth of the temporomandibular joint: a review developmental dynamics. Dev Dyn. 2014;243(7):864–874. doi:10.1002/dvdy.24130
15. Stocum DL, Roberts WE, Part I. Development and physiology of the temporomandibular joint. Curr Osteoporos Rep. 2018;16(4):360–368. doi:10.1007/s11914-018-0447-7
16. Shibata S, Sakamoto Y, Yokohama-Tamaki T, Murakami G, Cho BH. Distribution of matrix proteins in perichondrium and periosteum during the incorporation of Meckel’s cartilage into ossifying mandible in midterm human fetuses: an immunohistochemical study. Anat Rec. 2014;297:1208–1217. doi:10.1002/ar.22911
17. Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biological analysis of the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2006;44:124–128. doi:10.1016/j.bjoms.2005.05.002
18. Liang W, Li X, Gao B, et al. Observing the development of the temporomandibular joint in embryonic and post-natal mice using various staining methods. Exp Ther Med. 2016;11(2):481–489. doi:10.3892/etm.2015.2937
19. Tompson ST, Bacino CA, Safina NP, et al. Fibrochondrogenesis result from mutations in the COL11A1 type XI collagen gene. Am J Hum Genet. 2010;87:708–712. doi:10.1016/j.ajhg.2010.10.009
20. Li Y, Lacerda DA, Warman ML, et al. A fibrillar collagen gene, col11a1, is essential for skeletal morphogenesis. Cell. 1995;80(3):423–430. doi:10.1016/0092-8674(95)90492-1
21. Karki R, Pandya D, Elston RC, Ferlini C. Defining “mutation” and “polymorphism” in the era of personal genomics. BMC Med Genomics. 2015;8:37. doi:10.1186/s12920-015-0115-z
22. Kurniawati S, Soedarsono S, Aulanni’am A, Ni Made Mertaniasih NM. Single nucleotide polymorphism of eccb5 gene of mycobacterium tuberculosis complex isolates from suspected pulmonary Tb patients in Surabaya Indonesia. J Infect Dis. 2018;12(2):37–42.
23. Genetic Home Reference. What are Single Nucleotide Polymorphisms (Snps)? Lister Hill National Center for Biomedical Communications U.S. National Library of Medicine National Institutes of Health; 2019.
24. Egeblad M, Shen HCJ, Behonick DJ, et al. Type I collagen is a genetic Modifier of matrix metalloproteinase 2 in murine skeletal development. Dev Dyn. 2007;236(6):1683–1693. doi:10.1002/dvdy.21159
25. Baumert P, Consortium G-REX, Stewart CE, Lake MJ, Drust B, Erskine RM. Variations of collagen-encoding genes are associated with exercise-induced muscle damage. Physiol Genomics. 2018;50(9):691–693. doi:10.1152/physiolgenomics.00145.2017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


