Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Single-Inhaler Triple Therapy in Patients with Advanced COPD: Bayesian Modeling of the Healthcare Resource Utilization Data and Associated Costs from the IMPACT Trial
Authors Gabrio A, Gunsoy NB, Baio G , Martin A , Paly VF , Risebrough N, Halpin DMG , Singh D, Wise RA , Han MK, Martinez FJ , Criner GJ, Martin N, Lipson DA , Ismaila AS
Received 2 October 2021
Accepted for publication 25 June 2022
Published 25 July 2022 Volume 2022:17 Pages 1633—1642
DOI https://doi.org/10.2147/COPD.S342244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Andrea Gabrio,1 Necdet B Gunsoy,2 Gianluca Baio,1 Alan Martin,3 Victoria F Paly,4 Nancy Risebrough,5 David MG Halpin,6 Dave Singh,7 Robert A Wise,8 MeiLan K Han,9 Fernando J Martinez,10 Gerard J Criner,11 Neil Martin,12 David A Lipson,13,14 Afisi S Ismaila15,16
1UCL Statistical Science, University College London, London, UK; 2Value Evidence & Outcomes, GlaxoSmithKline, Brentford, UK; 3Value Evidence and Outcomes, GlaxoSmithKline, Uxbridge, UK; 4Global HTA, Health Economics, Reimbursement & Outcomes, ICON Plc., Philadelphia, PA, USA; 5Global HTA, Health Economics, Reimbursement & Outcomes, ICON plc., Toronto, ON, Canada; 6University of Exeter Medical School, College of Medicine and Health, University of Exeter, Exeter, UK; 7The Centre for Respiratory Medicine and Allergy, Institute of Inflammation and Repair, Manchester Academic Health Science Centre, University of Manchester, Manchester University NHS Foundation Trust, Manchester, UK; 8The Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 9University of Michigan, Pulmonary & Critical Care, Ann Arbor, MI, USA; 10Joan and Sandy Weill Department of Medicine, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY, USA; 11Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA; 12Global Medical Affairs, GlaxoSmithKline, Uxbridge, UK; 13Development Clinical Sciences, GlaxoSmithKline, Collegeville, PA, USA; 14Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 15Value Evidence and Outcomes, GlaxoSmithKline, Collegeville, PA, USA; 16Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Afisi S Ismaila, Value Evidence and Outcomes, GlaxoSmithKline, 1250 S. Collegeville Road, Collegeville, PA, 19426-0989, USA, Tel +19199320430; +19193158229, Email [email protected]
Abstract:
Objectives: In the IMPACT trial (NCT02164513), triple therapy with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) showed clinical benefit compared with dual therapy with either FF/VI or UMEC/VI in the treatment of chronic obstructive pulmonary disease (COPD). We used data from IMPACT to determine whether this translated into differences in COPD-related healthcare resource utilization (HRU) costs in a United Kingdom (UK) setting.
Methods: In a within-trial analysis, individual patient data from the IMPACT intention-to-treat (ITT) population were analyzed to estimate rates of COPD-related HRU with FF/UMEC/VI, FF/VI, or UMEC/VI. A Bayesian approach was applied to address issues typically encountered with this kind of data, namely data missing due to early study withdrawal, subjects with zero reported HRU, and skewness. Rates of HRU were estimated under alternate assumptions of data being missing at random (MAR) or missing not at random (MNAR). UK-specific unit costs were then applied to estimated HRU rates to calculate treatment-specific costs.
Results: Under each MNAR scenario, per patient per year (PPPY) rates of COPD-related HRU were lowest amongst those patients who received treatment with FF/UMEC/VI compared with those receiving either FF/VI or UMEC/VI. Although absolute HRU rates and costs were typically higher for all treatment groups under MNAR scenarios versus MAR, final economic conclusions were robust to patient withdrawals.
Conclusions: PPPY rates were typically lower with FF/UMEC/VI versus FF/VI or UMEC/VI.
Keywords: single-inhaler triple therapy, COPD, healthcare resource use, cost
Introduction
Triple pharmacological therapy, comprising an inhaled corticosteroid (ICS), a long-acting muscarinic antagonist (LAMA), and a long-acting β2-agonist (LABA), is recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) for the treatment of patients with chronic obstructive pulmonary disease (COPD) who remain symptomatic or at risk of exacerbations despite the use of dual-therapy regimens.1
COPD is associated with high healthcare resource utilization (HRU) and costs, with higher costs observed with increasing disease severity.1 A recent systematic review of 73 primary publications investigating the economic burden of COPD reported higher direct costs, such as mean cost per patient per year (PPPY), and mean costs per exacerbation, with increased severity of COPD and/or a history of more frequent or severe exacerbations.2 Thus, patients who have advanced symptomatic disease that is not effectively managed represent a high-cost subset of the COPD population.3 Clinical studies generally produce data that are characterized by missing information due to withdrawals. Technically, this is the reason why information is not present and thus one could argue that these are not “standard” missing data. However, because the process that determines withdrawal is not entirely due to chance, but is likely to be governed by some (possibly unobserved) features of the individuals, there are many similarities with the methodology usually applied to deal with missing data. More importantly, if these are not modeled correctly, it is likely that underlying systematic differences may cause bias in the estimation of the outcomes, particularly in a health economic evaluation where we need to consider two dimensions of interests (clinical benefits and cost). To address these withdrawals, analyses may apply Bayesian methods to allow a more flexible handling of the complexities that affect the HRU data (ie, excess zeros and skewness) while also imputing the missing values for individuals who withdrew early from the study under alternative missingness assumptions.
IMPACT was a phase 3, randomized, double-blind, three-arm, parallel-group, global multicenter study that compared the rate of moderate and severe exacerbations experienced by patients who received single-inhaler (ICS/LAMA/LABA) triple therapy (fluticasone furoate [FF, 100 μg], umeclidinium [UMEC, 62.5 μg], and vilanterol [VI, 25 μg]) or one of two different dual therapies (FF/VI [100/25 μg] or UMEC/VI [62.5/25 μg]). The trial included a 2-week run-in period during which patients continued to take their own medication (a LAMA, a LABA, or an ICS alone or in combination), a 52-week treatment phase, and a 1-week follow-up period after the treatment phase.4 The study demonstrated exacerbation reduction and lung function improvement with 52 weeks of once-daily single-inhaler FF/UMEC/VI treatment, compared with dual therapy with either FF/VI (100/25 μg) or UMEC/VI (62.5/25 μg).5 The rate of COPD-related hospitalization was also lower amongst those treated with FF/UMEC/VI compared with those who received UMEC/VI.5 A cost-effective analysis of IMPACT performed from the perspective of the Canadian public healthcare payer predicted that FF/UMEC/VI would be cost effective in Canada for the treatment of patients with COPD and a history of exacerbations.6 Furthermore, a post-hoc analysis of HRU and cost data from the intent-to-treat (ITT) population of IMPACT suggested that patients treated with FF/UMEC/VI had similar HRU to patients treated with FF/VI, and lower HRU and total costs than those who received UMEC/VI.7 However, this preliminary analysis showed higher rates of discontinuation in the dual-therapy arms compared with the FF/UMEC/VI group that could potentially bias the findings. To address this observation, Bayesian methods were used to conduct a within-trial analysis of individual patient data from the IMPACT trial to estimate COPD-related HRU differences between triple and dual therapies in the ITT population. This analysis also aimed to estimate the United Kingdom (UK)-specific COPD-related cost differences between triple and dual therapies. This was achieved by applying UK-specific unit costs to the estimated HRU rates from the ITT population.
Methods
Study Design
This study was a post-hoc analysis of data from the IMPACT trial (NCT02164513). The study design, methods, and results for IMPACT have been published in detail previously.4,5 Individual patient data for HRU were analyzed to estimate differences in COPD-related HRU and costs between FF/UMEC/VI and FF/VI or UMEC/VI.
Patient Population
The patient population for this analysis was the ITT population of the IMPACT trial. Patients eligible for IMPACT were ≥40 years of age, with symptomatic advanced COPD and an exacerbation in the previous 12 months. Full eligibility criteria and patient demographics have been previously published.5 Patient baseline characteristics were similar across all three treatment groups.5 All analyses in the current study were conducted on the ITT population from IMPACT. The total patient-years in each arm were 3780.6, 3523.3, and 1730.9 in the FF/UMEC/VI, FF/VI, and UMEC/VI arms, respectively.
Data Source
HRU data were collected via the electronic case report form at scheduled study visits during the on-treatment phase of the IMPACT study only. Data included all unscheduled (ie, non-study-related) visits to a physician office or clinic, urgent care or outpatient center, emergency department, home care visits, and hospitalizations (general ward days and intensive care unit days). In addition to collecting HRU data electronically at scheduled study visits, patients were provided with a paper diary to record any healthcare contacts occurring between scheduled visits. If a patient had no HRU reported, it was assumed that no unscheduled HRU contacts occurred.
All reported healthcare contacts were categorized as COPD-related (exacerbation), COPD-related (non-exacerbation), or non-COPD-related. Data on COPD-related and non-COPD-related concomitant medication use were also collected for both the on- and off-treatment phases of the IMPACT study. For the purposes of this analysis, only COPD-related HRU (exacerbation or non-exacerbation) was included.
Unit Costs
Unit costs were specific to the UK (GBP, £), with drug acquisition costs obtained from the Monthly Index of Medical Specialities.8 UK-specific unit costs for each resource category were obtained from the Personal Social Service Research Unit (PSSRU) and National Health Service (NHS)9,10 and inflated to 2018. Unit costs were applied to the average marginal rates for each HRU type to provide the different cost components.
Endpoints
Endpoints were COPD-related HRU rates for the ITT study population for the on-treatment phase of IMPACT together with associated costs, which were estimated by applying UK-specific unit costs to the HRU rates. Only COPD-related HRU data were analyzed, including exacerbation-related and non-exacerbation-related events. Results from all analyses are reported in terms of HRU rates per patient, which were averaged across patients. These were also annualized based on per patient use and the mean number of on-treatment days to give PPPY rates.
All endpoints were reported for COPD-related care and stratified by IMPACT treatment arm.
The following COPD-related HRU outcomes were included: number of days in hospital general ward; number of days in hospital intensive care; number of home care visits; number of office visits; number of urgent care/outpatient visits; and number of emergency room visits.
Analysis
When confronted with missing data, any model must make assumptions about the process that caused some of the values to be unobserved, also known as the missingness mechanism, and which cannot be validated from the available data.11,12 Thus, for each outcome variable, we first fitted the model under a base-case missingness assumption, which considered the most plausible scenario based on the available information from the trial. We then assessed the robustness of the results for each outcome to alternative missingness assumptions in a sensitivity analysis.
A Bayesian approach was applied to estimate the relevant uncertain quantities using full probability distributions, which allowed the computation of point and interval estimates for mean adjusted HRU rates.
The model was built using a modular structure in which different components were combined to estimate the main parameters of interest, accounting for excess of zeros (Module 1), skewness (Module 2), and missingness (Module 3) (Figure 1). These three modules were modeled at the same time within a Bayesian approach without the need for separation to impute missing values or estimate the mixing proportions for the main model for the analysis.13 Full details of the model and the assumptions of missingness applied are provided in the Supplementary Appendix.
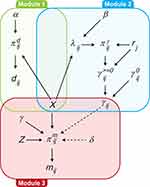 |
Figure 1 Graphical representation of the model. Abbreviations: HRU, healthcare resource utilization; MNAR, missing not at random. Notes: Graphical representation of the model at j=2, …, J, under MNAR1. Three modules form the framework. Module 1, denoted with a green box, is the model for the zero indicators d_ij, which allows separation of the zero and non-zero values in the HRU. Module 2, denoted with a blue box, is the model for the HRU data y_ij. Module 3, denoted with a red box, is the model for the missingness indicators m_ij, which accounts for the potential dependence between missingness probabilities and unobserved values at the same time. A set of base-case covariates X is included in all three modules. A set of additional missingness predictors Z is included in Module 3. The solid black arrows show the dependence relationships between the parameters within the modules, while the dashed arrows show the dependence between the parameters of the HRU and missingness module. Further details relating to the development of this model are provided in the Supplementary Appendix. |
According to the results from the descriptive analysis and assumptions about the longitudinal structure of the data, in our base-case scenario, we fitted the model under a missing not at random assumption (which we indicate as “MNAR3”). In particular, MNAR3 assumes that the probability of a patient being missing at any of the scheduled times at which data were collected over the duration of the study (ie, at 4, 16, 28, 40, and 52 weeks) depends on the patient’s HRU values at that, and the previous, visit. We informed this choice based on the descriptive analysis of the HRU data, which shows how individuals who drop out from the study are associated with systematically higher HRU values with respect to the completers at each follow-up time (Figures S1 and S2). This suggests that reasons for drop out at a given time are likely to be related to the actual unobserved value at the same time, therefore corresponding to a MNAR mechanism. In addition, we imposed a lag-one dependence model assumption, where the HRU value for each individual at a given time is linked to the value for the same individual at the previous time, thus accounting for the longitudinal nature of the data. We then fitted the model under three alternative missingness assumptions to assess the sensitivity of the results from the base-case. These included two MNAR scenarios where we assumed independence between missingness at some time point with respect to either the value at the previous time (MNAR1) or the values at the same time (MNAR2); and a missing at random (MAR) scenario where reasons for missingness are assumed not to depend on unobserved HRU values. We included the MAR scenario for comparison purposes, as this is a popular missingness assumption for trial-based cost-effectiveness analyses, even though we believe it is unlikely to hold in our case.
The findings presented here will focus on those observed with MNAR3, with findings from MNAR1, MNAR2, and MAR reported in the Supplementary Appendix as sensitivity analyses. Interval estimates were derived from the posterior distributions of each targeted quantity.
Results
HRU Rates
The mean and 95% credible intervals based on the posterior distribution of the aggregate PPPY adjusted rates in the trial by treatment group for each type of HRU are shown in Table 1 under MNAR3 (base-case scenario; MNAR1, MNAR2, and MAR shown in Tables S1–S3). PPPY rates of HRU were observed to be lowest amongst those patients who received treatment with FF/UMEC/VI when compared with those treated with either FF/VI or UMEC/VI (Figure 2). This is indicated by the rate ratios for FF/UMEC/VI compared with FF/VI or UMEC/VI, which are <1. In particular, there was a marked decrease in office visits for patients receiving FF/UMEC/VI compared with FF/VI or UMEC/VI. These findings were confirmed in the sensitivity analyses (Figures S3–S8). The per patient HRU rates were similar across all groups (Figure S9).
 |
Table 1 Mean and 95% Credible Intervals of the PPPY Rates for Each Type of HRU Over the IMPACT Trial Duration by Treatment Group Under MNAR3 (Base-Case Scenario) |
Total Costs
Mean and 95% credible intervals for PPPY total costs over the duration of the trial for MNAR3 (base-case scenario) are shown in Table 2. Mean total cost differences demonstrated that FF/UMEC/VI was less costly than either FF/VI or UMEC/VI for the management of patients with COPD. It is of note that the individuals who withdrew from the study had substantially different characteristics (including post-bronchodilator percent predicted FEV1, baseline eosinophil, race, and number of previous exacerbations treated with antibiotics, summarized in Table S4) compared with those who remained. Sensitivity analyses confirmed that the final economic conclusions were not affected by these withdrawals (Figure S10). Data for the three alternative missingness scenarios (MNAR1, MNAR2, and MAR) are included in Table S5.
 |
Table 2 Mean and 95% Credible Intervals of the PPPY Total Costs Over the IMPACT Trial Duration by Treatment Group. |
Discussion
The findings of this study demonstrated that PPPY HRU rates were consistently lower for those patients treated with FF/UMEC/VI when compared with treatment using either FF/VI or UMEC/VI. Moreover, UK costs associated with treatment with FF/UMEC/VI were shown to be lower than with either FF/VI or UMEC/VI. These findings were demonstrated in the base-case scenario and across all assumptions of missingness explored in this study.
It is of note that the results from the base-case scenario indicate relatively small differences between the per patient HRU rates across the three treatment groups for each type of HRU. In each group, similar point and interval estimates were obtained at each scheduled visit time, which resulted in aggregated per patient rates that were relatively similar for FF/UMEC/VI, FF/VI, and UMEC/VI. However, when these estimates were annualized using the mean number of treatment exposure days for study participants, the PPPY rates for individuals receiving FF/UMEC/VI were generally lower compared with those in the FF/VI and UMEC/VI groups for all HRU types. PPPY is the appropriate comparison, since it adjusts for exposure time. Calculation of PPPY total costs for each treatment based on estimated HRU rates and unit costs from published sources showed that under all scenarios, FF/UMEC/VI is on average cheaper than FF/VI and UMEC/VI.
Within this study, we set MNAR3 as the base-case scenario as this was considered the most plausible missingness assumption based on the results from the descriptive analysis. We reported the results under MAR as a sensitivity analysis since this is the default assumption used in previous published analyses.14,15 Although characteristics of the individuals who withdrew from the study were substantially different compared with those who remained, sensitivity analyses that consider both deviations from the base-case (MNAR1 and MNAR2) and even MAR as the “least plausible” scenario suggest that the final economic conclusions are robust to different assumptions on missingness. The three MNAR scenarios explored found that the individuals who withdrew from the study had greater HRU when compared with those who completed the study, with different variability across the scenarios (Figure S1). The MNAR assumption is used to explore whether HRU is being censored in the patients where HRU would be higher. Since rates are higher under MNAR, this may be the case. However, the patients who withdrew from the study are also associated with lower proportion of zeros at each time, indicating that the censoring had only a minimal effect on the analysis (Figure S2). Indeed, the treatment differences in HRU are robust to whether MAR or MNAR is assumed.
The results of the IMPACT trial demonstrated that treatment with FF/UMEC/VI was associated with lower rates of moderate or severe exacerbations of COPD than treatment with either FF/VI or UMEC/VI.5 Moreover, when compared with UMEC/VI, FF/UMEC/VI was associated with a lower rate of hospitalizations due to COPD.5 The higher rates of exacerbations and hospitalizations with FF/VI and UMEC/VI support that patients who withdrew early on these treatments would have had higher HRU in the unobserved period, and thus MNAR is more likely to be the correct assumption. These findings, together with the HRU results reported here, suggest that longer-term use of FF/UMEC/VI reduces the medical healthcare resource burden and economic consequences of COPD when compared with either FF/VI or UMEC/VI.
The potential offset in costs owing to the lower observed rates of moderate or severe exacerbations of COPD is further supported by an analysis of HRU in the FULFIL (Lung FUnction and quality of LiFe assessment in COPD with closed trIpLe therapy) trial, which showed that HRU and associated non-drug costs were lower amongst those patients treated with single-inhaler triple therapy with FF/UMEC/VI compared with patients receiving dual therapy with budesonide/formoterol.16 Moreover, a previous within-trial economic analysis of the IMPACT trial conducted from the United States healthcare perspective revealed reduced costs associated with FF/UMEC/VI compared with either FF/VI or UMEC/VI.3 It should be noted that Bogart et al only estimated costs relating to exacerbation events, whereas the current study assessed both exacerbation and non-exacerbation related events.3 Our findings are also supported by the recent systematic review that reported increased direct costs and higher rates of HRU, in terms of hospitalization, length of hospital stay, and primary care interactions, for patients with more severe COPD and COPD exacerbations.2
It is worth considering how deaths were accounted for in the study, since it could be the case that more missing people died in one arm, while more missing people in the other arms were associated with a continued use of HRU, affecting the average cost difference between arms. Evidence was not available regarding when and if individuals who dropped out from the study died, and we do not have information regarding which scenario is more plausible (ie, whether the chance of having a considerable difference in the proportions of dead between arms is high/low). The implicit assumption in our analyses is that the proportions do not massively differ between arms as otherwise we would need to explicitly model the mechanism associated with death. In such a case, we would need to make unverifiable assumptions about the time that will pass from the moment individuals drop out and when they die, as well as whether and how much these two aspects differ between arms.
Limitations of this study include that assessment of HRU using clinical trial data may not be generalizable to clinical practice. Moreover, these analyses were reliant on the completeness of the available data from the IMPACT trial. While the Bayesian modeling approach attempted to account for missingness, exploring MAR and MNAR are by necessity based on untestable assumptions. In addition, each HRU endpoint examined within this study was modeled independently using a sequential approach that did not consider any potential correlation amongst the outcomes. Nonetheless, IMPACT included >10,000 subjects in a randomized controlled trial setting, with the advantage of helping to balance baseline characteristics. Finally, our findings are UK-specific due to the use of UK unit costs and may or may not be representative of other markets; future analyses could examine treatment-specific costs associated with FF/UMEC/VI in other countries.
In conclusion, although the absolute HRU rates and costs are typically higher for all treatment groups under the MNAR scenarios compared with MAR, the final economic conclusions are robust to these different assumptions on missingness. Specifically, under each MNAR scenario, the per patient HRU rates were similar across all groups, while the PPPY rates were typically lower with FF/UMEC/VI compared with FF/VI or UMEC/VI. Similar conclusions hold for the results related to the PPPY total costs, indicating that FF/UMEC/VI, on average, reduces medical HRU costs compared with the competing treatments assessed herein across all missingness scenarios.
Abbreviations
COPD, chronic obstructive pulmonary disease; CrI, credible interval; ER, emergency room; FF, fluticasone furoate; FULFIL, Lung FUnction and quality of LiFe assessment in COPD with closed trIpLe therapy; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GW, general ward; HRU, healthcare resource utilization; HV, home visit; IC, intensive care; ICS, inhaled corticosteroid; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MAR, missing at random; MNAR, missing not at random; NHS, National Health Service; OV, office visit; PPPY, per patient per year; PSSRU, Personal Social Service Research Unit; UC, urgent care; UK, United Kingdom; UMEC, umeclidinium; VI, vilanterol.
Data Availability
The datasets supporting the results reported in this manuscript are not publicly available. Access to the raw data may be granted on reasonable request to the corresponding author dependent on the intended use and subject to third-party agreements.
Ethics Approval and Informed Consent
Not applicable; anonymized patient-level data were used in this analysis.
Acknowledgments
Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing, and referencing) was provided by Sarah Birch of Gardiner-Caldwell Communications (Macclesfield, UK) and Caroline McGown of Aura, a division of Spirit Medical Communications Group Ltd (Manchester, UK), and was funded by GlaxoSmithKline. Trademarks are the property of their respective owners.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The IMPACT trial (NCT02164513) and this associated study (HO-18-16283) were funded by GlaxoSmithKline.
Disclosure
Dr Gabrio has nothing to disclose. Dr Gunsoy reports personal fees from AbbVie (current employee, and owns stock), and GlaxoSmithKline (former employee). Dr Baio has nothing to disclose. Dr A. Martin is an employee of GlaxoSmithKline.
At the time of the study, Dr Paly was an employee of ICON. Dr Risebrough reports grants from GlaxoSmithKline. Dr Halpin reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer, and Sanofi. Dr Singh reports personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, personal fees from Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Sanofi, personal fees from Synairgen, personal fees from Teva, Theravance, and Verona. Dr Wise reports research grants from AstraZeneca, 4D Pharma, Chiesi, Savara, Vaxart, Polarean, Boehringer Ingelheim, GlaxoSmithKline, MedImmune, Pearl, and Sanofi-Aventis; is a consultant for AstraZeneca, Circassia, Galderma, MedImmune, Mylan/Theravance, Pearl, Pneuma, Propeller Health, and Verona; is on an advisory board for GlaxoSmithKline; data monitoring committee for AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, ChemRx, GlaxoSmithKline, Kamada, Kinevant, MedImmune, Merck, Pearl, Pulmonx, PureTech, and Roche-Genentech; steering committee for Boehringer Ingelheim, and GlaxoSmithKline; and clinical endpoint committee for Contrafect, GlaxoSmithKline, and Kiniksa. Dr Han reports analytic and publication support for this research from GlaxoSmithKline. She also reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Pulmonx, Teva, Verona, Merck, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, Cipla and Chiesi. She has received stock options from Meissa Vaccines. She has received either in kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation and the American Lung Association. She has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. Dr Martinez reports receiving honoraria and travel support from AstraZeneca, Boehringer Ingelheim, Canadian Respiratory Society, CME Outfitters, CSL Behring, Genentech, GlaxoSmithKline, Inova Fairfax, MDMagazine, Miller Communications, National Association for Continuing Education/Integritas, Novartis, NYP Methodist Hospital Brooklyn, Patara/Respivant, Peer View, Rare Diseases Healthcare Communications, Sanofi/Regeneron, Sunovion, Teva, University of Birmingham Alabama, and WebMD/MedScape; honoraria from Dartmouth University, New York University, Pearl, Physicians Education Resource, Rockpointe Communications, Theravance/Viatris, Medtronic, UpToDate, and Vindico; travel support from Chiesi and Zambon; research support from ProTerrix Bio and Zambon; publication support from Boehringer Ingelheim; is on an advisory board for AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Gala, Genentech, GlaxoSmithKline, Pearl, Sanofi/Regeneron, Sunovion, Teva, Zambon, and Verona; steering committee for AstraZeneca, AbbVie, Afferent/Merck, Bayer, Biogen, Boehringer Ingelheim, GlaxoSmithKline, Nitto, Patara/Respivant, ProMedior, Shionogi, United Therapeutics, and Veracyte; data monitoring committee for Biogen, Boehringer Ingelheim, Genentech Asthma, and GlaxoSmithKline; is a consultant for AbbVie, IQVIA, ProTerrix Bio, and Raziel; continuing medical education/study presentations for Boehringer Ingelheim, Canadian Respiratory Society, Dartmouth University, France Foundation, Inova Fairfax, National Association for Continuing Education/Integritas, New York University, Novartis, NYP Methodist Hospital Brooklyn, Peer View, Physicians Education Resource, Rare Diseases Healthcare Communications, Rockpointe Communications, University of Birmingham Alabama, Vindico, and WebMD/MedScape; and teleconference without compensation for Bristol Myers Squibb and twoXAR. Dr Criner reports consulting fees from Amgen, AstraZeneca, Auris Health, Boehringer Ingelheim, Broncus, BTG, Chiesi, CSA Medical, EOLO, Fisher Paykel, Gilead, GlaxoSmithKline, Intuitive Surgical Inc, Lungpacer, Medtronic Vascular, Mereo, NGM, Novartis Pharma AG, Nuvaira, Olympus, Pneumrx, Pulmonx, Regeneron Healthcare Solutions Inc, ResMed, Respironics, Sanofi, and Verona; and ownership interest in HGE Health Care Solutions LLC. At the time of the study, Dr N. Martin was an employee of GlaxoSmithKline. Dr Lipson is an employee of and owns stocks/shares in GlaxoSmithKline. Dr Ismaila is an employee of and owns stocks/shares in GlaxoSmithKline; and is an unpaid part-time Professor at McMaster University, Canada. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.1wms.pdf.
2. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460. doi:10.2147/COPD.S234942
3. Bogart MR, Hopson SD, Shih HC, Stanford RH, Coutinho AD. COPD exacerbation costs in the IMPACT study: a within-trial analysis. Am J Manag Care. 2020;26(5):e150–e154.
4. Pascoe SJ, Lipson DA, Locantore N, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur Respir J. 2016;48(2):320–330. doi:10.1183/13993003.02165-2015
5. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
6. Ismaila AS, Risebrough N, Schroeder M, et al. Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: the IMPACT trial. Int J Chron Obstruct Pulmon Dis. 2019;14:2681–2695. doi:10.2147/COPD.S216072
7. Ismaila AS, Paly V, Schroeder M, et al. IMPACT study single inhaler triple therapy (FF/UMEC/VI) versus FF/VI and UMEC/VI in patients with COPD: healthcare resource utilization and costs – UK. Eur Respir J. 2018;52(Suppl 62):OA2128.
8. MIMS; 2018. Available from: https://www.mims.co.uk/.
9. Personal Social Services Research Unit. Unit costs of health and social care; 2017. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/.
10. National Health Service. National health service reference costs 2016/17; 2016. Available from: https://improvement.nhs.uk/resources/reference-costs/.
11. Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. doi:10.1093/biomet/63.3.581
12. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987.
13. Gabrio A, Mason AJ, Baio G. A full Bayesian model to handle structural ones and missingness in economic evaluations from individual‐level data. Stat Med. 2019;8(38):1399–1420. doi:10.1002/sim.8045
14. Gabrio A, Mason AJ, Baio G. Handling missing data in within-trial cost-effectiveness analysis: a review with future recommendations. Pharmacoecon Open. 2017;1(2):79–97. doi:10.1007/s41669-017-0015-6
15. Leurent B, Gomes M, Carpenter JR. Missing data in trial‐based cost‐effectiveness analysis: an incomplete journey. Health Econ. 2018;27(6):1024–1040. doi:10.1002/hec.3654
16. Ismaila AS, Birk R, Shah D, et al. Once-daily triple therapy in patients with advanced COPD: healthcare resource utilization data and associated costs from the FULFIL trial. Adv Ther. 2017;34(9):2163–2172. doi:10.1007/s12325-017-0604-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

